We analyzed 112 patients with high-risk acute myeloid leukemia (61 in complete remission [CR]; 51 in relapse), who received human leukocyte-antigen (HLA)–haploidentical transplants from natural killer (NK) alloreactive (n = 51) or non-NK alloreactive donors (n = 61). NK alloreactive donors possessed HLA class I, killer-cell immunoglobulin-like receptor (KIR) ligand(s) which were missing in the recipients, KIR gene(s) for missing self recognition on recipient targets, and alloreactive NK clones against recipient targets. Transplantation from NK-alloreactive donors was associated with a significantly lower relapse rate in patients transplanted in CR (3% versus 47%) (P > .003), better event-free survival in patients transplanted in relapse (34% versus 6%, P = .04) and in remission (67% versus 18%, P = .02), and reduced risk of relapse or death (relative risk versus non-NK-alloreactive donor, 0.48; 95% CI, 0.29-0.78; P > .001). In all patients we tested the “missing ligand” model which pools KIR ligand mismatched transplants and KIR ligand-matched transplants from donors possessing KIR(s) for which neither donor nor recipient have HLA ligand(s). Only transplantation from NK-alloreactive donors is associated with a survival advantage.
Introduction
Transplantation of high doses of T-cell–depleted peripheral blood hematopoietic stem cells from human leukocyte antigen (HLA) haplotype-mismatched (haploidentical) family members is an option for patients with high-risk acute leukemia who urgently need a graft and do not have matched sibling or unrelated donors.1,–3 Because the graft-versus-leukemia (GvL) effect is conventionally achieved through T-cell–mediated alloreactions directed against histocompatibility antigens displayed on recipient leukemia cells, in the haploidentical transplantation setting the need for extensive T-cell depletion might have been expected to result in a weak or no GvL effect. However, another cell of the immune system influences outcomes of hematopoietic cell transplantation in a surprisingly favorable way. In a limited series of haploidentical transplantations (57 patients with acute myeloid leukemia [AML], 20 of whom were transplanted from natural killer [NK] alloreactive donors), we observed donor-versus-recipient NK cell alloreactivity reduced the risk of leukemia relapse, did not cause graft-versus-host disease (GvHD), and markedly improved event-free survival (EFS).4
As described in our original report,4 donor-versus-recipient NK cell alloreactions rest on NK cell recognition of missing self.5,,,–9 Human NK cells possess clonally distributed inhibitory receptors termed killer cell immunoglobulin-like receptors (KIRs) that recognize allotypic determinants (KIR ligands) shared by certain HLA class I allele groups.10,–12 KIR2DL1 recognizes HLA-C alleles with a Lys80 residue (HLA-Cw4 and related group 2 alleles), KIR2DL2 and KIR2DL3 recognize HLA-C with an Asn80 residue (HLA-Cw3 and related group 1 alleles), KIR3DL1 is the receptor for HLA-B alleles sharing the Bw4 supertypic specificity. In haploidentical transplantations that are KIR ligand mismatched in the graft-versus-host (GvH) direction, functional donor NK cells that express as their sole inhibitory receptor for self a KIR for the HLA class I group, which is absent in the recipient, sense the missing expression of the self class I ligand on allogeneic targets and mediate alloreactions (“missing self” recognition).4,13,,–16
An additional algorithm, termed the “missing ligand” or “receptor-ligand” model, has been proposed for predicting better outcome, ie, control of leukemia relapse and improved survival in haploidentical transplantations in children with acute leukemia,17,18 in (T-cell–depleted) matched sibling19 and in unrelated donor transplantations20 for AML. The missing-ligand model includes all donor-recipient pairs in whom there is a mismatch between at least one KIR gene in the donor and HLA molecule(s) in the recipient. Consequently, the model includes (1) all KIR ligand-mismatched transplants (in the GvH direction) which are all associated with a missing KIR ligand in the recipient, because the majority of donors possess the 3 inhibitory KIRs (for HLA-C and HLA-Bw4 allele group KIR ligands), whereas all recipients possess only 1 or 2 of these KIR ligands and (2) KIR ligand-matched transplants from donors possessing “extra” KIR(s) for which neither donor nor recipient have HLA ligand(s).17,,–20
Here, we analyze outcomes in all 112 adult patients with high-risk AML who have undergone haploidentical transplantation in our center from 1993 to 2006. These include 57 haploidentical transplantations which we presented in 20024 and all 55 who received transplants since then. We describe our KIR ligand mismatch-based process for selecting donors exerting NK cell alloreactivity (missing self recognition) against the recipients. In the same series we test the missing ligand model and demonstrate that only KIR ligand mismatching and, consequently, donor NK cell recognition of missing self on recipient targets is associated with a GvL effect and a marked survival advantage.
Patients and methods
Patients
One hundred twelve patients with AML who received haploidentical transplants were included in this study. Approval was obtained from the Umbria Region Ethics Committee and from the Perugia University institutional review board. Informed consent was provided in accordance with the Declaration of Helsinki. Table 1 shows, according to donor NK alloreactive status, clinical characteristics (disease status at transplantation and unfavorable prognostic features) of 57 haploidentical transplant recipients whom we presented in 2002,4 55 who received transplants since then, the combined group of 112, as well as details of graft content.
Donors
Donors who were classified as NK alloreactive against their recipients, termed NK alloreactive donors throughout, possessed (1) HLA class I KIR ligand(s) which were missing in the recipients, (2) KIR gene(s) for missing self recognition on recipient targets, and (3) alloreactive NK clones against recipient targets.
Candidates were assessed for HLA compatibility by serology (HLA-A, -B) and by high-resolution molecular analysis (HLA-C, -DR, -DP, -DQ). Donors were considered able to exert donor-versus-recipient NK cell alloreactivity when HLA-C and HLA-B typing showed KIR ligand mismatches in the GvH direction, ie, the recipient did not possess one HLA-C allele group (C1 or C2), the HLA-Bw4 group which were present in the donor, or both.
Genomic DNA was prepared from 2 × 106 to 1 × 107 peripheral blood mononuclear cells (PBMCs) using the Nucleospin Blood Kit (Macherey-Nagel, Düren, Germany). KIR typing of genomic DNA was performed by polymerase chain reaction (PCR) amplification with primers based on conserved regions specific to each KIR locus12 with modification.21
NK cell cloning and cytotoxicity assays
NK cell cloning by limiting dilution and cytotoxicity assays against recipient targets assessed alloreactive NK cell repertoires.4 PBMCs depleted of T cells by negative anti-CD3 immunomagnetic selection (Miltenyi, Bergisch Gladbach, Germany) were plated under limiting-dilution conditions, activated with phytohemagglutinin (PHA; Biochrom KG, Berlin, Germany), and cultured with interleukin-2 (Chiron BV, Amsterdam, Netherlands) and irradiated feeder cells. Cloning efficiencies ranged from 1 in 5 to 1 in 10 plated NK cells. Cloned NK cells were screened for alloreactivity by standard 51Cr release cytotoxicity at an effector-to-target ratio of 10:1 against allogeneic, KIR ligand-mismatched PHA lymphoblasts. Approximately 200 NK clones from each person were screened. Clones exhibiting greater than 30% lysis were scored as alloreactive. The assay was considered positive when the frequency of lytic clones was more than 1 in 50.
Antibodies and flow cytometric analyses
Four-color immunofluorescence analyses identified KIR+/NKG2A− versus KIR−/NKG2A+, CD56+/CD3− NK cells. The following mouse monoclonal antibodies: allophycocyanin (APC)–conjugated anti-CD56 (IgG1; Miltenyi), APC-Cy7–conjugated anti-CD3 (IgG1; BD Bioscience, San Diego, CA), unconjugated anti-NKG2A (clone Z199, IgG2b, kindly donated by Alessandro Moretta, University of Genova) developed with fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgG2b antibodies (Southern Biotech, Birmingham, AL), were used in combination with the following phycoerythrin-conjugated anti-KIR antibodies (Bechman Coulter, Fullerton, CA): either anti-KIR2DL2/3/S2 (clone GL183, IgG1), or anti-KIR2DL1/S1 (clone EB6B, IgG1), or anti-KIR3DL1/S1 (clone Z27, IgG1).
Two-color immunofluorescence analyses on NK cells purified by the RosetteSep human NK cell enrichment cocktail method (StemCell Technologies, Vancouver, BC, Canada) were used to visualize NK cells expressing, as their only inhibitory receptor for self, a KIR for which there was no class I ligand in the recipient.22 KIR2DL2/3 single-positive NK cells were identified with an unconjugated IgG2b isotype anti-KIR2DL2/3/S2 antibody (clone CH-L, kindly donated by Silvano Ferrini, Istituto Nazionale per la Ricerca sul Cancro, Genova) developed with FITC-conjugated goat anti–mouse IgG2b antibodies (SouthernBiotech), in combination with a cocktail of unconjugated IgG1 isotype anti-KIR2DL1/S1 (clone EB6), anti-KIR3DL1/S1 (clone Z27), and anti-NKG2A (clone Z270, all kindly donated by Alessandro Moretta, University of Genova) mouse antibodies, developed with PE-conjugated goat anti–mouse IgG1 antibodies (SouthernBiotech). KIR2DL1 single-positive NK cells were identified with an unconjugated IgG1 isotype anti-KIR2DL1/S1 antibody (clone EB6) developed with PE-conjugated goat anti–mouse IgG1 antibodies (SouthernBiotech), in combination with a cocktail of unconjugated IgG2a or IgG2b isotype anti-KIR2DL2/3S2 (clone CH-L, IgG2b), anti-KIR3DL1/S1 (clone AZ158, IgG2a, kindly donated by Silvia Parolini, University of Brescia) and anti-NKG2A (clone Z199, IgG2b) mouse antibodies, developed with FITC-conjugated goat anti–mouse IgG2a and anti-IgG2b antibodies (SouthernBiotech).
The missing ligand model
The missing ligand model includes (1) KIR ligand-matched transplants from donors possessing an “extra” KIR for which neither donor nor recipient has an HLA ligand and (2) all KIR ligand mismatched transplants.17,,–20 To apply the missing ligand model, the first step was to divide our non-NK alloreactive donor-recipient pairs into 2 groups according to the number of KIR ligands in donor and recipient, ie, 3 KIR ligands (“no missing ligand”) versus fewer than 3 (“missing ligand”). Probability of EFS was evaluated in each subgroup and was compared with NK alloreactive (KIR ligand mismatched) transplants. The second step was to pool the above missing ligand transplants and all KIR ligand-mismatched transplants (which corresponded to our NK alloreactive transplants) and to analyze EFS in these patients against EFS in patients with no missing ligand.
Transplantation procedures and graft processing
Patients were conditioned with total body irradiation (8 Gy; day −9), thiotepa (5 mg/kg per day on days −8, −7), fludarabine (40 mg/m2 per day on days −7 through −3), and rabbit antithymocyte globulin (ATG) (5 mg/kg per day on days −5 through −2).2,3 The first 9 patients received cyclophosphamide instead of fludarabine.1
Granulocyte colony-stimulating factor–mobilized hematopoietic progenitor cells were collected. T-cell depletion was by soybean-agglutination and E-rosetting1 in the first 9 transplants (graft, 13 ± 5 × 106 CD34+ and 1.5 ± 4.5 × 105 CD3+ cells/kg), E-rosetting and CD34+ cell selection with Cell-Pro columns (Bothell, Washington)2 in 22 transplants (graft, 14.5 ± 4 ×106 CD34+ and 3.2 ± 3.9 × 104 CD3+ cells/kg), and Clinimacs CD34+ cell selection (Miltenyi)2,3 in the last 81 transplants (graft, 17.5 ± 5 × 106 CD34+ and 3.5 ± 2 × 104 CD3+ cells/kg). No postgrafting GvHD prophylaxis was given.
Cytomegalovirus prophylaxis was gancyclovir (10 mg/kg per day on days −9 through −3) and foscarnet (90 mg/kg per day on days +4 through +21). Antifungal prophylaxis included liposomal amphotericin (1 mg/kg daily) from day −5 until the neutrophil count reached 1 × 109/L.
Blood and marrow chimerism was assessed bimonthly by PCR amplification of variable-number tandem repeat regions with different DNA polymorphism patterns (DNA microsatellites).
Outcome assessment
Engraftment and GvHD were assessed by consensus criteria23 and chimerism as described elsewhere.1 Transplantation-related mortality was defined as death from causes other than leukemia relapse, and EFS as the interval from transplantation to death from all causes, to leukemia relapse or to last follow-up.
Statistical analyses
Cohorts of patients transplanted from NK alloreactive versus non-NK alloreactive donors were compared using Fisher exact test, chi-square test, or Student t test, where appropriate. The Kaplan-Meier method evaluated EFS. The log-rank test assessed impact of donor-versus-recipient NK alloreactivity (presence versus absence) on EFS. Cumulative incidence estimates were used for relapse and nonleukemic mortality, because they are competing risks. Cumulative incidence of GvHD was calculated using death from any cause as competing risk. The Gray test compared univariate competing risk outcomes.24 In all 112 patients and in each series, ie, 57 haploidentical transplant recipients whom we presented in 20024 and 55 who received transplants since then, multivariate analysis (Cox regression model) assessed the impact of donor-versus-recipient NK alloreactivity, disease status at transplantation (remission versus chemoresistant relapse), age, cytogenetics, conditioning regimens, graft-processing procedures, and the number of CD34+ and T cells in the graft. Donor-versus-recipient NK alloreactivity was forced into all Cox models; all other variables were included in a conditional forward stepwise fashion. Because only donor-versus-recipient NK alloreactivity and disease status at transplantation had a significant impact on EFS, they were included in the final model and tested using a time-dependent covariate method. As donor-versus-recipient NK alloreactivity affected only late (< 6 months) survival, it did not meet the proportional hazard assumption and was modeled as a time-dependent covariate, considering its effects on early and late survival separately. Within these timeframes the proportional hazard assumption held. All reported P values are 2-sided and were considered statistically significant if greater than .05.
Results
Table 1 summarizes characteristics of patients who received transplants from NK-alloreactive versus non-NK alloreactive donors.
HLA haplotype-mismatched transplantations for AML with and without NK cell alloreactivity in the GVH direction according to patient series
| NK cell alloreactivity in GvH direction . | 1993–20014 . | 2002–2006 . | 1993–2006 . | |||
|---|---|---|---|---|---|---|
| No n = 37 . | Yes n = 20 . | No n = 24 . | Yes n = 31 . | No n = 61 . | Yes n = 51 . | |
| Status of disease at transplantation, n (%) | ||||||
| Bad-risk first and second CR | 7/37* (18.9) | 3/20* (15) | 19/24* (79.1) | 23/31* (74.2) | 26/61* (42.6) | 26/51* (51) |
| Myelodysplasia | 2/7 (28.6) | 1/3 (33.3) | 2/19 (10.5) | 5/23 (21.7) | 4/26 (15.5) | 6/26 (23) |
| Unfavorable cytogenetics | 2/7 (28.6) | 0/3 (0) | 4/19 (21.1) | 10/23 (43.5) | 6/26 (23) | 10/26 (38.5) |
| First-line induction therapy or autologous transplant failure | 3/7 (42.8) | 2/3 (66.7) | 13/19 (68.4) | 8/23 (34.8) | 16/26 (61.5) | 10/26 (38.5) |
| More than second complete remission | 4/37* (10.8) | 3/20* (15) | 1/24* (4.2) | 1/31* (3.2) | 5/61* (8.2) | 4/51* (7.8) |
| Chemoresistant relapse† | 26/37* (70.3) | 14/20* (70) | 4/24* (16.7) | 7/31* (22.6) | 30/61* (49.2) | 21/51* (41.2) |
| Graft composition (mean ± SD) | ||||||
| CD34+ × 106/kg | 14.7 ± 5* | 13.7 ± 4* | 16.7 ± 7* | 15.8 ± 6* | 15.9 ± 7* | 15.3 ± 4* |
| CD3+ × 104/kg | 5 ± 3.5* | 4.5 ± 4* | 3 ± 3.6* | 3.5 ± 3* | 3.5 ± 2* | 3.8 ± 3* |
| NK cell alloreactivity in GvH direction . | 1993–20014 . | 2002–2006 . | 1993–2006 . | |||
|---|---|---|---|---|---|---|
| No n = 37 . | Yes n = 20 . | No n = 24 . | Yes n = 31 . | No n = 61 . | Yes n = 51 . | |
| Status of disease at transplantation, n (%) | ||||||
| Bad-risk first and second CR | 7/37* (18.9) | 3/20* (15) | 19/24* (79.1) | 23/31* (74.2) | 26/61* (42.6) | 26/51* (51) |
| Myelodysplasia | 2/7 (28.6) | 1/3 (33.3) | 2/19 (10.5) | 5/23 (21.7) | 4/26 (15.5) | 6/26 (23) |
| Unfavorable cytogenetics | 2/7 (28.6) | 0/3 (0) | 4/19 (21.1) | 10/23 (43.5) | 6/26 (23) | 10/26 (38.5) |
| First-line induction therapy or autologous transplant failure | 3/7 (42.8) | 2/3 (66.7) | 13/19 (68.4) | 8/23 (34.8) | 16/26 (61.5) | 10/26 (38.5) |
| More than second complete remission | 4/37* (10.8) | 3/20* (15) | 1/24* (4.2) | 1/31* (3.2) | 5/61* (8.2) | 4/51* (7.8) |
| Chemoresistant relapse† | 26/37* (70.3) | 14/20* (70) | 4/24* (16.7) | 7/31* (22.6) | 30/61* (49.2) | 21/51* (41.2) |
| Graft composition (mean ± SD) | ||||||
| CD34+ × 106/kg | 14.7 ± 5* | 13.7 ± 4* | 16.7 ± 7* | 15.8 ± 6* | 15.9 ± 7* | 15.3 ± 4* |
| CD3+ × 104/kg | 5 ± 3.5* | 4.5 ± 4* | 3 ± 3.6* | 3.5 ± 3* | 3.5 ± 2* | 3.8 ± 3* |
*P = NS.
†Morphologic evidence of leukemic cells in the bone marrow and or blood after treatment.
Donor-versus-recipient NK cell alloreactivity
Fifty-one of the 112 haploidentical donor/recipient pairs were alloreactive in the donor-versus-recipient direction, 20 as reported in 20024 and 31 since then. These donors were KIR-ligand mismatched with their recipients in the GvH direction. Twenty-three were HLA-C group 1 mismatched, 20 were HLA-C group 2 mismatched, and 8 were HLA-Bw4 mismatched.
All 51 NK cell alloreactive donors possessed the inhibitory KIR gene that recognized missing expression of its HLA class I ligand on recipient cells. The 23 HLA-C group 1 mismatched donors possessed KIR2DL2 and/or KIR2DL3, the 20 HLA-C group 2 mismatched donors possessed KIR2DL1, and the 8 HLA-Bw4 mismatched had KIR3DL1.
Functional analyses showed alloreactive NK clones in all subjects (43 transplant donors and 25 healthy persons) whose NK clones were tested against allogeneic targets which did not express their HLA-C group. Frequencies of donor-versus-recipient alloreactive NK clones were 8 ± 6 cells in 100 (mean ± SD) for HLA-C group 2 mismatched pairs and 5 ± 3 cells in 100 for HLA-C group 1 mismatched pairs. In contrast, only 20 of 31 HLA-Bw4–positive healthy persons whose NK clones were tested against HLA-Bw4–negative allogeneic targets possessed NK alloreactive clones (that is, ≥ 1 cell in 50). Eleven displayed more than 1 alloreactive NK clone in 200 clones. The 8 HLA-Bw4 group–mismatched donors in this study all possessed the KIR3DL1 gene and possessed alloreactive NK clones which killed recipient targets in detectable frequencies (4 ± 2 cells in 100).
No alloreactive NK clones (> 1 in 200 clones) were detected in donors whose NK clones were tested against KIR ligand-matched targets, ie, when recipient target cells expressed the class I group(s) present in the donor.
Posttransplantation detection of alloreactive NK cells of donor origin in recipients
NK cells expressing CD94/NKG2A display no alloreactivity because all persons express HLA-E, the ligand of CD94/NKG2A, whereas KIRs are specific for HLA-C1, HLA-C2, and HLA-Bw4 allelele groups (reviewed in Velardi et al,14 Farag et al,15 , and Ruggeri et al16 ). Thus, potentially alloreactive NK populations are KIR+/NKG2A−. Figure 1 shows posttransplantation kinetics of KIR+/NKG2A− versus KIR−/NKG2A+ NK cell subsets of donor origin in a sample population of 10 recipients during the first 1 to 7 months after transplantation. KIR+/NKG2A− NK cell subsets were first detected in low numbers 1 month after transplantation and rose to donor levels by month 3 after transplantation. They remained high for as long as they were monitored (< 12 months).
Reconstitution of potentially alloreactive KIR+/NKG2A− NK cells of donor origin after haploidentical transplantation. Posttransplantation kinetics of KIR+/NKG2A− (CD56+/CD3−) NK cell subsets versus KIR−/NKG2A+ (CD56+/CD3−) NK cell subsets of donor origin in a sample population of 10 recipients during the first 1 to 7 months after transplantation (mean ± SD) compared with values in their donors (left). KIR−/NKG2A+ cells (open triangles), KIR2DL2/3/S2+/NKG2A− cells (open circles), KIR2DL1/S1+/NKG2A− cells (open squares), KIR3DL1/S1+/NKG2A− cells (closed circles).
Reconstitution of potentially alloreactive KIR+/NKG2A− NK cells of donor origin after haploidentical transplantation. Posttransplantation kinetics of KIR+/NKG2A− (CD56+/CD3−) NK cell subsets versus KIR−/NKG2A+ (CD56+/CD3−) NK cell subsets of donor origin in a sample population of 10 recipients during the first 1 to 7 months after transplantation (mean ± SD) compared with values in their donors (left). KIR−/NKG2A+ cells (open triangles), KIR2DL2/3/S2+/NKG2A− cells (open circles), KIR2DL1/S1+/NKG2A− cells (open squares), KIR3DL1/S1+/NKG2A− cells (closed circles).
Table 2 shows frequencies of donor-versus-recipient alloreactive NK clones (ie, clones killing recipient cryopreserved PHA blasts) in 24 randomly assayed NK alloreactive donors and their transplant recipients at different time points during 1 year after transplantation, along with the KIR ligand mismatch for each donor recipient pair. Alloreactive NK clones were detected in 17 of 24 recipients at 1 to 3 months after transplantation, in 7 of 24 at 4 to 7 months after transplantation, and in 2 of 24 at 8 to 12 months after transplantation. None was detected afterward (not shown).
Frequencies of donor vs recipient alloreactive NK clones in randomly assayed NK alloreactive donors and their transplant recipients, along with the KIR ligand mismatch
| Transplant pairs . | Isolated in donor, n (%) . | Isolated after transplant in recipient . | Missing donor KIR ligand in recipient . | ||
|---|---|---|---|---|---|
| 1-3 mo . | 4-7 mo . | 8-12 mo . | |||
| 1 | 5/24 (20.8) | 0/100 | ND | ND | HLA-C 2 |
| 2 | 8/14 (57.1) | 0/50 | ND | ND | HLA-Bw4 |
| 3 | 12/40 (30) | 0/40 | ND | ND | HLA-Bw4 |
| 4 | 4/25 (16) | 0/30 | ND | ND | HLA-C 1 |
| 5 | 6/36 (16.7) | 0/35 | ND | ND | HLA-C 1 |
| 6 | 2/40 (5) | 0/54 | ND | ND | HLA-C 2 |
| 7 | 12/20 (60) | 4/22 (18.2) | 0/48 | ND | HLA-C 1 |
| 8 | 2/33 (6.1) | 4/30 (13.3) | 0/35 | ND | HLA-C 1 |
| 9 | 3/75 (4) | 4/100 (4) | 0/52 | ND | HLA-Bw4 |
| 10 | 1/40 (2.5) | 1/40 (2.5) | 0/55 | ND | HLA-C 2 |
| 11 | 3/40 (7.5) | 2/45 (4.4) | 0/60 | ND | HLA-C 1 |
| 12 | 3/25 (12) | 2/17 (11.8) | ND | ND | HLA-C1 + HLA-Bw4 |
| 13 | 2/11 (18.2) | 2/37 (5.4) | ND | ND | HLA-C 2 |
| 14 | 1/41 (2.4) | 2/42 (4.8) | ND | ND | HLA-C 1 |
| 15 | 6/60 (10) | 2/45 (4.4) | 4/96 (4.2) | 2/80 (2.5) | HLA-C 2 |
| 16 | 6/30 (20) | 1/48 (2.1) | 2/47 (4.2) | 0/60 | HLA-C 1 |
| 17 | 2/48 (4.2) | 8/90 (8.9) | 5/48 (10.4) | 2/54 (3.7) | HLA-C 1 |
| 18 | 10/57 (17.5) | 4/66 (6.1) | 1/96 (1.1) | 0/86 | HLA-C 2 |
| 19 | 8/65 (12.3) | 4/97 (4.1) | 4/64 (6.2) | 0/58 | HLA-C 1 |
| 20 | 3/45 (6.7) | 0/90 | 0/113 | 0/130 | HLA-C 1 |
| 21 | 6/80 (7.5) | 2/36 (5.5) | 1/86 (1.2) | 0/97 | HLA-C 2 |
| 22 | 5/87 (5.7) | 20/78 (25.6) | 0/65 | ND | HLA-C 1 |
| 23 | 8/69 (11.6) | 10/46 (21.7) | 30/75 (40) | 0/79 | HLA-C 2 |
| 24 | 2/64 (3.1) | 3/43 (7) | 0/34 | 0/65 | HLA-Bw4 |
| Transplant pairs . | Isolated in donor, n (%) . | Isolated after transplant in recipient . | Missing donor KIR ligand in recipient . | ||
|---|---|---|---|---|---|
| 1-3 mo . | 4-7 mo . | 8-12 mo . | |||
| 1 | 5/24 (20.8) | 0/100 | ND | ND | HLA-C 2 |
| 2 | 8/14 (57.1) | 0/50 | ND | ND | HLA-Bw4 |
| 3 | 12/40 (30) | 0/40 | ND | ND | HLA-Bw4 |
| 4 | 4/25 (16) | 0/30 | ND | ND | HLA-C 1 |
| 5 | 6/36 (16.7) | 0/35 | ND | ND | HLA-C 1 |
| 6 | 2/40 (5) | 0/54 | ND | ND | HLA-C 2 |
| 7 | 12/20 (60) | 4/22 (18.2) | 0/48 | ND | HLA-C 1 |
| 8 | 2/33 (6.1) | 4/30 (13.3) | 0/35 | ND | HLA-C 1 |
| 9 | 3/75 (4) | 4/100 (4) | 0/52 | ND | HLA-Bw4 |
| 10 | 1/40 (2.5) | 1/40 (2.5) | 0/55 | ND | HLA-C 2 |
| 11 | 3/40 (7.5) | 2/45 (4.4) | 0/60 | ND | HLA-C 1 |
| 12 | 3/25 (12) | 2/17 (11.8) | ND | ND | HLA-C1 + HLA-Bw4 |
| 13 | 2/11 (18.2) | 2/37 (5.4) | ND | ND | HLA-C 2 |
| 14 | 1/41 (2.4) | 2/42 (4.8) | ND | ND | HLA-C 1 |
| 15 | 6/60 (10) | 2/45 (4.4) | 4/96 (4.2) | 2/80 (2.5) | HLA-C 2 |
| 16 | 6/30 (20) | 1/48 (2.1) | 2/47 (4.2) | 0/60 | HLA-C 1 |
| 17 | 2/48 (4.2) | 8/90 (8.9) | 5/48 (10.4) | 2/54 (3.7) | HLA-C 1 |
| 18 | 10/57 (17.5) | 4/66 (6.1) | 1/96 (1.1) | 0/86 | HLA-C 2 |
| 19 | 8/65 (12.3) | 4/97 (4.1) | 4/64 (6.2) | 0/58 | HLA-C 1 |
| 20 | 3/45 (6.7) | 0/90 | 0/113 | 0/130 | HLA-C 1 |
| 21 | 6/80 (7.5) | 2/36 (5.5) | 1/86 (1.2) | 0/97 | HLA-C 2 |
| 22 | 5/87 (5.7) | 20/78 (25.6) | 0/65 | ND | HLA-C 1 |
| 23 | 8/69 (11.6) | 10/46 (21.7) | 30/75 (40) | 0/79 | HLA-C 2 |
| 24 | 2/64 (3.1) | 3/43 (7) | 0/34 | 0/65 | HLA-Bw4 |
Number of donor vs recipient alloreactive NK clones (ie, killing recipient PHA blasts) in number of clones tested.
ND indicates not done.
Pretransplantation and posttransplantation immunofluorescence analysis visualized NK cells expressing the KIR for which there is no class I ligand in the recipient as their only inhibitory receptor for self. It detected potential donor-versus-recipient alloreactive NK cells when donors were homozygous for group A KIR haplotypes, as shown in a representative example illustrated in Figure 2A (donor) and 2B (recipient, 1 to 3 months after transplantation). This pair corresponds to donor-recipient pair no. 22 in Table 2. Note the concordance between immunofluorescence and functional analyses. Because of anti-KIR antibody cross-reactivity with activating KIRs, immunofluorescence detected fewer potential alloreactive NK cells when donors carried group B KIR haplotypes, which, unlike group A, include activating KIRs, as shown in a representative example illustrated in Figure 2C (donor) and 2D (recipient, 4 to 7 months after transplantation). This pair corresponds to donor-recipient pair no. 23 in Table 2. Note the discordance between immunofluorescence and functional analyses.
Pretransplantation and posttransplantation immunofluorescence analyses in 2 representative transplant pairs of NK cells of donor origin expressing the KIR for which there is no class I ligand in the recipient as their only inhibitory receptor for self. Panels A (donor) and B (recipient 1–3 months after transplantation) illustrate a transplant pair in whom the donor KIR ligand HLA-C 1 was missing in the recipient. KIR genotyping showed the donor possessed only group A haplotype genes, specifically KIR2DL1, KIR2DL3, and KIR3DL1 inhibitory KIR genes. Consequently the potentially alloreactive population is represented by cells expressing only KIR2DL3 (upper left quadrants in panels A and B where the percentage is indicated). Cells expressing or coexpressing all other KIRs and/or NKG2A are shown in the right quadrants. Panels C (donor) and D (recipient 4-7 months after transplantation) illustrate a transplant pair in whom the donor KIR ligand HLA-C 2 was missing in the recipient. KIR genotyping showed the donor possessed group B haplotype genes, specifically KIR2DL1, KIR2DL2, KIR2DL3, and KIR3DL1 inhibitory genes and KIR2DS1, KIR2DS2, KIR2DS3, and KIR3DS1 activating genes. Consequently, the potentially alloreactive population is represented by cells expressing only the KIR2DL1 inhibitory receptor (lower right quadrants in panels C and D where the percentage of cells expressing KIR2DL1 with or without KIR2DS1 is indicated). Cells expressing or coexpressing all other KIRs or NKG2A or both are shown in the upper quadrants.
Pretransplantation and posttransplantation immunofluorescence analyses in 2 representative transplant pairs of NK cells of donor origin expressing the KIR for which there is no class I ligand in the recipient as their only inhibitory receptor for self. Panels A (donor) and B (recipient 1–3 months after transplantation) illustrate a transplant pair in whom the donor KIR ligand HLA-C 1 was missing in the recipient. KIR genotyping showed the donor possessed only group A haplotype genes, specifically KIR2DL1, KIR2DL3, and KIR3DL1 inhibitory KIR genes. Consequently the potentially alloreactive population is represented by cells expressing only KIR2DL3 (upper left quadrants in panels A and B where the percentage is indicated). Cells expressing or coexpressing all other KIRs and/or NKG2A are shown in the right quadrants. Panels C (donor) and D (recipient 4-7 months after transplantation) illustrate a transplant pair in whom the donor KIR ligand HLA-C 2 was missing in the recipient. KIR genotyping showed the donor possessed group B haplotype genes, specifically KIR2DL1, KIR2DL2, KIR2DL3, and KIR3DL1 inhibitory genes and KIR2DS1, KIR2DS2, KIR2DS3, and KIR3DS1 activating genes. Consequently, the potentially alloreactive population is represented by cells expressing only the KIR2DL1 inhibitory receptor (lower right quadrants in panels C and D where the percentage of cells expressing KIR2DL1 with or without KIR2DS1 is indicated). Cells expressing or coexpressing all other KIRs or NKG2A or both are shown in the upper quadrants.
Transplantation outcomes
Primary engraftment was obtained in 103 of 112 patients. Rejection was reversed in 4 of 9 patients who rejected by transplanting CD34+ cells (from the same or other haploidentical donor) after reconditioning with cyclophosphamide (40 mg/kg × 2), rabbit ATG (2.5 mg/kg × 4), and fludarabine (40 mg/m2 × 4). Thus, 107 of 112 evaluable patients engrafted with no late rejection and full donor-type chimerism. Acute (≥ grade 2) GvHD developed in 11 of 112 patients. Relapses occurred in 28 of 112 patients. Deaths in remission were 48 (43%) of 112 with infectious deaths accounting for 42 of the 48. Event-free survivors were 36 of 112, 13 in the early series4 and 23 in the present study, at a median follow-up of 5.07 years (range, 0.95-13.22 years).
Rejection was 6% in transplants from NK-alloreactive donors versus 10% in transplants from non-NK alloreactive donors. The incidence of at least grade 2 acute GvHD was 10% in transplants from NK alloreactive donors versus 11% in transplants from non-NK alloreactive donors.
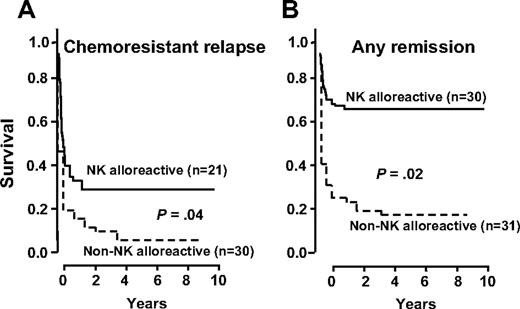
Figure 3 illustrates the cumulative incidences of relapse in patients transplanted in chemoresistant relapse (Figure 3A) versus patients transplanted in any CR (Figure 3B). The incidence of relapse was similar in patients transplanted in chemoresistant relapse from NK alloreactive donors (32% versus 37%, P = NS). In patients in any CR the cumulative incidence of relapse was significantly lower after transplantation from NK alloreactive donors (3% versus 47%, P > .003). To determine differences in relapse between the early and the late cohorts, patients transplanted in any CR were divided according to whether they had been transplanted before or after 2002. The incidence of relapse in the early series at a median follow-up of 4.18 years (range, 0.19-12.64 years) was 0% in patients transplanted from NK alloreactive donors versus 45% in patients transplanted from non-NK alloreactive donors (P > .01). The incidence of relapse in the later series with a median follow-up of 0.9 years (range, 0.1-4.9 years) was 5% in patients transplanted from NK alloreactive donors versus 25% in patients transplanted from non-NK alloreactive donors (P = .1). Thus, assessment of differences in relapse between the early and the late cohorts was apparently undermined by the much shorter follow-up in the later cohort.
Transplantation from haploidentical NK alloreactive donors controls AML relapse in patients transplanted in any remission. (A) Relapse in patients transplanted in chemoresistant relapse from NK alloreactive versus non-NK alloreactive donors. (B) Relapse in patients transplanted in any remission from NK alloreactive versus non-NK alloreactive donors.
Transplantation from haploidentical NK alloreactive donors controls AML relapse in patients transplanted in any remission. (A) Relapse in patients transplanted in chemoresistant relapse from NK alloreactive versus non-NK alloreactive donors. (B) Relapse in patients transplanted in any remission from NK alloreactive versus non-NK alloreactive donors.
Figure 4 shows EFS of patients transplanted in relapse (Figure 4A) versus remission (Figure 4B). Transplantation from NK alloreactive donors improved survival. Survival was poorest in patients in chemoresistant relapse transplanted from non-NK alloreactive donors (6%) but improved significantly when they received grafts from NK-alloreactive donors (30%, P = .04). In patients in any CR who received transplants from non-NK alloreactive donors the probability of event-free survival was 18%, which rose to 67% when they received grafts from NK-alloreactive donors (P = .02).
Transplantation from haploidentical NK alloreactive donors improves EFS. (A) EFS in patients transplanted in relapse from NK-alloreactive versus non-NK alloreactive donors. (B) EFS in patients transplanted in CR from NK alloreactive versus non-NK alloreactive donors.
Transplantation from haploidentical NK alloreactive donors improves EFS. (A) EFS in patients transplanted in relapse from NK-alloreactive versus non-NK alloreactive donors. (B) EFS in patients transplanted in CR from NK alloreactive versus non-NK alloreactive donors.
Figure 5A shows EFS in all patients with AML transplanted from non–NK-alloreactive versus NK-alloreactive donors. Transplantation from NK-alloreactive donors was associated with reduced risk of relapse or death (relative risk versus non–NK-alloreactive donor, 0.48; 95% CI, 0.29-0.78; P > .001). Multivariate analysis which included crucial transplantation variables affecting outcomes, such as, disease status at transplantation (any remission versus chemoresistant relapse), age, cytogenetics, conditioning regimens, graft-processing procedures, and the number of CD34+ and T cells in the graft, confirmed that transplantation from NK alloreactive donors is a strong independent factor for good prognosis. When these variables were tested for impact on NK cell alloreactivity, no variable had any significant impact in the total group of 112 patients and in the separate series of 57 haploidentical transplant recipients that we presented in 2002,4 and 55 who received a transplant since then.
EFS according to NK cell alloreactivity and to specific KIR ligand mismatches. (A) EFS after transplantation from NK-alloreactive donors versus non-NK alloreactive donors in the entire series of 112 haploidentical transplant recipients. (B) EFS after transplantation from NK-alloreactive donors according to the specific donor KIR ligand that was missing in the recipient. Solid line indicates HLA-C 1; dotted line, HLA-C 2; broken line, HLA-Bw4.
EFS according to NK cell alloreactivity and to specific KIR ligand mismatches. (A) EFS after transplantation from NK-alloreactive donors versus non-NK alloreactive donors in the entire series of 112 haploidentical transplant recipients. (B) EFS after transplantation from NK-alloreactive donors according to the specific donor KIR ligand that was missing in the recipient. Solid line indicates HLA-C 1; dotted line, HLA-C 2; broken line, HLA-Bw4.
Although transplantation from NK-alloreactive donors provided no advantage in the first 6 months after transplantation (relative risk versus non-NK alloreactive donor, 0.95; 95% CI, 0.61-1.92; P = NS), risk of relapse or death was greatly reduced in patients surviving fewer than 6 months after transplantation who had been transplanted from NK-alloreactive donors (relative risk versus non-NK alloreactive donor, 0.07; 95% CI, 0.02-0.26; P > .001).
Multivariate analyses showed disease status at transplantation was the only other prognostically significant pretransplantation variable (relative risk, 2.37 for patients transplanted in relapse versus remission).
Finally, whichever of the 3 KIR ligand mismatches was involved (missing HLA-C 1, HLA-C 2, HLA-Bw4), no significant differences emerged in survival advantage after transplantation from an NK alloreactive donor (Figure 5B).
Comparing NK cell alloreactivity and the missing ligand model
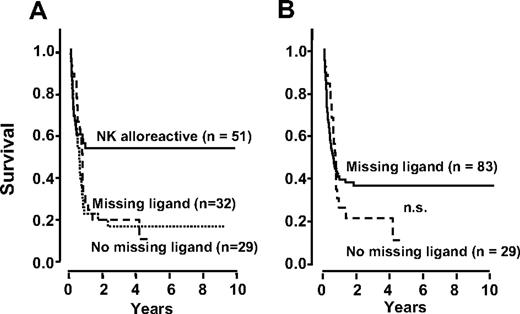
In applying the missing ligand model,17,,–20 the first step was to divide our 61 non-NK alloreactive (KIR ligand-matched) donor-recipient pairs according to the number of KIR ligands in donor and recipient, ie, 3 KIR ligands (no missing ligand, 29 patients; 15 in remission, 14 in relapse at transplantation) versus fewer than 3 (missing ligand, 32 patients; 16 in remission, 16 in relapse at transplantation). Figure 6A shows EFS did not differ in each subgroup. Both curves indicated worse survival than after transplantation from NK-alloreactive donors.
EFS is better predicted by NK alloreactivity than by the missing ligand model. (A) The missing ligand model breaks the non-NK alloreactive EFS curve, shown in Figure 5A, into 2 curves according to whether donors possessed an extra KIR(s) for which neither donor nor recipient had HLA ligand(s), ie, missing ligand, versus no missing ligand. The 2 curves do not differ significantly. (B) In the missing ligand model our NK alloreactive transplants (KIR ligand mismatched) are combined with the missing ligand transplants and compared with the no missing ligand transplants. No significant difference emerged (P = .28). EFS in the missing ligand cohort was worse than after transplantation from NK alloreactive donors (B versus Figure 4).
EFS is better predicted by NK alloreactivity than by the missing ligand model. (A) The missing ligand model breaks the non-NK alloreactive EFS curve, shown in Figure 5A, into 2 curves according to whether donors possessed an extra KIR(s) for which neither donor nor recipient had HLA ligand(s), ie, missing ligand, versus no missing ligand. The 2 curves do not differ significantly. (B) In the missing ligand model our NK alloreactive transplants (KIR ligand mismatched) are combined with the missing ligand transplants and compared with the no missing ligand transplants. No significant difference emerged (P = .28). EFS in the missing ligand cohort was worse than after transplantation from NK alloreactive donors (B versus Figure 4).
According to the missing ligand model,17,18 the second step was to group the above 32 missing ligand transplants and all 51 KIR ligand-mismatched transplants (which corresponded to all our NK-alloreactive transplants). We analyzed EFS in this pool of 83 patients (46 in remission, 37 in relapse at transplantation) against EFS in 29 patients with no missing ligand (15 in remission, 14 in relapse at transplantation) (Figure 6B). No significant difference emerged (P = .28). EFS in the missing ligand cohort (Figure 6B) was worse than after transplantation from NK-alloreactive donors (Figure 5A).
Discussion
This partially incremental study on NK cell alloreactivity in haploidentical transplantation for high-risk AML combines 57 patients whose outcome was reported in 20024 and 52 patients who received a transplant since then.
Transplantation from NK alloreactive donors did not cause GvHD. Unlike our previous findings in clinical transplantation4 and our observations in mice that received pretransplantation infusions of alloreactive NK cells,4,25 it did not significantly reduce rejection and incidence of GvHD. Thus, the present study with more than double the number of NK alloreactive transplants (20 versus 51; see Table 2) provides a more accurate picture of the clinical effects of the alloreactive NK cells that are generated in the patient on transplantation of purified CD34+ cells. The present findings reinforce evidence that not only does transplantation from NK-alloreactive donors not cause GvHD, but it also exerts a marked GvL effect which translates into a remarkable survival advantage (ie, 65% EFS in patients in any CR).
Several observations illustrate the biology underlying the GvL effect. Transfer of human alloreactive NK cells to nonobese diabetic/severe combined immunodeficient mice eradicates AML cells.4 In clinical transplantation, donors who are KIR ligand-mismatched with their recipients in the GvH direction possess alloreactive NK clones which kill recipient hematopietic target cells, including the leukemic (present data).4 One to 3 months after transplantation recipients displayed an NK cell repertoire of donor origin which, when assayed in vitro, contained donor-versus-recipient alloreactive NK clones.26 The present study extends these observations and shows alloreactive NK clones persist for up to 12 months in some patients.
Posttransplantation immunofluorescence analyses may visualize potentially alloreactive NK cells.22 In a donor who was homozygous for group A KIR haplotypes, immunofluorescence detected potential donor-versus-recipient alloreactive NK cells in frequencies concurring with NK clone cytotoxicity assays. In another donor who was positive for group B KIR haplotypes, which, unlike group A, include activating KIRs, immunofluorescence detected fewer potential NK alloreactive cells than NK clone cytotoxicity assays, probably because of anti-KIR antibody cross-reactivity with activating KIRs.
Thus, regardless of posttransplantation frequencies and time kinetics of donor-type alloreactive NK clones and independently of the KIR ligand mismatch, donor-versus-recipient NK alloreactivity emerged as a crucial factor in improving outcomes of haploidentical transplantation. When it is incorporated as a criterion for donor selection the random 33% chance of finding an NK-alloreactive donor increases to 49%, which approaches the maximum because one third of the population expresses all 3 class I allele groups recognized by KIRs and blocks NK cells from every donor. The approximate 50% chance of finding NK-alloreactive donors compares favorably with the odds of finding an unrelated donor and has the advantage of no delay between decision making and transplantation because haploidentical donors are immediately available.
Our functional assessment of donor-versus-recipient NK cell alloreactivity showed all donors who were HLA-C group-mismatched with their recipients (in the GvH direction) possessed alloreactive NK clones against allogeneic targets which did not express their HLA-C group. Thus, for HLA-C group mismatches, which are the most frequent, HLA-C typing predicted NK alloreactivity. One limitation, occurring in approximately 1.5% of HLA-C group mismatched transplantations, is the combination of HLA-C group 2–positive donor/HLA-C group 2–negative recipient. Concurring with other reports,12,27,28 in a survey of 198 persons, we observed that, although the HLA-C group 1 receptor genes (KIR2DL2 and/or KIR2DL3) are present in 100% of persons, the HLA-C group 2 receptor gene (KIR2DL1) is present in only 97% (A.M., unpublished data, June 2006). When this combination presents, KIR2DL1 gene typing of the donor is necessary.
The 8 HLA-Bw4 KIR ligand mismatched donors in the present transplant series possessed the KIR3DL1 HLA-Bw4 receptor gene and alloreactive NK clones against recipient target cells. However, in analyzing HLA-Bw4 mismatches in 31 candidate donors, we detected alloreactive NK clones in only 20. Indeed, as shown by others,12,27,28 KIR3DL1 was found in only ∼90% of individuals (A.M., unpublished data, June 2006). Moreover, in a few people, allelic variants may not allow receptor expression at the cell membrane.29 Thus, in HLA-Bw4 mismatches the donor NK clone repertoire should be assessed.
In unrelated donor transplantations, some retrospective studies show no advantage in transplantation from KIR ligand-mismatched donors.20,30,,,–34 Unrelated donor transplantation protocols are heterogeneous in conditioning regimens, patient populations, and underlying diseases (including adult common phenotype acute lymphoblastic leukemia which is resistant to alloreactive NK killing26 ). They use T-cell–replete bone marrow harvests (or, less frequently, peripheral blood progenitors) which contain approximately 4-log more T cells and up to 1-log fewer stem cells than haploidentical grafts. Relatively few transplanted stem cells, combined with the high T-cell graft content and posttransplantation immune suppression have been associated with poor reconstitution of potentially alloreactive, KIR-bearing NK cells.35,36 Nonetheless, other studies have observed an increased GvL effect in transplants from NK alloreactive unrelated donors.37,,,,–42 In particular, a marked survival advantage was reported in patients who received antithymocyte globulins before transplantation (which provided in vivo T-cell depletion) and a graft containing 2- to 3-fold more nucleated cells than usual in unrelated-donor transplants.37 Prospective studies are needed to determine whether strategies (high doses of stem cells, T-cell depletion, no posttransplantation immune suppression), which in haploidentical transplantation harness donor-versus-recipient NK cell alloreactivity, can be implemented to improve outcome in unrelated donor transplants.
Because our original report that NK cell alloreactivity in haploidentical transplantation rests on KIR ligand mismatching and donor NK cell recognition of missing self on recipient targets,4 the missing ligand model has been proposed as a powerful algorithm for predicting favorable transplantation outcomes not only in haploidentical transplants17,18 but also in matched sibling19 and in unrelated donor transplants.20 Under the perturbed conditions that exist after a hematopoietic stem cell transplantation, it hypothesizes NK alloreactions occur when KIR ligand-matched donors possess an extra KIR for which neither donor nor recipient have an HLA ligand. These donors may carry KIR-bearing NK cells in an anergic or regulated state which, on transfer into the recipient, are hypothesized to become activated and exert a GvL effect. Unfortunately, no informative results emerged when we analyzed outcomes of missing ligand transplantations in our patients who were transplanted from haploidentical KIR ligand-matched donors. Differences in diseases, age of patients, and transplantation protocols, such as ATG (our series) versus no ATG in the conditioning17,,–20 or peripheral blood CD34+ cells (our series) versus bone marrow19,20 as a source of hematopoietic cells, may account for these discrepancies. In any case, no studies have as yet determined whether anergic NK cells acquire or resume effector function after transplantation, even though self-tolerant NK cells which do not express inhibitory receptors for self-MHC have been described in mice43,,–46 and humans.47
The missing ligand model also includes all haploidentical transplantations that are KIR ligand-mismatched in the GvH direction,17,18 because the majority of donors possess the 3 inhibitory KIRs, whereas all recipients possess only 1 or 2 HLA KIR ligands. Even when weighted with our KIR ligand-mismatched transplantations, the missing ligand donor-recipient combination disappointingly identified a group of patients with worse prognosis than those transplanted from NK-alloreactive donors. Therefore, the present analysis leaves no doubt that KIR ligand mismatches, ie, donor NK cell recognition of missing self on recipient targets, are essential for triggering powerful NK cell alloreactions that impact beneficially on transplantation outcomes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Mauro Di Ianni for DNA polymorphism analyses, Dr Antonella Santucci for statistical analyses, Drs. Alessandro Moretta (University of Genova), Silvano Ferrini (Istituto Nazionale per la Ricerca sul Cancro, Genova) and Silvia Parolini (University of Brescia) for the generous gift of anti-KIR antibodies, and Dr Geraldine Anne Boyd for editorial assistance.
This work was supported by a Translational Research Grant from the Leukemia and Lymphoma Society and by grants from the Italian Association for Cancer Research, the Italian Ministry of Further Education, and the Italian Ministry of Health, by the European Community (contract no. LSHB-CT-2004-503319) and by the National Institutes for Health (project no. 1 PO1 CA100265).
L.R. is a Leukemia and Lymphoma Society Special Fellow in Clinical Research. A.M. is a student of the International PhD Program of Molecular Medicine of Vita Salute San Raffaele University, Milan, Italy. M.S. is the recipient of fellowships by the Swiss Cancer League (grant BIL OCS 01597-08-2004) and the Swiss National Science Foundation (grant PBBSB-107328).
Authorship
Contribution: L.R. performed research, collected data, and analyzed data and data layouts; A.M. performed KIR gene typing; M.C., E.U., F.T., E. Bianchi., and D.P. performed NK clonal and immunofluorescence analyses; A.C., T.A., K.P., and E. Burchielli took care of patients; M.S. performed statistical analyses; F.A. was responsible for patient care; M.F.M. directs the transplantation program; A.V. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea Velardi, Division of Hematology and Clinical Immunology, Department of Clinical and Experimental Medicine, University of Perugia, Policlinico Monteluce, Via Brunamonti 51-06122 Perugia, Italy; e-mail: velardi@unipg.it.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal