Almost 5 decades after the first clinical transplantations, delayed immune reconstitution remains a considerable hurdle in bone marrow transplantation, and the mechanisms regulating immune reconstitution after transplantation remain to be established. Whereas adult fms-like tyrosine kinase 3 ligand–deficient (FL−/−) mice have reduced numbers of early B- and T-cell progenitors, they sustain close to normal levels of mature B and T cells. Herein, we demonstrate that adult bone marrow cells fail to reconstitute B-cell progenitors and conventional B cells in lethally irradiated FL−/− recipients, which also display delayed kinetics of T-cell reconstitution. Similarly, FL is essential for B-cell regeneration after chemotherapy-induced myeloablation. In contrast, fetal progenitors reconstitute B lymphopoiesis in FL−/− mice, albeit at reduced levels. A critical role of FL in adult B lymphopoiesis is further substantiated by an age-progressive decline in peripheral conventional B cells in FL−/− mice, whereas fetally and early postnatally derived B1 and marginal zone B cells are sustained in a FL-independent manner. Thus, FL plays a crucial role in sustaining conventional B lymphopoiesis in adult mice and, as a consequence, our findings implicate a critical role of FL in promoting immune reconstitution after myeloablation and bone marrow transplantation.
Introduction
The period of immune deficiency after high-dose chemotherapy and bone marrow (BM) transplantation (BMT) results in significant morbidity and mortality.1 Whereas early recovery of innate immunity (granulocytes, monocytes/macrophages and natural killer [NK] cells) results in reconstitution of protective immunity against many bacterial pathogens, the levels of functional lymphocytes (components of adaptive immunity) frequently remain abnormal for many months or years.2 Low levels of B cells and immunoglobulins, as well as reduced CD4 T cells, predispose transplant recipients to severe bacterial (such as Streptococcus pneumoniae or Haemophilus influenzae) and opportunistic viral infections (such as cytomegalovirus, Epstein-Barr virus, and herpes).1,3,,–6 Consequently, significant efforts have been pursued to develop more efficient means of accelerating immune recovery after BMT. Whereas the identification of granulocyte colony–stimulating factor and granulocyte/monocyte colony–stimulating factor has been used to successfully promote clinical granulocyte recovery,7 no cytokine treatment has been developed to accelerate B- and T-cell reconstitution after myeloablation and BMT.
The fms-like tyrosine kinase 3 (FLT3, also called Flk2) receptor and its ligand (FL) have been shown to play an important role in T- and particular B-cell development.8,,–11 However, despite having extensively reduced B-cell progenitors, mature B cells are reduced to a much less extent in adult FL−/−11 and Flk2−/−8 mice, suggesting a redundant role of FL in peripheral B-cell homeostasis. The role of FL in sustaining mature B cells in older mice when B-cell progenitors in the BM normally are reduced12,13 has not, however, been investigated.
Notably, patients undergoing chemotherapy-induced myeloablation and BMT have increased serum levels of FL,14,15 initially thought to reflect an important role of FL in hematopoietic stem cell reconstitution after BMT. However, more recent studies in FL−/− mice suggest that FL is not important in regulation of hematopoietic stem cell numbers or function, but rather in early lymphoid development.10,16 Thus, in the present study we explored whether FL might play an important role in B- and T-cell reconstitution after BMT and chemotherapy-induced myeloablation. Herein, we provide compelling evidence for a fundamental role of FL in regeneration of the B-cell compartment after myeloablation of adult recipients, and in line with this, an age-progressive loss of B-cell progenitors in adult FL−/− mice, ultimately resulting in depletion of conventional B cells. In contrast, the peripheral pool of B1 and marginal zone (MZ) B cells, derived from progenitors existing predominantly during fetal and early postnatal development, are sustained and expanded in a FL-independent manner.
Materials and methods
Animals
Mice deficient in FL expression on a pure C57BL/6 (CD45.2) background were generated as previously described.9 FL−/− CD45.1 mice were obtained by cross breeding of FL−/− CD45.2 mice and wild-type (WT) CD45.1 C57BL/6 mice and subsequent interbreeding of heterozygous FL+ /− CD45.1/CD45.2 mice. Mice used for experiments were obtained through breeding of homozygous FL−/− CD45.1 mice. FL−/− CD45.2 and FL−/− CD45.1 as well as WT congenic C57BL/6 CD45.1 and CD45.2 mice were used in transplantation experiments. Mice were maintained under specific pathogen-free conditions and fed irradiated chow and autoclaved acidified water. For the analysis of embryonic B-cell development, timed pregnancies were established, with the day of vaginal plug observed considered as day 0.5.11 All animal protocols were approved by the local Ethical Committee at Lund University (Lund, Sweden).
Antibodies
All antibodies were from Becton Dickinson (Franklin Lakes, NJ) unless otherwise indicated. Monoclonal antibodies (conjugated with different fluorochromes) used to stain cell surface antigens were CD16/32 (clone 2.4G2), B220 (RA3–6B2), CD11b/Mac-1 (M1/70), CD4 (H129.9), CD8α (53-6.7), Ter-119 (LY-76), SCA-1 (E13-161.7), C-KIT (2B8), CD45.1 (A20), CD45.2 (104), FLT3 (AZF10.1), CD43 (S7), IL-7Rα (A7R34), Gr-1 (RB6–8C5), CD5 (53-7.3), CD3 (17A2), CD21 (7G6), CD23 (B3B4), AA4.1, IgD (II/41), NK1.1 (PK136), and anti-IgM (R6-60.2). Biotinylated antibodies were visualized by streptavidin-PE (phycoerythrin), or streptavidin-PECy7, and purified antibodies by polyclonal goat antirat-Tricolor (both Caltag, Buckingham, United Kingdom). In some experiments 7-amino-actinomycin D (Sigma, St. Louis, MO) was added to exclude dead cells from the analysis.
BMT experiments
Noncompetitive and competitive reconstitution assays were performed using C57BL/6 CD45.1 and CD45.2 congenic WT as well as FL−/− strains as previously described.17 In this model, test cells can be separated from host and competitor cells, using antibodies specific for CD45.1 and CD45.2. Importantly, B cells (B220+ or B220+IgM+), T cells (CD4/CD8+), and myeloid cells (Mac-1+) in peripheral blood (PB) as well as all BM stem and progenitor subsets ubiquitously express the pan-hematopoietic CD45 antigen. To evaluate their ability to reconstitute the different blood cell lineages and B-cell progenitors, lethally irradiated (900-975 rads) WT or FL−/− (CD45.1 or CD45.2) recipient mice were transplanted with 0.2-1.0 × 106 (as specified) freshly isolated WT and FL−/− BM or embryonic day 14.5 fetal liver (test) cells expressing the other CD45 isoform. In competitive transplantation experiments, mice were also cotransplanted with the same number of unfractionated BM cells expressing the same CD45 isoform as the recipients; 3, 5, and 16 weeks after transplantation, PB, BM, and/or spleens were collected from the recipient mice and analyzed for donor-derived reconstitution by flow cytometry.
Analysis of hematopoietic reconstitution in mice with transplanted BM or fetal liver cells
PB was collected from the retro-orbital sinus venous plexus and stained against CD45.1 and CD45.2 to determine the level of donor reconstitution. To determine reconstitution of different blood lineages, PB was analyzed using a modification of a previously reported protocol.18 Briefly, nucleated cells were stained with lineage specific anti mouse antibodies against CD45.1, CD45.2, Mac-1 (myeloid cells), B220, and anti-IgM (B cells), CD4, and CD8α (T cells). Positively reconstituted mice were defined as having a minimum of 0.1% total and 0.02% of each of the B, T, and myeloid test cell contribution toward total PB cells.
5-FU treatment
The 9- to 15-week-old mice were injected subcutaneously with 0.2 mL 5-fluorouracil (5-FU; Sigma; 150 mg/kg mouse body weight). At 7, 14, and 42 days after treatment, PB was drawn from orbital sinux plexus and analyzed. At 68 to 83 days after 5-FU treatment, BM was harvested and B-cell progenitors were analyzed.
Treatment with FLT3 ligand
The 27- to 33-week-old FL−/− mice or BM-transplanted FL−/− lethally irradiated recipients were injected subcutaneously with FL at 10 μg or 0.4 μg per mouse, or with phosphate-buffered saline (PBS, BioWhittaker, Walkersville, MD) as control, every other day for 2 to 3 weeks. The last injection was performed 8 hours before PB and BM cellularities were established and tissues were analyzed by flow cytometry for levels of mature B cells and distinct stages of B-cell progenitors.
Identification and staging of early B-cell progenitors
Common lymphoid progenitors (CLPs), pre-pro-, pro-, and pre-B cells were identified as previously described.19,–21 Briefly, CLPs were defined as Lin−SCA1loC-KITloIL-7Rα+, pre-pro-B cells as B220+CD43+AA4.1+CD19−, pro-B cells as B220+CD43+AA4.1+CD19+, and pre-B cells as B220+CD43−IgM−. MZ B cells were defined as IgMhiCD21hiCD23loIgDlo, B1 cells in the spleen as IgM+CD23loCD43+ 22,23 and B1a cells in the fetal liver as B220+IgM+CD5+NK1.1−.23 All flow cytometry analyses were performed on FACSCalibur, FACSDiva, or FACSAria (Becton Dickinson) using FlowJo software (Tree Star Inc.).
Statistical analysis
The statistical significance of differences between WT and FL−/− mice was determined using the 2-tailed Mann-Whitney test.
Results
Critical role of FLT3 ligand in B-cell reconstitution after BMT and myeloablation
To address the role of FL in promoting B- and T-cell reconstitution after BM transplantation, it was essential to develop an experimental strategy in which not only the recipients but also the donor mice were FL-deficient, because it has been demonstrated that BM-derived cells, such as T cells, stromal cells, and endothelial cells, represent a major source of FL.10,24,–26 To accomplish this and at the same time be able to make the important distinction between donor- and host-derived cells post-transplantation, we generated FL−/− donor mice on a pure C57BL/6 background expressing the pan-hematopoietic marker CD45.1, whereas recipients expressed CD45.2 (see Materials and methods). Unfractionated WT BM cells (CD45.1) were transplanted into lethally irradiated WT (CD45.2) recipients, whereas FL−/− BM cells (CD45.1) were transplanted into FL−/− (CD45.2) recipients. Strikingly, virtually no PB B-cell reconstitution could be observed in FL−/− mice (Figure 1A,B), translating into as much as 43-fold and 208-fold reduced numbers of donor-derived B cells (compared with WT recipients) at 3 and 5 weeks after transplantation, respectively. Although much less significant, reconstitution of T cells was almost 3-fold lower in FL−/− compared with WT mice at both 3 and 5 weeks after transplantation. At 16 weeks after transplantation, B-cell reconstitution in FL−/− mice remained almost undetectable (61-fold lower than in WT recipients), whereas T cells remained slightly but significantly reduced (Figure 1C). The reduction in mature B cells in transplanted FL−/− mice was not a result of a redistribution to other sites, as comparable reductions were observed in the spleen (25-fold; Figure 1D) and BM (60-fold; Figure 1E).
Critical role of FLT3 ligand in B-cell regeneration after BM transplantation. Lethally irradiated adult WT mice were transplanted with 2 × 105 unfractionated BM cells from 9- to 11-week-old WT mice (WT into WT), and FL−/− recipient mice were transplanted with 2 × 105 unfractionated FL−/− BM cells (FL−/− into FL−/−). In all experiments, donor- and recipient-derived blood cells could be separated based on expression of different CD45 isoforms (“Materials and methods”). Donor-derived B- and T-cell reconstitution of PB at (A) 3, (B) 5, and (C) 16 weeks after transplantation. FACS plots show donor-derived B-cell (B220+ or B220+IgM+) and T-cell (CD4/CD8+) reconstitution from representative mice transplanted with WT and FL−/− cells. Numbers indicate percentage of total donor-derived cells within the indicated quadrants. Bar graphs show mean (standard error of mean [SEM]) values from 19-27 recipient mice of each genotype, from 3-4 independent experiments. Donor-derived B cells (B220+CD19+) in the spleen (D) and BM (per 2 tibiae and 2 femora) (E) 16 weeks after transplantation. Mean (SEM) values from 9-21 recipients and 2-3 independent experiments. **P < .01, ***P < .001, compared with WT mice.
Critical role of FLT3 ligand in B-cell regeneration after BM transplantation. Lethally irradiated adult WT mice were transplanted with 2 × 105 unfractionated BM cells from 9- to 11-week-old WT mice (WT into WT), and FL−/− recipient mice were transplanted with 2 × 105 unfractionated FL−/− BM cells (FL−/− into FL−/−). In all experiments, donor- and recipient-derived blood cells could be separated based on expression of different CD45 isoforms (“Materials and methods”). Donor-derived B- and T-cell reconstitution of PB at (A) 3, (B) 5, and (C) 16 weeks after transplantation. FACS plots show donor-derived B-cell (B220+ or B220+IgM+) and T-cell (CD4/CD8+) reconstitution from representative mice transplanted with WT and FL−/− cells. Numbers indicate percentage of total donor-derived cells within the indicated quadrants. Bar graphs show mean (standard error of mean [SEM]) values from 19-27 recipient mice of each genotype, from 3-4 independent experiments. Donor-derived B cells (B220+CD19+) in the spleen (D) and BM (per 2 tibiae and 2 femora) (E) 16 weeks after transplantation. Mean (SEM) values from 9-21 recipients and 2-3 independent experiments. **P < .01, ***P < .001, compared with WT mice.
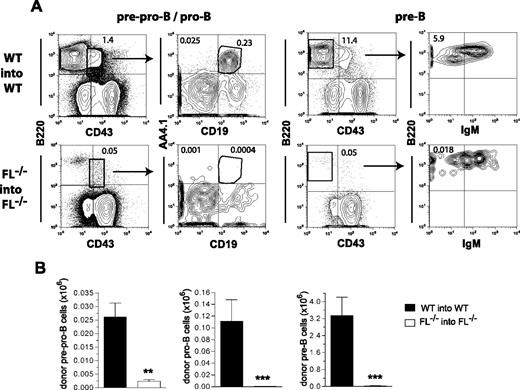
At 16 weeks after transplantation we also investigated whether the impaired recovery of mature B cells in FL−/− recipients was attributable to deficient reconstitution of B-cell progenitors in the BM using established procedures for B-cell progenitor staging.19,20 A severe reduction in pre-pro-B (B220+CD43+CD19−AA4.1+; 11-fold), pro-B (B220+CD43+CD19+AA4.1+; 156-fold), and pre-B (B220+CD43−IgM−; 97-fold) cells were observed (Figure 2), demonstrating a severely impaired ability of BM stem/progenitor cells to reconstitute B-cell progenitors in the absence of FL. Taken together, these results clearly demonstrate a critical role of FL in the recovery of B-cell lymphopoiesis after transplantation.
Role of FLT3 ligand in regeneration of B-cell progenitors after BM transplantation. Lethally irradiated adult WT mice were transplanted with 2 × 105 unfractionated BM cells from 9- to 10-week-old WT mice, whereas FL−/− recipient mice were transplanted with 2 × 105 unfractionated BM cells from FL−/− mice. In all experiments, donor- and recipient-derived BM cells could be separated based on expression of different CD45 isoforms. The frequency and absolute numbers of donor-derived B-cell progenitors in the BM of transplanted mice were determined 16 weeks after transplantation. (A) FACS profiles from representative mice showing donor-derived (gated on CD45.1 vs CD45.2) pre-pro-B (B220+CD43+AA4.1+CD19−), pro-B (B220+CD43+AA4.1+CD19+), and pre-B (B220+CD43−IgM−) cells. Numbers indicate percentage of total donor-derived cells within the indicated gates or quadrants. (B) Bar graphs show mean (SEM) numbers of donor-derived pre-pro-B, pro-B, and pre-B cells (per 2 tibiae and 2 femora) from 7 recipient mice of each genotype from 1 of 2 representative experiments. **P < .01, ***P < .001, compared with WT mice.
Role of FLT3 ligand in regeneration of B-cell progenitors after BM transplantation. Lethally irradiated adult WT mice were transplanted with 2 × 105 unfractionated BM cells from 9- to 10-week-old WT mice, whereas FL−/− recipient mice were transplanted with 2 × 105 unfractionated BM cells from FL−/− mice. In all experiments, donor- and recipient-derived BM cells could be separated based on expression of different CD45 isoforms. The frequency and absolute numbers of donor-derived B-cell progenitors in the BM of transplanted mice were determined 16 weeks after transplantation. (A) FACS profiles from representative mice showing donor-derived (gated on CD45.1 vs CD45.2) pre-pro-B (B220+CD43+AA4.1+CD19−), pro-B (B220+CD43+AA4.1+CD19+), and pre-B (B220+CD43−IgM−) cells. Numbers indicate percentage of total donor-derived cells within the indicated gates or quadrants. (B) Bar graphs show mean (SEM) numbers of donor-derived pre-pro-B, pro-B, and pre-B cells (per 2 tibiae and 2 femora) from 7 recipient mice of each genotype from 1 of 2 representative experiments. **P < .01, ***P < .001, compared with WT mice.
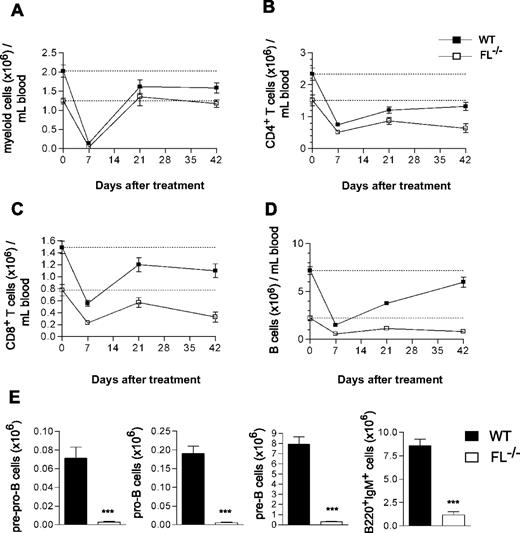
To further investigate the role of FL in regeneration of B and T lymphopoiesis after clinically relevant chemotherapy-induced myeloablation, adult mice were next treated with 5-FU, resulting in efficient ablation of hematopoietic progenitor cells, whereas activating quiescent hematopoietic stem cells are resistant to 5-FU.27,28 Whereas recovery of myeloid cells was unaffected by FL deficiency (Figure 3A), the recovery of CD4+ and CD8+ T cells was impaired in FL−/− mice (Figure 3B,C). Specifically, whereas 36% and 58% of the reductions induced in CD4+ and CD8+ T cells were recovered in WT mice by 42 days, only 13% (P < .05 WT vs FL−/−) and 18% (P = .2 WT vs FL−/−), respectively, were recovered in FL−/− mice (Figure 3B,C). Whereas the reduced B cells in WT mice recovered almost completely by 42 days (78%), the recovery was only marginal in FL−/− mice (15%; Figure 3D; P < .01 WT vs FL−/−). Furthermore, the recovery of B-cell progenitors in the BM was severely impaired in FL−/− mice, as demonstrated by pre-pro-B, pro-B, and pre-B cells being reduced 96%, 97%, and 96%, respectively, in 5-FU–treated FL−/− mice (Figure 3E), as well as by reaching only 34%, 36%, and 22%, respectively, of the already considerably reduced steady-state levels in FL−/− mice (data not shown). These data establish an essential role of FL in B- and T-cell regeneration after chemotherapy-induced myeloablation.
Impaired B-cell recovery after chemotherapy-induced myeloablation of FL−/− mice. The 9- to 15-week-old WT and FL−/− mice were treated with a single dose of 5-FU (150 mg/kg mouse weight). PB cellularity and lineage composition were determined at different time points post-treatment. At 7 days before treatment, PB was analyzed to establish the baseline levels of all blood cell lineages (depicted as day 0 and indicated by a dotted line). Data are expressed as mean (SEM) number of Mac-1+ myeloid cells (A), CD4+ T cells (B), CD8+ T cells (C), and B220+IgM+ B cells (D) per mL blood. (E) At day 68-83 of treatment, BM cells were harvested and counted from 5-FU–treated mice and B-cell progenitor subsets analyzed (“Materials and methods”). Data are expressed as mean (SEM) number of each progenitor subset (from 2 tibiae and 2 femora) from 11-14 treated mice per genotype from 2 experiments. ***P < .001 compared with WT mice.
Impaired B-cell recovery after chemotherapy-induced myeloablation of FL−/− mice. The 9- to 15-week-old WT and FL−/− mice were treated with a single dose of 5-FU (150 mg/kg mouse weight). PB cellularity and lineage composition were determined at different time points post-treatment. At 7 days before treatment, PB was analyzed to establish the baseline levels of all blood cell lineages (depicted as day 0 and indicated by a dotted line). Data are expressed as mean (SEM) number of Mac-1+ myeloid cells (A), CD4+ T cells (B), CD8+ T cells (C), and B220+IgM+ B cells (D) per mL blood. (E) At day 68-83 of treatment, BM cells were harvested and counted from 5-FU–treated mice and B-cell progenitor subsets analyzed (“Materials and methods”). Data are expressed as mean (SEM) number of each progenitor subset (from 2 tibiae and 2 femora) from 11-14 treated mice per genotype from 2 experiments. ***P < .001 compared with WT mice.
FL is increasingly important with age in sustaining conventional B lymphopoiesis from multipotent stem/progenitor cells
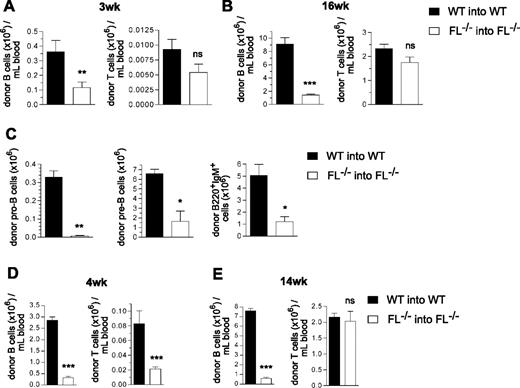
To investigate whether the severely impaired ability of multipotent FL−/− stem/progenitor cells to reconstitute B cells after transplantation is ontogeny-dependent, we next transplanted adult lethally irradiated recipients with embryonic day 14.5 fetal liver hematopoietic cells, because the liver is the site of fetal B lymphopoiesis.29 Although reduced compared with the reconstitution observed with WT cells (3.1- and 6.5-fold after 3 and 16 weeks, respectively), a much higher level of PB B cell reconstitution was observed in adult FL−/− recipients transplanted with fetal liver cells (Figure 4A,B) than with adult BM cells (43-fold and 61-fold, respectively; Figure 1A,C). Furthermore, T-cell reconstitution was not significantly affected in FL−/− recipients of fetal liver cells (Figure 4A,B). Notably, BM analysis at 17 weeks post-transplantation also revealed a less dramatic reduction in pro-B and pre-B cells (47-fold and 4.0-fold, respectively; Figure 4C) than in recipients of adult BM cells (156-fold and 97-fold, respectively; Figure 2A,B), and B220+IgM+ cells were reduced 4.2-fold (Figure 4C).
B-cell reconstitution in FL−/−mice after transplantation of fetal liver cells or BM cells from 2 week old mice. Lethally irradiated adult WT and FL−/− mice were transplanted with 2 × 105 unfractionated day 14.5 fetal liver cells (A-C) or 5 × 105 unfractionated 2-week-old BM cells (D,E), from WT and FL−/− mice, respectively. Donor- and recipient-derived blood cells could be separated based on expression of different CD45 isoforms (Materials and methods). (A,B) Donor-derived PB B-cell and T-cell reconstitution 3 and 16 weeks after fetal liver cell transplantation. Mean (SEM) values from 9 recipient mice of each genotype. (C) At 17 weeks post-transplantation, recipient mice were killed, and BM cells were counted and analyzed for donor-derived B-cell progenitors. Mean (SEM) values for total number of each progenitor subset (per 2 tibiae and 2 femora) of 6 mice per genotype. (D,E) Donor-derived PB B-cell and T-cell reconstitution at 4 and 14 weeks after transplantation of BM from 2-week-old mice. Mean (SEM) values from 7 recipients of each genotype. *P < .05, **P < .01, ***P < .001, ns indicates not significant compared with WT mice.
B-cell reconstitution in FL−/−mice after transplantation of fetal liver cells or BM cells from 2 week old mice. Lethally irradiated adult WT and FL−/− mice were transplanted with 2 × 105 unfractionated day 14.5 fetal liver cells (A-C) or 5 × 105 unfractionated 2-week-old BM cells (D,E), from WT and FL−/− mice, respectively. Donor- and recipient-derived blood cells could be separated based on expression of different CD45 isoforms (Materials and methods). (A,B) Donor-derived PB B-cell and T-cell reconstitution 3 and 16 weeks after fetal liver cell transplantation. Mean (SEM) values from 9 recipient mice of each genotype. (C) At 17 weeks post-transplantation, recipient mice were killed, and BM cells were counted and analyzed for donor-derived B-cell progenitors. Mean (SEM) values for total number of each progenitor subset (per 2 tibiae and 2 femora) of 6 mice per genotype. (D,E) Donor-derived PB B-cell and T-cell reconstitution at 4 and 14 weeks after transplantation of BM from 2-week-old mice. Mean (SEM) values from 7 recipients of each genotype. *P < .05, **P < .01, ***P < .001, ns indicates not significant compared with WT mice.
BM cells from 2-week-old FL−/− mice showed an intermediate ability (compared with adult BM and fetal liver cells) to reconstitute B lymphopoiesis, showing a 9-fold and 13-fold reduction in B-cell generation at 4 and 14 weeks post-transplantation (Figure 4D,E). As for adult BM cells, a significantly (4-fold) reduced T-cell reconstitution was observed from 2-week-old FL−/− BM cells at 4 weeks (Figure 4D) but not 14 weeks (Figure 4E) post-transplantation.
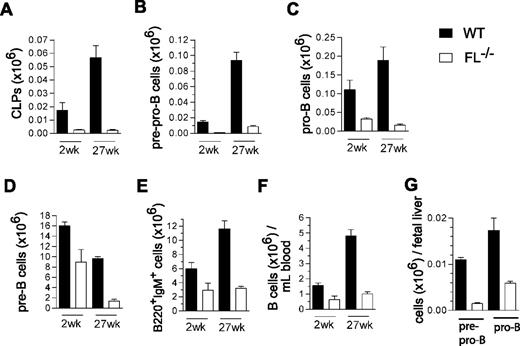
Collectively, these data suggested that FL plays a more crucial role in maintenance of B-cell progenitors in adult than fetal hematopoiesis, as previously also demonstrated for IL-7.30 Because adult FL−/− mice failed to reconstitute B lymphopoiesis on BM but not fetal liver transplantation, we speculated that FL might prove to be increasingly important for maintenance of B-cell progenitors and potentially even peripheral B-cell homeostasis in older mice, when B cell progenitors are normally present at reduced levels.12,13 Thus, whereas previous studies of FL and FLT3 (Flk2)-deficient mice had only investigated B lymphopoiesis in young adult mice,8,–10 we compared the different subsets of early B-cell progenitors as well as mature B cells in 2-week-old and 27-week-old WT and FL−/− mice. In agreement with previous studies,10 CLPs were reduced in FL−/− mice. Whereas CLPs were reduced 7-fold in 2-week-old mice, they were reduced 24-fold in 27-week-old FL−/− mice (summarized in Figure 5A and FACS profiles in Figure S1A, available on the Blood website; see Supplemental Materials link at the top of the online article). While pre-pro-B cells were reduced to a similar extent in 2-week-old and 27-week-old FL−/− mice (13-fold and 11-fold, respectively; Figure 5B and Figure S1B), pro-B (2-week-old, 3.4-fold; 27-week-old, 12-fold; Figure 5C and Figure S1B) and pre-B cells (2-week-old, 1.8-fold; 27-week-old, 7-fold; Figure 5D and Figure S1C) showed a progressive reduction with age in FL−/− mice. Most notably, whereas these reductions in B-cell progenitors only resulted in a slight reduction in mature B cells in the BM and PB of 2-week-old mice, a more pronounced reduction was seen in 27-week-old FL−/− mice, translating into a 3.6-fold and 4.8-fold reduction of B220+IgM+ cells in the BM (Figure 5E and Figure S1D) and PB (Figure 5F and Figure S1D), respectively. Pre-pro-B and pro-B-cell progenitors were already reduced (11-fold and 2.5-fold) in the fetal liver of FL−/− mice (Figure 5G), comparable with 2-week-old BM.
FLT3 ligand is essential for sustaining the peripheral B-cell compartment in adult mice. Mean (SEM) values (from 3-8 mice of each genotype and age) of CLPs (A; Lin−SCA-1loKITloIL-7Rα+), pre-pro-B (B; B220+CD43+AA4.1+CD19−), pro-B (C; B220+CD43+AA4.1+CD19+), pre-B (D; B220+CD43−IgM−), and mature/immature B cells (E: B220+IgM+) in BM, and of B cells (F; B220+IgM+) in PB in 2-week-old and 27-week-old WT and FL−/− mice. (G) Mean (SEM) values (from 5 fetuses per genotype) of pre-pro-B (B220+CD43+AA4.1+CD19−) and pro-B (B220+CD43+AA4.1+CD19+) cells in day 14.5 fetal liver from WT and FL−/− mice.
FLT3 ligand is essential for sustaining the peripheral B-cell compartment in adult mice. Mean (SEM) values (from 3-8 mice of each genotype and age) of CLPs (A; Lin−SCA-1loKITloIL-7Rα+), pre-pro-B (B; B220+CD43+AA4.1+CD19−), pro-B (C; B220+CD43+AA4.1+CD19+), pre-B (D; B220+CD43−IgM−), and mature/immature B cells (E: B220+IgM+) in BM, and of B cells (F; B220+IgM+) in PB in 2-week-old and 27-week-old WT and FL−/− mice. (G) Mean (SEM) values (from 5 fetuses per genotype) of pre-pro-B (B220+CD43+AA4.1+CD19−) and pro-B (B220+CD43+AA4.1+CD19+) cells in day 14.5 fetal liver from WT and FL−/− mice.
Taken together, these findings demonstrate an age-dependent increasingly important role of FL in maintaining B-cell progenitors in the BM, ultimately resulting in severe reductions also in the peripheral mature B-cell compartment.
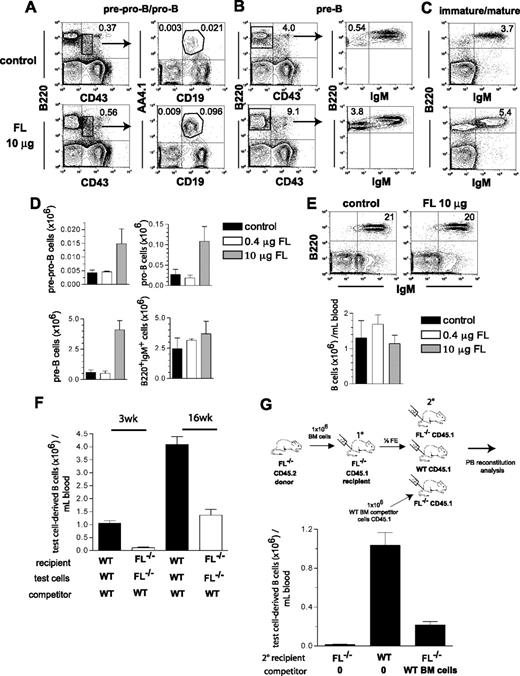
Although the main focus of the present studies was to establish the role of FL in B-cell and T-cell reconstitution after transplantation and chemotherapy, we also performed experiments to investigate to what degree systemic treatment with FL might enhance B lymphopoiesis under conditions of endogenous FL deficiency. This was not obvious, in part because endogenous FL is primarily expressed in a membrane-bound rather than soluble form,31 and in part because recent studies failed to demonstrate a positive effect of FL administration on B lymphopoiesis.32 As in the studies of Ceredig et al,32 mice were treated with high (10 μg) and low (0.4 μg) doses of FL every second day for 2 weeks. Although the total numbers of pre-pro-B, pro-B, and pre-B cell progenitors in FL−/− mice injected with the high (10 μg) dose of FL increased 3.4-fold, 4-fold, and 6.8-fold, respectively, this did not translate into a correction of the reduced levels of B220+IgM+ B cells in BM or PB (Figure 6A-E). Further, FL treatment did not enhance B cells in PB in BM-transplanted FL−/− recipients (Figure S2).
Effect of in vivo FLT3 ligand administration on B lymphopoiesis. FL−/− mice (27-33 weeks old) were treated with FL (0.4 μg or 10 μg per mouse) or PBS (control) every second day for 2 weeks, at which time mice were killed and BM cells counted and analyzed for presence of distinct stages of B-cell development in the BM. (A-C) FACS profiles from representative mice treated with PBS (control) or 10 μg FL. Numbers indicate percentage of total BM cells within the indicated gates or quadrants. (D) Total number of B-cell progenitor subsets in the BM (per 2 tibiae and 2 femora) of mice treated with PBS (control), 0.4 μg, or 10 μg FL (based on the gated cell populations indicated in A-C). Mean (SEM) numbers from 3 mice per group. (E) PB B220+IgM+ cell analysis of mice treated with PBS (control) or 10 μg FL. Numbers in FACS plots indicate frequency of B220+IgM+ B cells among total blood cells. Bar graphs show mean (SEM) values from 3 mice per group. (F) Lethally irradiated adult WT and FL−/− recipients were transplanted with 106 WT and FL−/− BM (test) cells, in competition with 106 WT cells as indicated. Donor- and competitor-derived blood cells could be separated based on expression of different CD45 isoforms (Materials and methods). Mice were analyzed for PB B cell (B220+) reconstitution at 3 and 16 weeks post-transplantation. Mean (SEM) values from 14 recipient mice in each group, from 2 independent experiments. (G) BM (test) cells from adult FL−/− (CD45.2) mice were transplanted into lethally irradiated primary (10) FL−/− (CD45.1) recipients. Four weeks after transplantation, mice were killed and ½ femur equivalent (FE) of BM cells from pooled 10 recipients were transplanted into 3 different groups of lethally irradiated secondary (20) recipients as indicated: either FL−/− CD45.1 mice without competitor, WT CD45.1 mice without competitor, or FL−/− CD45.1 mice along with 106 WT CD45.1 competitor BM cells. PB was analyzed at 6 weeks post-transplantation for donor-derived B-cell reconstitution. Bar graph shows mean (SEM) total donor-derived B220+IgM+ cells per mL blood from 5-7 secondary recipient mice per group.
Effect of in vivo FLT3 ligand administration on B lymphopoiesis. FL−/− mice (27-33 weeks old) were treated with FL (0.4 μg or 10 μg per mouse) or PBS (control) every second day for 2 weeks, at which time mice were killed and BM cells counted and analyzed for presence of distinct stages of B-cell development in the BM. (A-C) FACS profiles from representative mice treated with PBS (control) or 10 μg FL. Numbers indicate percentage of total BM cells within the indicated gates or quadrants. (D) Total number of B-cell progenitor subsets in the BM (per 2 tibiae and 2 femora) of mice treated with PBS (control), 0.4 μg, or 10 μg FL (based on the gated cell populations indicated in A-C). Mean (SEM) numbers from 3 mice per group. (E) PB B220+IgM+ cell analysis of mice treated with PBS (control) or 10 μg FL. Numbers in FACS plots indicate frequency of B220+IgM+ B cells among total blood cells. Bar graphs show mean (SEM) values from 3 mice per group. (F) Lethally irradiated adult WT and FL−/− recipients were transplanted with 106 WT and FL−/− BM (test) cells, in competition with 106 WT cells as indicated. Donor- and competitor-derived blood cells could be separated based on expression of different CD45 isoforms (Materials and methods). Mice were analyzed for PB B cell (B220+) reconstitution at 3 and 16 weeks post-transplantation. Mean (SEM) values from 14 recipient mice in each group, from 2 independent experiments. (G) BM (test) cells from adult FL−/− (CD45.2) mice were transplanted into lethally irradiated primary (10) FL−/− (CD45.1) recipients. Four weeks after transplantation, mice were killed and ½ femur equivalent (FE) of BM cells from pooled 10 recipients were transplanted into 3 different groups of lethally irradiated secondary (20) recipients as indicated: either FL−/− CD45.1 mice without competitor, WT CD45.1 mice without competitor, or FL−/− CD45.1 mice along with 106 WT CD45.1 competitor BM cells. PB was analyzed at 6 weeks post-transplantation for donor-derived B-cell reconstitution. Bar graph shows mean (SEM) total donor-derived B220+IgM+ cells per mL blood from 5-7 secondary recipient mice per group.
One possible explanation for the inability of systemic FL administration to significantly correct the B-cell deficiency in FL−/− mice might be the limited bioavailability of systemically injected FL in areas of active B lymphopoiesis and/or that the dominant membrane-bound form of FL might be required. Thus, we next performed experiments in which we investigated the ability of cotransplanted WT hematopoietic cells (known to express FL10,24,–26,33,–35 ) to enhance B lymphopoiesis from FL−/− BM cells (Figure 6F,G). Whereas B-cell generation from FL−/− BM cells was reduced by as much as 43-fold at 3 weeks after transplantation in a noncompetitive setting (Figure 1A), the reduction was only 9.5-fold when WT BM cells were cotransplanted with the FL−/− BM cells (Figure 6F). Even more notably, at 16 weeks when the reduction in B-cell generation from FL−/− BM cells in the noncompetitive setting remained 61-fold (Figure 1C), in FL−/− mice cotransplantated with WT BM cells, the reduction in generation of B cells from FL−/− BM cells was only 3-fold (Figure 6F).
To further address the ability of cell-derived FL to rescue B lymphopoiesis from FL−/− BM cells, a second set of experiments was performed, in which the goal was to use BM cells from primary FL−/− recipients of FL−/− BM cells, because these are virtually devoid of B-cell progenitors (Figure 2) and thereby investigate the ability of predominantly uncommitted progenitors in FL−/− BM to re-establish B lymphopoiesis in the absence and presence of endogenous FL (Figure 6G). As expected, BM cells from primary FL−/− recipients failed to generate almost any B cells in secondary FL−/− recipients. Strikingly, if rather transplanted into WT recipients, FL−/− BM cells from primary recipients generated 80-fold more B cells than in FL−/− recipients (P < .01), and if transplanted into FL−/− secondary recipients along with WT competitor BM cells, B-cell generation from primary FL−/− BM cells was enhanced 17-fold (P < .001) (Figure 6G).
Redundant role of FLT3 ligand in regulation of B1 and MZ B cells
Although peripheral B cells were progressively reduced in older FL−/− mice, we also found evidence for maintenance of a pool of peripheral B cells even in old FL−/− mice (up to 1-year-old, data not shown). However, peripheral B cells are of different types and are generated during distinct stages of ontogeny.23 Whereas replenishment of conventional B (B2) cells is strictly dependent on the persistence of B-cell progenitors in the BM, B1 cells and MZ B cells are derived from precursors existing predominantly during fetal and early postnatal hematopoiesis.23 However, B1 and MZ B cells are long-lived and can be sustained through activation-dependent peripheral expansion.23 Thus, we next investigated to what degree the maintenance of B1 and MZ B cells in adult mice is also FL-dependent.
Notably, whereas the total numbers of B cells in the spleen were reduced in adult FL−/− mice, IgM+CD23loCD43+ B1 cells and IgMhiCD21hiCD23loIgDlo MZ B cells were present at enhanced frequencies and at similar absolute numbers in FL−/− as in WT mice, at 12 to 15 weeks, 25 weeks, as well as 16 months of age (Figure 7A-E). The total number of B cells (B220+IgM+) was also reduced in FL−/− day 18.5 fetal livers (Figure 7F), and at variance with what we observed for B1 cells in adult mice, B220+IgM+CD5+NK1.1− B1a cells were reduced 3.6-fold in embryonic day 18.5 FL−/− fetal livers (Figure 7G). However, when FL−/− fetal liver cells were transplanted into adult FL−/− recipients, they generated less conventional B cells (Figure 7H) but, in fact, higher numbers of B1 cells (Figure 7I), suggesting that FL is important for B1 B-cell genesis in the fetal liver but not for their peripheral expansion in adult mice.
FLT3 ligand is redundant for maintenance of MZB and B1 cell compartments with age and post-transplantation. Representative FACS plots of IgM+CD23loCD43+ B1 (A) and IgMhiCD21hiCD23loIgDlo MZB (B) cells from spleens of 12- to 15-week-old WT and FL−/− mice. Numbers shown represent frequencies of gated cell populations of total spleen cells. Mean (SEM) numbers of total IgM+ B cells (C), B1 cells (D), and MZ B cells (E) in the spleens of 12- to 15-weekold (n = 5), 25-week-old (n = 5), and 16- to 18-month-old (n = 5-11) WT and FL−/− mice. Mean (SEM) numbers (from 10 fetuses per genotype) of total B220+IgM+ B cells (F) and B220+IgM+CD5+NK1.1− B1a cells (G) in the fetal liver of day 18.5 WT and FL−/− mice. Lethally irradiated adult WT and FL−/− mice (both CD45.2) were transplanted with WT and FL−/− day 14.5 fetal liver cells (both CD45.1), respectively, and 17 weeks after transplantation their spleens were analyzed for donor-derived total B220+IgM+ (H) and B1 (IgM+CD23loCD43+) cells (I). Mean (SEM) values from 9 recipients per genotype. **P < .01, ***P < .001, ns indicates not significant compared with WT mice.
FLT3 ligand is redundant for maintenance of MZB and B1 cell compartments with age and post-transplantation. Representative FACS plots of IgM+CD23loCD43+ B1 (A) and IgMhiCD21hiCD23loIgDlo MZB (B) cells from spleens of 12- to 15-week-old WT and FL−/− mice. Numbers shown represent frequencies of gated cell populations of total spleen cells. Mean (SEM) numbers of total IgM+ B cells (C), B1 cells (D), and MZ B cells (E) in the spleens of 12- to 15-weekold (n = 5), 25-week-old (n = 5), and 16- to 18-month-old (n = 5-11) WT and FL−/− mice. Mean (SEM) numbers (from 10 fetuses per genotype) of total B220+IgM+ B cells (F) and B220+IgM+CD5+NK1.1− B1a cells (G) in the fetal liver of day 18.5 WT and FL−/− mice. Lethally irradiated adult WT and FL−/− mice (both CD45.2) were transplanted with WT and FL−/− day 14.5 fetal liver cells (both CD45.1), respectively, and 17 weeks after transplantation their spleens were analyzed for donor-derived total B220+IgM+ (H) and B1 (IgM+CD23loCD43+) cells (I). Mean (SEM) values from 9 recipients per genotype. **P < .01, ***P < .001, ns indicates not significant compared with WT mice.
Discussion
Although clinical BMT has been used for decades as a treatment modality for severe hematologic and nonhematologic diseases, delayed immune reconstitution remains a considerable hurdle,1 and the mechanisms regulating immune reconstitution remain to be better established. While myeloid and NK cells recover early after transplantation, the levels of B and T lymphocytes frequently remain low for months, and although substantial efforts have been made to accelerate and enhance immune recovery after transplantation, such a treatment remains elusive.
FL had previously been demonstrated to be important for B lymphopoiesis in adult BM, but redundant for sustaining peripheral B cell numbers.9,–11 Herein, we investigated its potential role in promoting B- and T-cell reconstitution after myeloablation, and provide compelling evidence establishing a nonredundant and essential role of FL in promoting B-cell recovery after transplantation in adult recipients.
Whereas the inability of transplanted BM cells to regenerate peripheral B cells and B lymphopoiesis in the BM could potentially be explained by a role of FL in promoting homing of stem/progenitor cells to the BM, this possibility was virtually ruled out by the demonstration of a similar deficiency in B-cell recovery in 5-FU–treated FL-deficient mice. Although of less magnitude, FL also proved essential for accelerating early recovery of T cells after transplantation and myeloablation, and consequently FL might also prove of value to facilitate T cell recovery after these treatment modalities.
The enhanced reconstitution of peripheral B cells and BM B lymphopoiesis after transplantation of fetal liver or BM cells from 2-week-old mice, compared with adult BM cells, suggested an ontogeny-dependent role of FL in promoting B lymphopoiesis. Further evidence for this were obtained by establishing an age-dependent increasingly important role of FL, not only in sustaining CLPs and B cell progenitors in the BM but also in peripheral conventional B-cell homeostasis. Notably, and in contrast to conventional B2 cells, we found that fetally and early postnatally derived B1 and MZ B cells are sustained through peripheral FL-independent mechanisms, and in fact the loss of conventional B2 cells in FL−/− mice after transplantation (of fetal liver cells), as well as with age, were in part compensated through an expansion of B1 and activated MZ B cells, although our data show that the genesis of B1a cells during fetal development is, in fact, also partially FL-dependent. The finding that adult BM cells transplanted into FL−/− recipients almost completely failed to produce peripheral B cells of any type, further supports that FL-independent maintenance and expansion of peripheral B1 and MZ B cells occurs almost entirely from cells derived from progenitors present predominantly during fetal and early postnatal stages of development. In addition, we had previously shown that the B1 cell compartment in the peritoneal cavity is not affected by the absence of FLT3 signaling.11 Taken together, these findings help to explain the unequivocal demonstration of an essential role of FL in adult immune reconstitution after myeloablation and BM transplantation.
The finding that patients after myeloablation and BMT have increased FL production14,15 is compatible with a crucial role of FL in immune reconstitution of human BMT recipients, and it has been suggested that FL levels in patients undergoing chemotherapy could serve as a bioindicator for immune reconstitution after transplantation.15 The herein documented ability of endogenous FL levels to promote rapid B-cell recovery in normal recipients could explain why previous studies in mice have failed to demonstrate a positive effect of FL treatment on B-cell regeneration.32,36 Because the level of FL produced in patients varies considerably,14 it is possible that patients with low levels of FL after myeloablation and with delayed immune recovery could benefit from FL treatment after transplantation.
Our initial attempts to enhance B lymphopoiesis in old and BM-transplanted FL−/− mice through systemic administration of FL were not effective, and suggest that it will not be straightforward to enhance B- and T-cell recovery after transplantation with FL. However, through a series of informative cotransplantation experiments we demonstrated that hematopoietic cells known to express FL10,24,–26,33,–35 were much more efficient at enhancing B-cell regeneration from FL−/− BM cells than systemically administered FL. Although this remains to be established, it is tempting to speculate that the reason for this might be that cotransplanted WT BM cells will largely deliver the membrane-bound rather than soluble form of FL, or alternatively provide the soluble ligand in a more optimal manner. FL has been shown to be predominantly expressed in a membrane-bound rather that soluble form,31 and although not investigated for FL, previous studies have elegantly demonstrated that the membrane-bound ligand for another cytokine tyrosine kinase receptor, C-KIT, has essential functions that cannot be replaced by the soluble form of C-KIT ligand.37,–39 Thus, further studies are needed to establish whether the herein documented critical role of FL in immune reconstitution after BMT and chemotherapy can be developed toward clinical applications.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Peschon for kindly providing the FL−/− mice and Dr Lyman for recombinant human FL. The expert technical assistance and advice from Lilian Wittmann, Christina T. Jensen, Jessica Wahlgren, Hong Qian, Zhi Ma, Kees-Jan Pronk, Stuart Walsh, and Anna Fossum are highly appreciated.
This work was supported by grants from ALF (Governmental Public Health Grant), The Swedish Paediatric Cancer Society, The Swedish Cancer Society, The Göran Gustafsson Foundation, Tobias Foundation, The Swedish Research Council, Alfred Österlund Foundation, Gunnar Nilsson Foundation, Crafoord Foundation, and Funds of Lund Sjukvårdsdistrikt. E.S. has an Assistant Professor position from the Swedish Cancer Society. The Lund Stem Cell Center is supported by a Center of Excellence grant from the Swedish Foundation for Strategic Research.
Authorship
Contribution: N.B.V. and E.S. designed and performed research, analyzed data, and wrote the manuscript; S.E.W.J. designed research, analyzed data, and wrote the manuscript; M.C., S.D., and H.N. performed research and analyzed data.
S.E.W.J. and E.S. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sten Eirik W. Jacobsen, Hematopoietic Stem Cell Laboratory, Lund Strategic Research Center for Stem Cell Biology and Cell Therapy, Lund University, 221 84 Lund, Sweden; e-mail: sten.jacobsen@med.lu.se.

![Figure 1. Critical role of FLT3 ligand in B-cell regeneration after BM transplantation. Lethally irradiated adult WT mice were transplanted with 2 × 105 unfractionated BM cells from 9- to 11-week-old WT mice (WT into WT), and FL−/− recipient mice were transplanted with 2 × 105 unfractionated FL−/− BM cells (FL−/− into FL−/−). In all experiments, donor- and recipient-derived blood cells could be separated based on expression of different CD45 isoforms (“Materials and methods”). Donor-derived B- and T-cell reconstitution of PB at (A) 3, (B) 5, and (C) 16 weeks after transplantation. FACS plots show donor-derived B-cell (B220+ or B220+IgM+) and T-cell (CD4/CD8+) reconstitution from representative mice transplanted with WT and FL−/− cells. Numbers indicate percentage of total donor-derived cells within the indicated quadrants. Bar graphs show mean (standard error of mean [SEM]) values from 19-27 recipient mice of each genotype, from 3-4 independent experiments. Donor-derived B cells (B220+CD19+) in the spleen (D) and BM (per 2 tibiae and 2 femora) (E) 16 weeks after transplantation. Mean (SEM) values from 9-21 recipients and 2-3 independent experiments. **P < .01, ***P < .001, compared with WT mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-09-047480/2/m_zh80130702940001.jpeg?Expires=1765993819&Signature=v9h2ka8fg3FgdSr6Ez2I5tKaLEuJ2AODxUglATtM3O8PWhve5CyszUHMoovP9kWnO-7QO1QVm36zMRDuAcl-0O2ELZnPDonZDqpFsHxMVX-YYBpmopDZuCBCQqQ8TlQdzrc5hih7iCneEJMO7mBc8EcZpUJdwAW1Wb2cqPHPG2245wkXU-GIfNGkhqzLDUqTnCaubHdAy~GlvupbNww4ZRrojZkX9xmFQ~UX8Ec1JA63GqiCqXmEff54kXYwfnVpVYYTmVTMnuBRVJjo2k3RSC1CKjZpSIBqNUvLSrEwPfG2yvPPAmGJQ3PA0Swwlq9-OFkq~VBrjpqOzM4MKBYYRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal