Abstract
The stem cell leukemia (SCL) gene encodes a basic helix-loop-helix transcription factor expressed in erythroid, megakaryocyte, and mast-cell lineages. SCL is essential for growth of megakaryocyte and erythroid progenitors. We have used a conditional knockout of SCL (SCL−/Δ) to examine its function in mast cells, critical effectors of the immune system. SCL−/Δ mice had markedly increased numbers of mast-cell progenitors (MCPs) within the peritoneal fluid, bone marrow, and spleen. Fractionation of bone marrow myeloid progenitors demonstrated that these MCPs were present in the megakaryocyte-erythroid–restricted cell fraction. In contrast, unilineage MCPs from control mice were present in the cell fraction with granulocyte-macrophage potential. The aberrant mast-cell differentiation of SCL−/Δ megakaryocyte-erythroid progenitors was associated with increased expression of GATA-2. Despite increased numbers of MCPs in SCL−/Δ mice, numbers of mature tissue mast cells were not increased unless SCL−/Δ mice were treated with IL-3 and stem-cell factor. In part, this may be due to a requirement for SCL in normal mast-cell maturation: SCL−/Δ mast cells had reduced expression of the high-affinity IgE receptor and mast cell proteases, MCP-5 and MCP-6. Together, these studies suggest that loss of SCL leads to aberrant mast-cell differentiation of megakaryocyte-erythroid progenitors.
Introduction
The stem cell leukemia gene (Scl/Tal1/Tcl5) is a member of the basic helix-loop-helix (bHLH) gene family.1 SCL is essential for hematopoietic specification of mesoderm during development: SCL-null embryos die due to absence of hematopoiesis.2,3 In adult hematopoiesis, we have used a conditional knockout to demonstrate an essential role for SCL in growth of erythroid and megakaryocyte progenitors.4 Remarkably, in vivo production of erythroblasts and platelets in SCL-deleted mice can continue indefinitely despite the severe in vitro growth defects.5,6 This ongoing hematopoiesis in the absence of SCL may be due to redundancy by other related bHLH factors such as LYL-1, although this is yet to be demonstrated. SCL functions by forming heterodimers with ubiquitous E proteins, which then bind to a specific E-box DNA motif CANNTG. SCL can also be found within larger protein complexes that include GATA factors, ETO2 and Gfi1.7 The critical target genes of SCL that regulate cell fate are unknown, and their discovery is made even more complex by the observation that SCL-containing complexes can either activate or repress gene transcription.8-10
In addition to the erythroid and megakaryocyte lineages, SCL is expressed in mature mast cells.11 Mast cells are important regulatory cells of the innate immune system and mediators of allergic diseases.12 Defining the factors that regulate mast-cell number and function will be critical for developing therapies for disorders of mast cells. Mast cells are derived from mast cell progenitors (MCPs), which arise from lineage−c-kit+ bone marrow (BM) cells.13 The relationship between MCPs and other myeloid progenitors is controversial, however assays of BM or spleen cell fractions suggest MCPs arise from either an early multipotent progenitor or from the granulocyte-macrophage lineage.14,15 MCPs do not appear to be closely related to megakaryocyte-erythroid progenitors (MEPs) despite all 3 lineages expressing a common set of transcription factors including SCL and GATA-1.16 MCPs have several distinct properties including maturation within tissues rather than the BM and the ability to proliferate indefinitely in culture in the presence of IL-3 and stem cell factor (SCF).17
GATA-2 and PU.1 are transcription factors critical for early mast cell development. GATA-2–null yolk sacs generate macrophages but not mast cells in the presence of IL-3.18 PU.1-null embryos have no mature mast cells in their skin, while fetal liver cultures stimulated with IL-3 can form only immature mast-cell lines.19 GATA-1 appears to be more important for later mast-cell development.20 The BM and spleens of GATA-1lo mice contain trilineage progenitor cells capable of giving rise to erythroblasts, megakaryocytes, and mast cells. This observation raises the possibility that mast cells arise from an MEP rather than a multipotent progenitor or granulocyte-macrophage progenitor (GMP) as postulated by recent cell-sorting experiments.14,15 The function of SCL in mast-cell development is unknown, although the association of SCL with GATA factors in erythroid cells raises the possibility that SCL may act in concert with the GATA factors for early or later mast-cell development.21 To address the role of SCL in mast-cell commitment, growth, and differentiation, we have analyzed the mast-cell phenotype of conditional SCL knockout mice.
Materials and methods
Mice and tissues
Mice with loxP sites flanking exons encoding the essential bHLH domain of SCL (Sclfl), an SCL-null allele (Scl−), and MxCre transgenic and PU.1gfp knock-in mice have been previously reported.4,22-24 Poly(I:C)-treated MxCreSCL+/+, MxCreSCL+/fl, and MxCreSCL−/fl mice were used to generate Scl wild-type (Scl+/+), SCL-heterozygous (Scl+/Δ), and SCL-deleted (Scl−/Δ) mice. All analyses were performed at least 4 weeks after administration of poly(I:C) in age-matched adult mice (10 to 26 weeks) on a congenic C57BL/6 genetic background. Southern blot confirmed more than 90% deletion of the Sclfl allele in BM, spleen, and thymus (data not shown).
The numbers of peritoneal mast cells were calculated by multiplying the total peritoneal cell count by the percentage of mast cells determined by morphology or flow cytometry. Organs including skin and gastrointestinal tissue were fixed in 10% formalin for toluidine blue staining or Carnoy for staining with safranin red/Alcian blue. The numbers of tissue mast cells were determined by counting at least 10 high-power fields (100 ×) using a Nikon Optiphot-2 upright microscope (Nikon, Melville, NY) equipped with an AxioCam Mrc5 digital imaging system (Carl Zeiss, North Ryde, Australia). Images were processed using Axiovision (Carl Zeiss) AC Rel 4.5 acquisition software and Adobe (San Jose, CA) Photoshop version 8.0.
Antibodies and fluorescence-activated cell sorting (FACS) analyses
Antibodies used for analyses were obtained from BD Pharmingen (San Diego, CA): Sca-1 (E13–161-7), IgE (R35–72), and CD34 (RAM34) as fluorescein isothiocyanate (FITC) conjugates; c-kit (Ack45), β7 integrin (M293), and FcRIII/II (2.4G2) as phycoerythrin (PE) conjugates; c-kit (Ack45) as an allophycocyanin (APC) conjugate; and biotinylated IL-7Rα (B12–1), CD3ϵ (145–2C11), CD11b (M1/70), B220 (RA3–6B2), Gr-1 (RB6–8C5), TER-119, and IgE. Second-stage fluorescent reagents were either SAv-APC or SAv-PerCP-Cy5.5. Viability was determined by exclusion of propidium iodide (Sigma, St Louis, MO) or hydroxystilbamidine (Molecular Probes, Eugene, OR).
Expression of FcϵRI was determined by preincubation with 2.5 μg/mL αDNP IgE (Sigma) for 1 hour on ice or overnight stimulation for up-regulation of FcϵRI. Calcium flux was performed by loading for 45 minutes at 30°C in the dark with 2.1 μg/mL Indo-1 AM cell permanent, 0.02% pluronic F127, and 4 mM probenecid (Molecular Probes). Cells were stimulated with 10 μg/mL DNP-HSA and the FL5/FL4 ratio was analyzed on a LSR flow cytometer (BD Biosciences, San Jose, CA).
Isolation of myeloid progenitor populations
The following monoclonal antibodies were used as supernatants for immunomagnetic bead depletion of lineage marker–positive BM cells: anti-CD3 (KT3–1.1), anti-CD8 (53–6.7), anti-CD2 (RM2–1), anti-B220 (RA3–6B2), anti–Mac-1 (M1/70), anti–Gr-1 (RA6–8C5), and antierythrocyte antigen (TER-119). For isolation of CMPs, GMPs, and MEPs, the lineage-depleted cell fraction was stained with goat anti–rat immunoglobulin Texas red, anti–FcRIII/II-FITC, anti–IL-7Rα–Alexa 594, anti–Sca-1–Alexa 594, anti–c-kit–APC, and anti–CD34-biotin followed by SAv-PE. For isolation of myeloid progenitors based upon PU.1gfp expression, the lineage-depleted cell fraction was stained with anti–IL-7Rα–biotin, anti–Sca-1–biotin, anti–FcRIII/II-PE, anti–c-kit–APC followed by SAv-PerCP-Cy5.5. Cells were sorted using a FACSVantage SE system (BD Biosciences), and after sorting, the purity of sorted cells was determined by reanalyzing a small sample of the collected cells and was 80% to 90%.
Semisolid culture of BM progenitors
BM cells were cultured in 0.3% agar and analyzed as described previously.25 Recombinant cytokines were used at the following concentrations: 10 ng/mL mIL-3, 50 ng/mL rSCF, 4 IU/mL human erythropoietin (Epo; Amgen, Thousand Oaks, CA), 25 ng/mL mFlt-3L (R&D Systems, Minneapolis, MN), 50 ng/mL mIL-6 (R&D Systems), 10 ng/mL GM-CSF (PeproTech, Rocky Hill, NJ), and 10 ng/mL human megakaryocyte growth and differentiation factor (MGDF; Amgen). For growth of blast-forming unit–erythroid (BFU-E), BM and spleen cells were plated in 0.9% methylcellulose, 20% fetal bovine serum (FBS), Iscoves modified Dulbecco medium (IMDM; GIBCO, Carlsbad, CA) supplemented with 10 ng/mL IL-3, 50 ng/mL SCF, and 4 U/mL human Epo.
Real-time PCR
RNA was isolated using TRIZol (Invitrogen). Real-time polymerase chain reaction (PCR) was carried out in 20-μL reactions using SYBR green fluorescent DNA labeling (Molecular Probes) at a dilution of 1:10 000, and PCR was performed using the Rotorgene 2000 PCR machine (Corbett Research, Sydney, Australia). Relative quantitation was performed using the standard curve method, which was generated for each amplicon using 5-fold serial dilutions of normal mouse BM cDNA. All values were normalized to those obtained via amplification with primers specific for HPRT. Gene specific primer sequences are available on request.
In vitro cell culture
For limit dilution analysis, myeloid progenitor populations were plated at limiting dilution in 96-well plates with IMDM and 10% FBS supplemented with IL-3, SCF, Epo, MGDF, GM-CSF, and IL-6. Wells containing single colonies were identified at 10 days and harvested for morphologic and flow cytometric analyses. Liquid cultures for erythroid progenitors were performed using serum-free media as previously described.26 BM-derived mast-cell lines were generated in RPMI (GIBCO) supplemented with 10% FBS, 10% WEHI-3B conditioned media, and 50 ng/mL rSCF (Amgen).
Retrovirus infection
Retroviral supernatants were prepared using transient transfection of HEK-293T cells with the retroviral plasmids MSCViresGFP, MSCVSCLiresGFP, or pCre-ERT2 together with the pGag-Pol and pCAG-Eco packaging plasmids (a gift of Dr Derek Persons). BM cells from mice treated with 150 mg/kg 5-flurouracil (5-FU; Sigma) were cultured at 5 × 105 cells/mL in Stem Pro-34 SFM (GIBCO) with nutrient supplement, 2 mM glutamine, 10 ng/mL IL-3, 50 ng/mL IL-6, 50 ng/mL SCF, and 25 ng/mL Flt-3L for 48 to 72 hours prior to 3 to 6 rounds of infection. To confirm expression of SCL, sorted GFP+ BM cells were grown for 3 weeks in IL-3 and SCF. Cell extracts were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to PVDF, immunoblotted with a rabbit polyclonal SCL antibody,27 and developed with enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ).
Results
SCL−/Δ mice have increased mast cell progenitors
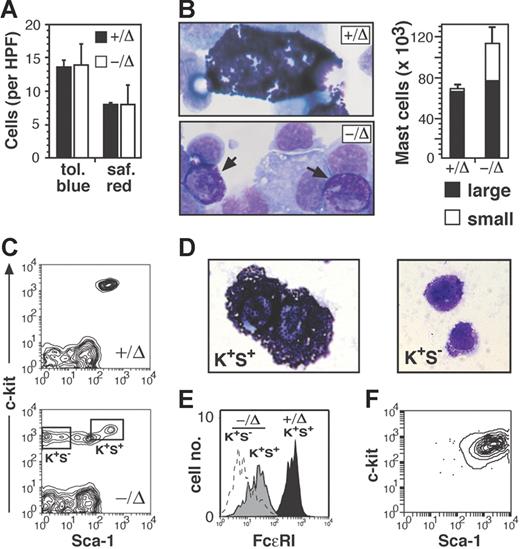
We treated adult MxCreSCL−/fl mice with poly(I:C) to generate SCL-deleted hematopoiesis (SCL−/Δ). With respect to the mast-cell phenotype, there was no difference between poly(I:C)-treated SCL wild-type and SCL-heterozygous mice (SCL+/Δ) except for gene expression studies (Figure 5). Therefore, for all functional studies, SCL+/Δ mice, in which one allele of SCL was deleted by treatment with poly(I:C), were used as controls. Mucosal mast cells of the jujenal epithelium, as determined by Alcian blue staining, were rare (< 1 per 20× power field) and not altered by deletion of SCL (data not shown). The numbers of mast cells of the skin, as determined by safranin red staining, were also not significantly different between SCL+/Δ and SCL−/Δ mice (Figure 1A). However, mast cells of the peritoneal fluid were increased 2-fold in SCL−/Δ mice due to the presence of small mast cells containing fine, basophilic granules (Figure 1B). Flow cytometry of peritoneal fluid demonstrated an abnormal population of c-kit+Sca-1neg/lo cells (Figure 1C). Morphologic examination of sorted cell fractions showed that these c-kit+Sca-1neg/lo cells represented the small mast cells, while the c-kit+Sca-1+ cells represented the larger mast cells such as those observed in control SCL+/Δ peritoneal fluid (Figure 1D). Expression of the high-affinity IgE receptor (FcϵRI) on the c-kit+Sca-1neg/lo cells was not detectable by flow cytometry, while the c-kit+Sca-1+ peritoneal mast cells of SCL−/Δ mice expressed 10-fold reduced levels of FcϵRI compared with control mast cells (Figure 1E).
SCL−/Δ mice have increased mast-cell progenitors in the peritoneal cavity. (A) Numbers of toluidine blue+ and safranin red+ mast cells in connective tissue of the ears of SCL+/Δ and SCL−/Δ mice (n = 3 for each genotype). Results are the mean and standard error of the mean (SEM) of mast cells per 100× high-power field (HPF). (B) Wright-Giemsa stain of peritoneal fluid demonstrating the small, granular mast cells (→) seen only in the SCL−/Δ mice. A normal, large granular mast cell from an SCL+/Δ mouse is shown for comparison. The mean and SEM numbers of each type of mast cell were calculated by absolute peritoneal cell count × proportion of mast cells using flow cytometry. Data represent the mean and SD from 4 mice of each genotype. (C) Flow cytometric analysis of peritoneal cells from SCL+/Δ and SCL−/Δ mice stained for c-kit and Sca-1 expression. (D) Wright-Giemsa stain of sorted c-kit+Sca-1+ (K+S+) and c-kit+Sca-1− (K+S−) peritoneal cells from an SCL−/Δ mouse. (E) Expression of the high-affinity receptor for IgE (FcϵRI) on peritoneal mast- cell subsets from SCL+/Δ and SCL−/Δ mice. (F) Flow cytometric analysis of SCL−/Δ K+S− mast cells cultured for 3 weeks in media containing IL-3 and SCF.
SCL−/Δ mice have increased mast-cell progenitors in the peritoneal cavity. (A) Numbers of toluidine blue+ and safranin red+ mast cells in connective tissue of the ears of SCL+/Δ and SCL−/Δ mice (n = 3 for each genotype). Results are the mean and standard error of the mean (SEM) of mast cells per 100× high-power field (HPF). (B) Wright-Giemsa stain of peritoneal fluid demonstrating the small, granular mast cells (→) seen only in the SCL−/Δ mice. A normal, large granular mast cell from an SCL+/Δ mouse is shown for comparison. The mean and SEM numbers of each type of mast cell were calculated by absolute peritoneal cell count × proportion of mast cells using flow cytometry. Data represent the mean and SD from 4 mice of each genotype. (C) Flow cytometric analysis of peritoneal cells from SCL+/Δ and SCL−/Δ mice stained for c-kit and Sca-1 expression. (D) Wright-Giemsa stain of sorted c-kit+Sca-1+ (K+S+) and c-kit+Sca-1− (K+S−) peritoneal cells from an SCL−/Δ mouse. (E) Expression of the high-affinity receptor for IgE (FcϵRI) on peritoneal mast- cell subsets from SCL+/Δ and SCL−/Δ mice. (F) Flow cytometric analysis of SCL−/Δ K+S− mast cells cultured for 3 weeks in media containing IL-3 and SCF.
Based upon their morphology and immunophenotype, we hypothesized that the c-kit+Sca-1neg/loFcϵRIneg cells represented promastocytes similar to that found in fetal blood.28 To test this hypothesis, we examined their proliferative capacity by culturing sorted cell populations in IL-3 alone, SCF alone, or the combination of IL-3 and SCF. Like fetal promastocytes, the c-kit+Sca-1neg/loFcϵRIneg cells proliferated only in the combination of IL-3 and SCF (data not shown). In contrast, the c-kit+Sca-1+FcϵRIhi mast cells from both SCL+/Δ and SCL−/Δ mice had minimal proliferative capacity (data not shown). Phenotypic analysis of c-kit+Sca-1neg/loFcϵRIneg cells after 3-week culture demonstrated morphology indistinguishable from normal bone marrow–derived mast cell lines and expression of c-kit, Sca-1, and FcϵRI (Figure 1F and data not shown). Together, these results show that loss of SCL promoted the accumulation of promastocytes within the peritoneal cavity.
Mast cell progenitors (MCPs) do not form readily identifiable colonies in semisolid agar cultures of BM cells.29 However, culture of SCL−/Δ BM and spleen cells generated large numbers of mast cell colonies (Table 1; Figure 2A). The mast-cell phenotype was demonstrated by the presence of toluidine blue+ granules and cell surface expression of c-kit, Sca-1, and FcϵRI (Figure 2B,C). Similar to the peritoneal promastocytes, growth of SCL−/Δ mast cell colonies from the BM and spleen required the combination of IL-3 and SCF (data not shown). As previously reported, the major erythroid progenitor and megakaryocyte progenitors were not detected in SCL−/Δ cultures (Table 1). However, occasional acetylcholinesterase-positive megakaryocytes were observed within or near mast cell colonies (Figure 2D). This suggested that SCL−/Δ mast cells and megakaryocytes might have a common cell of origin similar to that reported in GATA-1lo mice.20
Hematopoietic progenitors in SCL+/D and SCL−/D mice
| Tissue/genotype . | No. of colonies* . | ||||||
|---|---|---|---|---|---|---|---|
| BFU-E† . | G . | GM . | M . | Meg . | Blast . | Mast . | |
| Bone marrow | |||||||
| +/Δ | 10 ± 3 | 14 ± 4 | 31 ± 12 | 18 ± 6 | 8 ± 4 | 3 ± 1 | 4 ± 2 |
| −/Δ | 0‡ | 17 ± 7 | 19 ± 5 | 12 ± 3 | 1 ± 1‡ | 3 ± 1 | 50 ± 13‡ |
| Spleen | |||||||
| +/Δ | 2 ± 1 | 4 ± 1 | 4 ± 3 | 4 ± 3 | 2 ± 1 | 2 ± 1 | 0 |
| −/Δ | 0 | 7 ± 2 | 6 ± 3 | 3 ± 2 | 0 | 4 ± 1 | 66 ± 25‡ |
| Tissue/genotype . | No. of colonies* . | ||||||
|---|---|---|---|---|---|---|---|
| BFU-E† . | G . | GM . | M . | Meg . | Blast . | Mast . | |
| Bone marrow | |||||||
| +/Δ | 10 ± 3 | 14 ± 4 | 31 ± 12 | 18 ± 6 | 8 ± 4 | 3 ± 1 | 4 ± 2 |
| −/Δ | 0‡ | 17 ± 7 | 19 ± 5 | 12 ± 3 | 1 ± 1‡ | 3 ± 1 | 50 ± 13‡ |
| Spleen | |||||||
| +/Δ | 2 ± 1 | 4 ± 1 | 4 ± 3 | 4 ± 3 | 2 ± 1 | 2 ± 1 | 0 |
| −/Δ | 0 | 7 ± 2 | 6 ± 3 | 3 ± 2 | 0 | 4 ± 1 | 66 ± 25‡ |
Twenty-five-thousand cells cultured for 7 days in 0.3% agar with IL-3, SCF, and MGDF or methylcellulose with IL-3, SCF, and Epo.
BFU-E indicates blast-forming unit-erythroid; G, granulocyte colonies; GM, granulocyte-macrophage colonies; M, macrophage colonies; and Meg, megakaryocyte colonies.
The numbers of colonies are the mean plus or minus SD from 4 mice of each genotype: SCL heterozygous (+/Δ,) and SCL deleted (−/Δ).
BFU-Es were grown in methylcellulose, while all other colonies were grown in agar.
Statistical comparison with SCL-heterozygous cells using Student t test; P < .05.
SCL−/Δ mice have increased bone marrow and splenic mast-cell progenitors. (A) Morphology of typical colony grown from agar culture of SCL−/Δ bone marrow. The colony was floated and fixed on a slide and then stained with hematoxylin. (B) Toluidine blue stain of cells picked from a typical colony and spun onto a slide. (C) Flow cytometric analysis of a single colony stained with c-kit, Sca-1, and FcϵRI. The dotted line in the histogram represents the isotype control. (D) Acetylcholinesterase stain of floated and fixed mast cell colony demonstrating megakaryocytes (Megs). (E) Flow cytometric analysis of CD34 and β7-integrin expression on lineage marker–negative (Mac-1, B220, TER119, CD3), c-kit+ Sca-1− IL-7R− bone marrow (BM) and spleen cells from SCL+/Δ and SCL−/Δ mice. The numbers represent the mean percentages within the gates of total nucleated cells from 2 mice of each genotype. (F) Mean colony numbers from bone marrow cells cultured in methylcellulose for blast-forming unit–erythroid (Ery) or agar for megakaryocyte (Meg), granulocyte/macrophage (G/M), and mast-cell colonies. Numbers have been standardized for 25 000 cultured cells from mice reconstituted with either SCL+/Δ or SCL−/Δ bone marrow cells (n = 4 for each genotype).
SCL−/Δ mice have increased bone marrow and splenic mast-cell progenitors. (A) Morphology of typical colony grown from agar culture of SCL−/Δ bone marrow. The colony was floated and fixed on a slide and then stained with hematoxylin. (B) Toluidine blue stain of cells picked from a typical colony and spun onto a slide. (C) Flow cytometric analysis of a single colony stained with c-kit, Sca-1, and FcϵRI. The dotted line in the histogram represents the isotype control. (D) Acetylcholinesterase stain of floated and fixed mast cell colony demonstrating megakaryocytes (Megs). (E) Flow cytometric analysis of CD34 and β7-integrin expression on lineage marker–negative (Mac-1, B220, TER119, CD3), c-kit+ Sca-1− IL-7R− bone marrow (BM) and spleen cells from SCL+/Δ and SCL−/Δ mice. The numbers represent the mean percentages within the gates of total nucleated cells from 2 mice of each genotype. (F) Mean colony numbers from bone marrow cells cultured in methylcellulose for blast-forming unit–erythroid (Ery) or agar for megakaryocyte (Meg), granulocyte/macrophage (G/M), and mast-cell colonies. Numbers have been standardized for 25 000 cultured cells from mice reconstituted with either SCL+/Δ or SCL−/Δ bone marrow cells (n = 4 for each genotype).
To exclude the possibility that the mast cell colonies were derived from multipotent progenitors, we picked single colonies and cultured them in a cytokine cocktail containing IL-3, SCF, Flt3 ligand, IL-6, Epo, and MGDF. Consistent with a unilineage potential, 31 of 72 independent colonies generated homogeneous mast-cell lines with no detectable macrophages, granulocytes, or basophils. The remaining 41 colonies failed to proliferate. Given SCL is not required for the growth of macrophages or granulocytes,4 these results suggest that loss of SCL led to an accumulation of progenitors with limited differentiation capacity rather than multipotent progenitors.
The majority of BM and spleen MCPs express β7 integrin.14,15 Consistent with increased numbers of MCPs in SCL−/Δ BM and spleen, there was a 4-fold and 7-fold increase in the numbers of myeloid progenitors (Linnegc-kit+Sca-1−IL-7R−) expressing β7 integrin in the BM and spleen of SCL−/Δ mice (Figure 2E).
Expression of the MxCre transgene is not restricted to hematopoietic cells.23,30 To exclude the possibility that the mast-cell phenotype was due to deletion of SCL in nonhematopoietic cell types, we generated BM mouse chimeras in which lethally irradiated wild-type mice were reconstituted with either SCL+/Δ or SCL−/Δ bone marrow. Cultures of BM cells from SCL+/Δ chimeras demonstrated the same abnormalities as de novo SCL−/Δ mice with absence of erythroid and megakaryocyte colonies and the presence of numerous mast-cell colonies (Figure 2F). Thus, the increased number of MCPs in SCL−/Δ mice was a cell-intrinsic defect of hematopoiesis.
SCL−/Δ MCPs are derived from the megakaryocyte-erythroid cell fraction
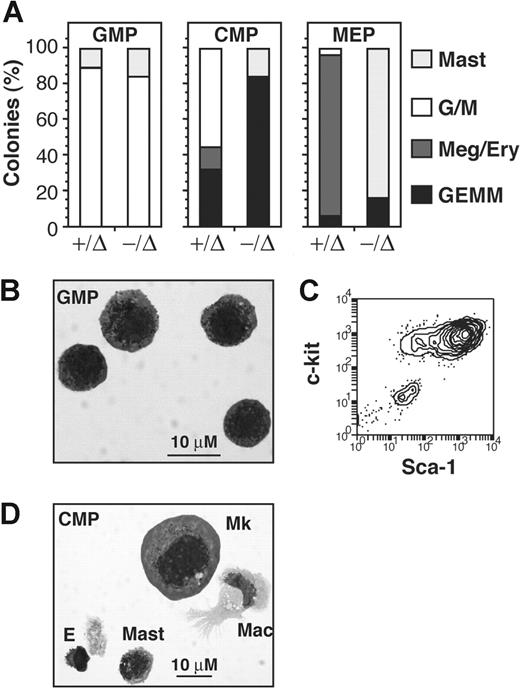
The presence of occasional megakaryocytes within or near mast-cell colonies suggested a common cell of origin for megakaryocytes and mast cells. To determine the ontogeny of SCL−/Δ MCPs, we assayed the mast-cell potential of myeloid progenitors separated into common myeloid progenitors (CMPs; CD34+FcRIII/IIlo), granulocyte-macrophage progenitors (GMPs; CD34+FcRIII/IIhi), and megakaryocyte-erythroid progenitors (MEPs; CD34loFcRIII/IIlo). As previously reported,5 SCL-deleted mice had 2-fold more MEPs (Figure 3A). The purity of sort fractions was 80% to 90% (data not shown). Clonal assay of cell fractions stimulated with IL-3, SCF, and Epo was performed in methylcellulose for erythroid progenitors (BFU-E) or agar for myeloid progenitors including megakaryocyte and mast cell colonies. Consistent with a megakaryocyte-erythroid cell origin, greater than 90% of colonies derived from SCL−/Δ MEP cultures were mast-cell colonies (Figure 3B). Similar to nonfractionated BM cultures, occasional mast- cell colonies contained acetylcholinesterase+ megakaryocytes (Figure 3C). SCL−/Δ CMP cultures also generated mast-cell colonies, but these represented only one third of all colonies. The SCL−/Δ GMP cell fraction did not contain any mast-cell activity. As expected, mast-cell colonies were not detectable at 7 days in any cell fraction from control SCL+/Δ BM (Figure 3B). Together, these results show that abnormal mast-cell growth from SCL−/Δ BM was derived predominantly from the MEP cell fraction.
Mast-cell progenitors in SCL−/Δ mice arise from the megakaryocyte-erythroid progenitor cell fraction. (A) Flow cytometric analysis used for isolating common myeloid progenitors (CMPs), megakaryocyte-erythroid progenitors (MEPs), and granulocyte-macrophage progenitors (GMPs) from SCL+/Δ or SCL−/Δ bone marrow cells. The gate used for lineageneg c-kit+ cells is shown in the left dot plot and gates for CMPs, MEPs, and GMPs in the right dot plots. The numbers represent the mean percentage of MEPs of total nucleated cells from 4 mice of each genotype. (B) Mean numbers of colonies from 500 sorted cells from SCL+/Δ or SCL−/Δ mice cultured in methylcellulose or agar containing IL-3, SCF, and Epo. Results are the means from 2 independent experiments. Ery indicates blast forming unit-erythroid; Meg, megakaryocyte colonies; and G/M, granulocyte and granulocyte-macrophage colonies. (C) Acetylcholinesterase stain of a floated and fixed mast cell colony grown from SCL−/Δ MEP cells. (D) Flow cytometric analysis used for isolating myeloid progenitors according to PU.1gfp and FcRIII/II expression on lineageneg Sca-1− IL-7Rα− c-kit+ bone marrow cells from SCL+/Δ or SCL−/Δ mice. The gated regions used for cell sorting and the mean percentage of R3 cells calculated from 3 mice of each genotype are shown. (E) Relative PU.1 mRNA expression in sorted cell fractions measured by real-time PCR. Expression was normalized for HPRT and expression of PU.1 in SCL+/Δ R2 cells was arbitrarily set at 1.0. (F) Mean numbers of colonies from 500 sorted cells from SCL+/Δ or SCL−/Δ mice cultured in methylcellulose or agar containing IL-3, SCF, and Epo. Results are the mean from 3 independent experiments.
Mast-cell progenitors in SCL−/Δ mice arise from the megakaryocyte-erythroid progenitor cell fraction. (A) Flow cytometric analysis used for isolating common myeloid progenitors (CMPs), megakaryocyte-erythroid progenitors (MEPs), and granulocyte-macrophage progenitors (GMPs) from SCL+/Δ or SCL−/Δ bone marrow cells. The gate used for lineageneg c-kit+ cells is shown in the left dot plot and gates for CMPs, MEPs, and GMPs in the right dot plots. The numbers represent the mean percentage of MEPs of total nucleated cells from 4 mice of each genotype. (B) Mean numbers of colonies from 500 sorted cells from SCL+/Δ or SCL−/Δ mice cultured in methylcellulose or agar containing IL-3, SCF, and Epo. Results are the means from 2 independent experiments. Ery indicates blast forming unit-erythroid; Meg, megakaryocyte colonies; and G/M, granulocyte and granulocyte-macrophage colonies. (C) Acetylcholinesterase stain of a floated and fixed mast cell colony grown from SCL−/Δ MEP cells. (D) Flow cytometric analysis used for isolating myeloid progenitors according to PU.1gfp and FcRIII/II expression on lineageneg Sca-1− IL-7Rα− c-kit+ bone marrow cells from SCL+/Δ or SCL−/Δ mice. The gated regions used for cell sorting and the mean percentage of R3 cells calculated from 3 mice of each genotype are shown. (E) Relative PU.1 mRNA expression in sorted cell fractions measured by real-time PCR. Expression was normalized for HPRT and expression of PU.1 in SCL+/Δ R2 cells was arbitrarily set at 1.0. (F) Mean numbers of colonies from 500 sorted cells from SCL+/Δ or SCL−/Δ mice cultured in methylcellulose or agar containing IL-3, SCF, and Epo. Results are the mean from 3 independent experiments.
Using a PU.1gfp reporter mouse strain, we have shown previously that down-regulation of PU.1 is one of the earliest transcriptional changes associated with commitment to the megakaryocyte-erythroid cell lineages.24 To define the origin of mast-cell colonies in SCL−/Δ BM with respect to PU.1 expression, MxCreSCLfl mice were mated with the PU.1gfp reporter strain to permit cell sorting of myeloid progenitor cells on the basis of PU.1gfp expression. SCL−/Δ mice contained 2-fold more PU.1loFcRIII/IIlo cells (Figure 3D). Real-time PCR analysis confirmed that GFP expression reflected PU.1 expression in SCL−/Δ cell fractions (Figure 3E). Consistent with our previous report,24 the PU.1lo cell fraction from control SCL+/Δ bone marrow was highly enriched for megakaryocyte-erythroid progenitors, while the PU.1hiFcRIII/IIhi cell fraction was enriched for granulocyte-macrophage progenitors (Figure 3F). The PU.1hiFcRIII/IIlo myeloid progenitors behaved like CMP, with a mixture of megakaryocyte-erythroid and granulocyte-macrophage progenitors. Assay of SCL−/Δ BM cells demonstrated mast-cell activity almost exclusively within the megakaryocyte-erythroid–restricted PU.1loFcRIII/IIlo cell fraction (Figure 3F). There was no significant mast-cell activity in the granulocyte-macrophage PU.1hiFcRIII/IIhi cell fraction. Thus, MCPs in SCL−/Δ mice were derived from the PU.1lo progenitor cell fraction, which is normally megakaryocyte-erythroid restricted.
Normal mast cells are derived from progenitors with granulocyte-macrophage potential
The ontogeny of mast cells and their relationship with other myeloid cell lineages remains unclear but MCPs most likely arise from an early multipotent progenitor or a granulocyte-macrophage progenitor.14,15 Given that normal MCPs are not easily discernible in 7-day agar cultures, we used a limit dilution liquid culture assay to compare the origins of normal MCPs with SCL−/Δ MCPs. After 10-day culture with IL-3, SCF, Epo, MGDF, GM-CSF, and IL-6, GMP was the only cell fraction containing pure mast-cell colonies in control SCL+/Δ cultures (Figure 4A-C). Cultures from SCL+/Δ CMPs also contained mast cells, but these were always present within multipotent granulocyte-erythrocyte-megakaryocyte-macrophage (GEMM) colonies (Figure 4D). The SCL+/Δ MEP cell fraction had no significant mast-cell activity (Figure 4A). In contrast and consistent with the semisolid cultures, the SCL−/Δ MEP cell fraction was highly enriched for mast-cell activity. Thus, liquid culture assays demonstrated a distinct origin of mast cells in the presence of SCL: unilineage mast cells were derived from myeloid progenitors with granulocytic-macrophage potential rather than from megakaryocyte-erythroid potential. This suggests that mast cells derived from SCL−/Δ MEP cells were due to aberrant differentiation rather than expansion of the normal mast-cell progenitor pool.
Normal mast-cell progenitors arise from GMPs rather than MEPs. (A) Proportion of progenitor cell types generated from 10-day liquid cultures of GMPs, CMPs, or MEPs from SCL+/Δ or SCL−/Δ mice. Wells containing only single colonies were analyzed by morphology and flow cytometry. At least 20 colonies were analyzed for each cell type. (B) Wright-Giemsa stain of a single colony from SCL+/Δ GMP culture. (C) Flow cytometric analysis of a single colony from SCL+/Δ GMP culture. (D) Wright-Giemsa stain of a single colony from a SCL+/Δ CMP culture.
Normal mast-cell progenitors arise from GMPs rather than MEPs. (A) Proportion of progenitor cell types generated from 10-day liquid cultures of GMPs, CMPs, or MEPs from SCL+/Δ or SCL−/Δ mice. Wells containing only single colonies were analyzed by morphology and flow cytometry. At least 20 colonies were analyzed for each cell type. (B) Wright-Giemsa stain of a single colony from SCL+/Δ GMP culture. (C) Flow cytometric analysis of a single colony from SCL+/Δ GMP culture. (D) Wright-Giemsa stain of a single colony from a SCL+/Δ CMP culture.
Increased expression of GATA-2 in the absence of SCL
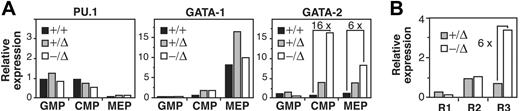
Cross-talk between lineage-determining transcription factors may be an important mechanism of achieving coordinated expression of stage-specific transcription factors that regulate hematopoietic cell lineage fate.31 For example, down-regulation of GATA-2 by PU.1 promotes macrophage differentiation, while coexpression of GATA-2 and PU.1 promotes mast-cell differentiation.19 We hypothesized aberrant mast-cell differentiation might be associated with an abnormal transcriptional profile in SCL−/Δ MEP cells. Therefore, we examined the expression of PU.1, GATA-1, and GATA-2 in sorted myeloid progenitors from SCL+/+, SCL+/Δ, and SCL−/Δ mice. Absence of SCL did not alter the expression of PU.1 or GATA-1 in MEP cells (Figure 5A). However, GATA-2 expression was markedly increased in SCL−/Δ MEPs (6-fold greater than SCL+/+ cells) and SCL−/Δ CMPs (16-fold increased). Interestingly, loss of one SCL allele (SCL+/Δ cells) also increased expression of GATA-2 by 2- to 3-fold (Figure 5A). Abnormal expression of GATA-2 was also observed in the SCL−/Δ PU.1lo cell fraction (R3) but not the PU.1hi cell fractions (Figure 5B). Thus, deletion of SCL was associated with increased expression of GATA-2 in megakaryocyte-erythroid–restricted progenitors.
Elevated expression of GATA-2 in SCL+/Δ CMPs and MEPs. (A) Relative expression of PU.1, GATA-1, and GATA-2 mRNA in GMPs, CMPs, or MEPs isolated from wild-type (+/+), SCL-heterozygous (+/Δ), and SCL-deleted (−/Δ) mice and measured by real-time PCR. Expression was normalized for HPRT and expression of the gene in SCL+/+ CMP cells was arbitrarily set at 1.0. (B) Relative expression of GATA-2 in progenitor cells isolated on the basis of PU.1gfp and FcRIII/II expression as described in Figure 3D. Expression was normalized for HPRT and expression of GATA-2 in SCL+/Δ R2 cells was arbitrarily set at 1.0.
Elevated expression of GATA-2 in SCL+/Δ CMPs and MEPs. (A) Relative expression of PU.1, GATA-1, and GATA-2 mRNA in GMPs, CMPs, or MEPs isolated from wild-type (+/+), SCL-heterozygous (+/Δ), and SCL-deleted (−/Δ) mice and measured by real-time PCR. Expression was normalized for HPRT and expression of the gene in SCL+/+ CMP cells was arbitrarily set at 1.0. (B) Relative expression of GATA-2 in progenitor cells isolated on the basis of PU.1gfp and FcRIII/II expression as described in Figure 3D. Expression was normalized for HPRT and expression of GATA-2 in SCL+/Δ R2 cells was arbitrarily set at 1.0.
Enforced expression of SCL does not prevent aberrant mast- cell growth
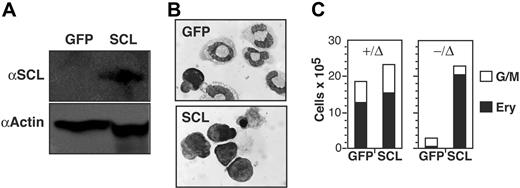
To further explore the function of SCL in mast-cell lineage commitment, we infected SCL−/Δ BM cells with an SCL-expressing retrovirus. Western blot confirmed SCL protein levels far greater than endogenous SCL protein, which was not detectable in control SCL+/Δ mast cells using a rabbit polyclonal anti-SCL antibody (Figure 6A). We predicted that enforced expression of SCL would rescue erythroid and megakaryocyte progenitor cell growth and inhibit growth of mast cells from SCL−/Δ BM. In our hands, BFU-Es from retrovirus-infected BM cells grew poorly in methylcellulose. Therefore, we used liquid culture in erythroid-defined media to determine the effect of SCL expression on erythroid cell growth. As expected, erythroid cultures of SCL−/Δ BM cells infected with a control GFP-expressing retrovirus contained granulocytes and only rarely erythroblasts (Figure 6B). In contrast, SCL−/Δ BM cells infected with the SCL-expressing retrovirus generated large numbers of erythroblasts (Figure 6C). To address the effects of SCL on megakaryocytes and mast cells, retrovirus-infected SCL−/Δ BM cells were grown for 7 days in agar cultures containing IL-3, SCF, and MGDF. Expression of SCL rescued megakaryocyte progenitor cell growth (Table 2). However, expression of SCL did not inhibit mast-cell growth despite rescuing erythroid and megakaryocyte cell growth. This suggested that deletion of SCL produced immutable changes in progenitor cells that could not be easily reversed by enforced expression of SCL.
Enforced expression of SCL does not prevent mast-cell formation from SCL−/Δ cells. (A) Western blot of SCL+/Δ bone marrow cells infected with either an MSCV-GFP retrovirus (GFP) or MSCV-SCLiresGFP retrovirus (SCL). Cells were harvested 3 weeks after sorting for GFP expression and growth in IL-3 and SCF. (B) Wright-Giemsa stain of SCL−/Δ bone marrow cells infected with the GFP or SCL retrovirus and cultured for 6 days in erythroid media containing SCF, Epo, IGF-1, and dexamethasone. (C) Numbers of erythroblasts (Erys) and granulocyte-macrophage cells (G/Ms) generated from 105 infected SCL+/Δ or SCL−/Δ bone marrow cells cultured in erythroid media for 6 days. Data are representative of 3 independent experiments.
Enforced expression of SCL does not prevent mast-cell formation from SCL−/Δ cells. (A) Western blot of SCL+/Δ bone marrow cells infected with either an MSCV-GFP retrovirus (GFP) or MSCV-SCLiresGFP retrovirus (SCL). Cells were harvested 3 weeks after sorting for GFP expression and growth in IL-3 and SCF. (B) Wright-Giemsa stain of SCL−/Δ bone marrow cells infected with the GFP or SCL retrovirus and cultured for 6 days in erythroid media containing SCF, Epo, IGF-1, and dexamethasone. (C) Numbers of erythroblasts (Erys) and granulocyte-macrophage cells (G/Ms) generated from 105 infected SCL+/Δ or SCL−/Δ bone marrow cells cultured in erythroid media for 6 days. Data are representative of 3 independent experiments.
Enforced expression of SCL in SCL−/Δ bone marrow cells
| Retrovirus . | No. of colonies* . | |||||
|---|---|---|---|---|---|---|
| G . | GM . | M . | Meg . | Blast . | Mast . | |
| GFP | 14 ± 8 | 11 ± 5 | 7 ± 6 | 0 | 5 ± 5 | 6 ± 2 |
| SCL | 29 ± 12† | 12 ± 5 | 5 ± 3 | 6 ± 1† | 4 ± 1 | 16 ± 10† |
| Retrovirus . | No. of colonies* . | |||||
|---|---|---|---|---|---|---|
| G . | GM . | M . | Meg . | Blast . | Mast . | |
| GFP | 14 ± 8 | 11 ± 5 | 7 ± 6 | 0 | 5 ± 5 | 6 ± 2 |
| SCL | 29 ± 12† | 12 ± 5 | 5 ± 3 | 6 ± 1† | 4 ± 1 | 16 ± 10† |
One-thousand GFP+ sorted cells cultured for 7 days in 0.3% agar with IL-3, SCF, and MGDF.
G indicates granulocyte colonies; GM, granulocyte-macrophage colonies; M, macrophage colonies; and Meg, megakaryocyte colonies.
The numbers of colonies are the mean plus or minus SD from 3 independent infections of SCL-deleted (−/Δ) bone marrow cells.
Statistical comparison with cells infected with the GFP retrovirus; P < .01.
SCL is required for normal mast-cell maturation
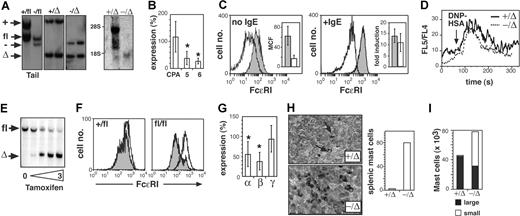
While loss of SCL resulted in an accumulation of progenitors with mast- cell potential, the numbers of tissue mast cells were normal, suggesting that SCL might be required for maturation of mast-cells. The reduced expression of FcϵRI on peritoneal mast cells was consistent with this hypothesis (Figure 2C). To examine mast-cell maturation in more detail, we generated BM-derived mast-cell lines (BMMCs) from control and SCL−/Δ mice. Southern blot of BMMCs confirmed complete deletion of SCL, and Northern blot demonstrated the absence of the SCL transcript (Figure 7A). Absence of SCL did not affect the morphology, growth, or survival of BMMCs (data not shown). However, SCL−/Δ BMMCs had reduced expression of mast-cell protease 5 (MCP-5) and MCP-6 (Figure 7B). Expression of the mast cell–specific enzyme, carboxypeptidase A (MC-CPA), which is expressed earlier than MCP-5 and MCP-6, was not affected by loss of SCL. Similar to SCL−/Δ peritoneal mast cells, expression of FcϵRI was reduced 4-fold (Figure 7C). Induction of FcϵRI expression by IgE was normal, suggesting that IgE signaling was not impaired (Figure 7C). The reduced expression of FcϵRI did not affect IgE-induced Ca2+ flux (Figure 7D).
SCL is required for mast-cell maturation. (A) Southern blot (left) and Northern blot (right) of mast-cell lines derived from SCL+/Δ or SCL−/Δ bone marrow cells. Tail DNA from MxSCL+/fl and MxSCL−/fl mice is shown to demonstrate the position of the SCLfl allele. The faint band running at 18S may represent cross-hybridization with LYL1. (B) Expression of the MC-CPA (CPA), MCP-5, and MCP-6 in SCL−/Δ mast-cell lines relative to SCL+/Δ mast-cell lines. Data represent analysis of at least 4 lines from each genotype. *P < .05 using Student t test. (C) Surface FcϵRI expression on resting (no IgE) or IgE-stimulated SCL+/Δ (shaded histogram) and SCL−/Δ (open histogram) mast-cell lines. The mean cell fluorescence (MCF) of FcϵRI expression was calculated from 4 cell lines of each genotype. The fold induction of FcϵRI expression by IgE compared with no IgE is shown for 4 lines of each genotype. (D) Ca2+ flux in SCL+/Δ or SCL−/Δ mast-cell lines determined by flow cytometry based on the change of FL5/FL4 ratio. Data are representative of 3 cell lines of each genotype. (E) Southern blot of a mast-cell line derived from an SCLfl/fl mouse. The cells were infected with a tamoxifen-inducible Cre recombinase retrovirus and treated for 48 hours with various concentrations of tamoxifen (0 to 3 μM). (F) Surface FcϵRI expression on Cre retrovirus-infected mast-cell lines derived from SCL+/fl mouse or SCLfl/fl mouse. FcϵRI expression was measured in the absence (open histogram) or presence (shaded histogram) of 1 μM tamoxifen. (G) Expression of the α-, β-, and γ- chains of FcϵRI in SCL−/Δ mast-cell lines relative to SCL+/Δ mast-cell lines. Data represent analysis of 8 cell lines of each genotype. (H) Spleen sections from SCL+/Δ and SCL−/Δ mice treated with 25 μg/kg per day mIL-3 (PeproTech) and 50 μg/kg per day rSCF (Amgen) for 5 days. Sections are stained with toluidine blue to detect cells with metachromatic mast-cell granules (→). Data represent the mean number of mast cells per high-power field from 2 mice. (I) The mean number of mast cells in peritoneal space from cytokine-treated mice shown in panel H was calculated by multiplying the absolute peritoneal cell count by the percentage of mast cells determined by flow cytometry: small c-kit+Sca-1− and large c-kit+Sca-1+.
SCL is required for mast-cell maturation. (A) Southern blot (left) and Northern blot (right) of mast-cell lines derived from SCL+/Δ or SCL−/Δ bone marrow cells. Tail DNA from MxSCL+/fl and MxSCL−/fl mice is shown to demonstrate the position of the SCLfl allele. The faint band running at 18S may represent cross-hybridization with LYL1. (B) Expression of the MC-CPA (CPA), MCP-5, and MCP-6 in SCL−/Δ mast-cell lines relative to SCL+/Δ mast-cell lines. Data represent analysis of at least 4 lines from each genotype. *P < .05 using Student t test. (C) Surface FcϵRI expression on resting (no IgE) or IgE-stimulated SCL+/Δ (shaded histogram) and SCL−/Δ (open histogram) mast-cell lines. The mean cell fluorescence (MCF) of FcϵRI expression was calculated from 4 cell lines of each genotype. The fold induction of FcϵRI expression by IgE compared with no IgE is shown for 4 lines of each genotype. (D) Ca2+ flux in SCL+/Δ or SCL−/Δ mast-cell lines determined by flow cytometry based on the change of FL5/FL4 ratio. Data are representative of 3 cell lines of each genotype. (E) Southern blot of a mast-cell line derived from an SCLfl/fl mouse. The cells were infected with a tamoxifen-inducible Cre recombinase retrovirus and treated for 48 hours with various concentrations of tamoxifen (0 to 3 μM). (F) Surface FcϵRI expression on Cre retrovirus-infected mast-cell lines derived from SCL+/fl mouse or SCLfl/fl mouse. FcϵRI expression was measured in the absence (open histogram) or presence (shaded histogram) of 1 μM tamoxifen. (G) Expression of the α-, β-, and γ- chains of FcϵRI in SCL−/Δ mast-cell lines relative to SCL+/Δ mast-cell lines. Data represent analysis of 8 cell lines of each genotype. (H) Spleen sections from SCL+/Δ and SCL−/Δ mice treated with 25 μg/kg per day mIL-3 (PeproTech) and 50 μg/kg per day rSCF (Amgen) for 5 days. Sections are stained with toluidine blue to detect cells with metachromatic mast-cell granules (→). Data represent the mean number of mast cells per high-power field from 2 mice. (I) The mean number of mast cells in peritoneal space from cytokine-treated mice shown in panel H was calculated by multiplying the absolute peritoneal cell count by the percentage of mast cells determined by flow cytometry: small c-kit+Sca-1− and large c-kit+Sca-1+.
To determine if loss of SCL had a direct effect on FcϵRI expression, we used a tamoxifen-inducible Cre recombinase retrovirus (pCre-ERT2) to induce deletion of SCL in an established BMMC. Treatment of a pCre-ERT2–infected BMMC with tamoxifen produced efficient deletion of the SCLfl allele within 48 hours (Figure 7E). Deletion of SCL reduced the expression of FcϵRI 4-fold within 72 hours (Figure 7F). Absence of one SCL allele had no effect on expression of FcϵRI. To determine which receptor subunit may be regulated by SCL, we examined the expression of the IgE receptor subunits in BMMCs by real-time PCR. Overall, the expression of both the FcϵRI α and β chains were significantly reduced 2-fold compared with SCL+/Δ BMMCs, while expression of the γ chain was unaltered (Figure 7G). Together, these analyses of BMMCs show that SCL was required for normal expression of mast-cell proteases and FcϵRI.
While defects in mast-cell maturation might explain the failure of SCL−/Δ MCPs to generate large numbers of mature mast cells in vivo, an alternate explanation might be lack of in vivo stimulus. We hypothesized that tissue mast cells might be increased in SCL−/Δ mice only under appropriate physiologic challenge. To test the in vivo proliferative response of SCL−/Δ MCPs, we treated mice with IL-3 and SCF for 5 days and then examined the numbers of tissue mast cells. Compared with control mice, there was a 20-fold increase in toluidine blue+ mast cells within the spleens of SCL−/Δ mice (Figure 7H). These mast cells were present in discrete clusters, suggesting clonal origin. This effect was limited to the spleen: mast cell numbers in the skin, peritoneal fluid, and gastrointestinal mucosa were not increased after 5 days of IL-3 and SCF (Figure 7I and data not shown). Treatment with SCF alone for up to 3 weeks had no effect on splenic mast-cell numbers in SCL−/Δ mice (data not shown), suggesting that the effect was IL-3 dependent. Thus, IL-3 treatment can increase the numbers of splenic mast cells but not nonhematopoietic tissue mast cells in SCL−/Δ mice.
Discussion
SCL is expressed in 3 hematopoietic cell types: erythroid, megakaryocyte, and mast cells. The function of SCL in mast-cell development is unknown. Our results show that loss of SCL produced markedly increased numbers of progenitors with mast- cell potential within the BM, spleen, and peritoneal fluid (Table 1; Figures 1,2). This finding of progenitors with mast-cell potential is in stark contrast to the role of SCL in the erythroid and megakaryocyte lineages where SCL is essential for the in vitro growth of BFU-Es and megakaryocyte–colony-forming units (Mk-CFUs).4 Assays of myeloerythroid cell fractions demonstrated that unilineage mast cell progenitors in SCL−/Δ mice were derived from the CD34lo PU.1lo cell fraction, the same cell fraction that normally contains erythroid and megakaryocyte progenitor cell fractionation (Figure 3). In contrast, we identified unilineage mast-cell progenitors in control animals within the CD34hi PU.1hi cell fraction (Figure 4). Thus, loss of SCL led to aberrant mast-cell differentiation rather than expansion of the normal MCP pool.
Our studies do not definitively prove a common origin for erythroid, megakaryocyte, and mast-cell progenitors in SCL−/Δ mice. It is possible that they arise from a distinct progenitor within the same cell fraction, however the occasional megakaryocytes observed within SCL−/Δ mast cell colonies support a common origin. The mast-cell phenotype of SCL−/Δ mice has many similarities with GATA-1 mutant mice: increased mast-cell progenitors, normal numbers of tissue mast cells, and defects in mast-cell maturation including reduced expression of FcϵRI.20,32 In the case of GATA-1lo mice, BM and spleen cultures contain abnormal trilineage colonies (erythroid, megakaryocyte, and mast cells), suggestive of a common progenitor.20 During review of this paper, single-cell cloning and replating experiments of GATA-1lo BM progenitors confirmed the presence of an MEP with a unique capacity to generate mast cells in vitro.33 One obvious difference with GATA-1lo mice is the absence of significant megakaryocytes or erythroblasts within SCL−/Δ mast-cell colonies. GATA-1lo erythroblasts can grow to the proerythroblast stage, while megakaryocytes are hyperproliferative.34,35 In contrast, SCL is essential for in vitro growth of the major erythroid and megakaryocyte progenitors.4 Thus, it is possible that the mast-cell colonies observed in SCL−/Δ cultures are restricted to mast-cell growth rather than truly unilineage MCPs because unlike GATA-1, SCL is essential for in vitro growth of megakaryocytes and erythroblasts.
Expression of specific transcription factors is critical for lineage specification.31 In their absence, hematopoietic progenitors committed to a single lineage can maintain multipotency. For example, loss of GATA-1 in erythroid-committed cells maintains myeloid and mast-cell potential when cultured in the appropriate conditions.36 Our results suggest a similar scenario for SCL: loss of SCL in cells normally fated for megakaryocyte-erythroid lineage can generate mast cells under appropriate conditions. The maintenance of multipotency may, in part, be due to cross-talk between lineage-determining transcription factors.31 For example, GATA-1 can both repress transcription of PU.1 as well as directly bind to PU.1 to block myeloid cell differentiation.19,37,38 Repression of GATA-2 transcription by GATA-1 is required for proliferation arrest and erythroid maturation.39,40 The elevated levels of GATA-2 observed in SCL−/Δ CMP and MEP cells may contribute to the propensity for mast cell formation (Figure 5). GATA-2 is essential for mast-cell progenitor formation.18 Furthermore, enforced expression of GATA-2 in C/EBPα-deficient progenitors led to mast-cell differentiation.41 We propose that the high levels of GATA-2 in SCL−/Δ CMP and MEP cells, which have relatively low levels of C/EBPα, contributed to aberrant mast-cell differentiation. At this stage, we do not know whether SCL is a direct regulator of GATA-2 expression. However, it is possible that SCL participates in a protein complex with GATA-1 to regulate GATA-2 transcription.
Rescue of the erythroid and megakaryocyte growth defects by reintroduction of SCL in SCL−/Δ BM cells confirms a direct role for SCL in erythroid and megakaryocyte cells. The inability of SCL to reduce mast-cell growth suggests that the aberrant mast-cell formation is more complex. We did not examine the GATA-2 levels following enforced expression of SCL. It is possible that loss of SCL leads to chromatin changes that are not rapidly reversible by reintroduction of SCL.
The reduced expression of MCP-5/6 and FcϵRI in SCL−/Δ mast cells suggests that SCL is required for terminal mast-cell differentiation, which occurs in tissues rather than the BM or spleen. Reduced FcϵRI expression within 72 hours of deleting SCL suggests that components of the FcϵRI may be a direct target of SCL. Both the α and β chains of the IgE receptor may be regulated by GATA-1.42,43 The promoters of the α chain but not the β chain have GATA and E-box motifs.44 Coimmunoprecipitation experiments have recently found a physical association between SCL and GATA-1 in mast-cell lines (J.M.S., unpublished data, October 2006). Chromatin immunoprecipitation assays will be required to colocalize SCL and GATA-1 on potential target genes.
The ongoing red cell and platelet production in SCL−/Δ mice despite the marked in vitro growth defects remains enigmatic. Similarly, the normal numbers of mature tissue mast cells despite large numbers of mast-cell progenitors in the absence of SCL remain unexplained. A similar discrepancy occurs in GATA-1lo mice that has been attributed to a requirement for GATA-1 in mast- cell maturation.20 Similarly, SCL may be required for maturation and/or homing of mast-cell progenitors to the tissues. Unlike other hematopoietic lineages, mast-cell progenitors complete their maturation in connective tissue.13 The reduced expression of FcϵRI and mast cell proteases may reflect a more global defect in mast-cell maturation. While these defects alone would not be sufficient to explain the normal numbers of mast cells (IgE receptor knockout mice have normal resting mast-cell numbers45 ), SCL may regulate other factors required for mast-cell maturation.17 For example, it is possible that SCL−/Δ mast cells derived from megakaryocyte-erythroid fated cells lack factors essential for homing and this explains the increased numbers of splenic mast cells but not other tissues in response to IL-3 and SCF (Figure 7H).
An alternate explanation for normal numbers of tissue mast cells is that the fate of SCL−/Δ megakaryocyte-erythroid progenitors is determined by physiologic need. In vivo production of SCL−/Δ erythroblasts and megakaryocytes continues relatively normally, even under conditions of stress, despite the inability to grow in vitro.5,6 This suggests that in vivo factors can maintain normal growth of erythroid and megakaryocyte progenitors in the absence of SCL. Defining the in vivo factors that promote appropriate growth and differentiation of megakaryocyte-erythroid progenitors rather than mast cells will provide important insights into the regulation of these cell lineages.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) project grant 332502.
We thank Lan Ta, Meagan James, and Jenny Davis for animal husbandry; Sumitha Vasudevan for technical assistance; Wu Li, Angela D'Amico, Alice Holloway, and Dean Hewish for cell sorting.
National Institutes of Health
Authorship
Contribution: J.M.S., N.J.S., M.A.H., and M.P.M. designed and performed research and analyzed data; S.L.N. contributed vital new reagents; S.M.J. designed research; D.J.C. designed and performed research, analyzed data, and wrote the paper. J.M.S. and N.J.S. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David J. Curtis, Rotary Bone Marrow Research Laboratories, P.O. Royal Melbourne Hospital, Grattan St, Parkville, Melbourne 3050, Australia; e-mail:dcurtis@wehi.edu.au.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal