Abstract
Epigenetic modifications of chromatin may play a role in maintaining viral latency and thus persistence of the human T-lymphotropic virus type 1 (HTLV-1), which is responsible for HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP). A major determinant of disease progression is increased peripheral blood proviral load (PVL), possibly via the accumulation of infected cells in the central nervous system (CNS) creating a damaging inflammatory response. Current therapeutic approaches that focus on reducing either cell proliferation, viral replication, or tissue invasion are still unsatisfactory. Contrasting with these inhibitory strategies, we evaluated the efficacy of a novel approach aimed, paradoxically, at activating viral gene expression to expose virus-positive cells to the host immune response. We used valproate (VPA), a histone deacetylase inhibitor that has been used for decades as a chronic, safe treatment for epileptic disorders. Based on in vitro and in vivo data, we provide evidence that transient activation of the latent viral reservoir causes its collapse, a process that may alleviate the condition of HAM/TSP. This represents the first such approach to treating HAM/TSP, using gene activation therapy to tilt the host-pathogen balance in favor of an existing antiviral response. This trial is registered at http://clinicaltrials.gov/as no. NCT00519181.
Introduction
Of the 10 to 20 million human T-lymphotropic virus 1 (HTLV-1)–infected people worldwide, a significant fraction (approximately 2%-3%) will develop adult T-cell leukemia (ATL), while a similar proportion will develop HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP), a neuroinflammatory disease of the central nervous system (CNS).1-3 Palliative treatments for HAM/TSP include steroids to decrease inflammation.4-6 Attempts to treat HAM/TSP by interfering with cell invasion into the CNS using inhibitors of matrix metalloproteinases7 have been unsuccessful. Similarly, other strategies aimed at inhibiting cell activation and/or viral replication with cytokines (IFN-α or -β)6 or antiviral compounds8-10 did not show efficacy. We propose here a completely new approach based on the likely pathogenic mechanisms of HAM/TSP, and tested with success by us11 in lymphomatous sheep infected by bovine leukemia virus and suggested to eliminate the viral reservoir in human immunodeficiency virus type 1 (HIV-1)–infected subjects.12
CD4+ and, to a lesser extent, CD8+ T lymphocytes represent the main reservoirs of HTLV-1 in vivo.13 Histopathological studies have shown that HAM/TSP lesions are associated with perivascular and parenchymal infiltration of HTLV-1–infected T cells and activated cytotoxic T lymphocytes (CTLs).13,14 It is assumed that the immune response can be beneficial when directed toward the virus but becomes deleterious when excessive or autoimmune.13,15 Indeed, virus-specific CTLs or antibodies can cross-react with host proteins, a phenomenon known as antigen mimicry.16 Another nonexclusive process is collateral damage resulting from the release of proinflammatory, neurotoxic cytokines such as IFN-γ and TNF-α by invading CD4+ and CD8+ T cells. Whatever the mechanism, cytotoxic, bystander, or autoimmune, a key determinant of the development of HAM/TSP is the level of HTLV-1–infected cell burden. Indeed, the HTLV-1 proviral load (PVL) in peripheral mononuclear cells (PBMCs) is 5- to 20-fold higher in patients with HAM/TSP than in asymptomatic carriers.17,18 Interestingly, the proviral load is remarkably stable over periods of several years within each of these 2 phases.17,19-21 This equilibrium level, which appears genetically determined, is tightly controlled by host immune responses, mainly directed by cytotoxic T cells.13 This selective pressure seems so effective that the viral load consists almost exclusively of infected cells containing transcriptionally silent viruses. In the apparent absence of viral expression, such latently infected cells persist and accumulate over years by a mechanism referred to as clonal expansion.22 Although the pathways leading to this expansion are not fully understood, the most likely hypothesis supposes (1) that there is a degree of persistent spontaneous onset of proviral transcription in vivo, (2) that this process is driven by the viral Tax oncoprotein, and (3) that infected cells escape from immune surveillance after silencing virus transcription.
There are thus 2 main categories of infected lymphocytes: those expressing viral proteins that are rapidly eliminated by host immunity and those carrying silent provirus that have the capacity to accumulate by cell mitosis. Although cells of the former type potentially generate inflammation, major problems result from the long-term accumulation of provirus-carrying cells. When a threshold level of these cells is reached in peripheral blood, a proportion migrates into the CNS.23 Since a close correlation exists between the number of proviral copies and the amount of expressed viral RNAs,24 an increase in the PVL is expected to worsen indirectly inflammation, although there is an overlap in the absolute levels of proviral load between patients with HAM/TSP and asymptomatic carriers. Inversely, a long-term reduction in the number of provirus-carrying cells would be protective against collateral damage responsible for HAM/TSP. In this context, we postulated that transient transcriptional activation of this viral reservoir out of hiding would result in rapid depletion of the infected cells by the host immune system.
Patients, materials, and methods
HAM/TSP patients included in the study were followed at the University Hospital of Fort-de-France, French West Indies. The study was approved by the Local and Regional Research Ethics Committee, and all procedures were carried out with informed consent obtained in accordance with the Declaration of Helsinki.
Gene reporter assays
HeLa cervix epithelial cells were grown in minimal essential medium (Sigma, St Louis, MO) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U penicillin, and 100 μg streptomycin per milliliter. Twenty-four hours prior to transfection, cells were cultured at a density of 3 × 105 cells per well (6-well dish; Nunc, Rochester, NY) and transfected with different plasmid combinations. The reporter plasmid (pCRLuc, a gift of Ralph Grassmann, Erlangen, Germany) contains the HTLV-1 long terminal repeat promoter cloned upstream the luciferase gene into pGem-luc (Promega, Madison, WI). The pCMVTax1 effector plasmid (provided by Françoise Bex, Free University of Brussels, Brussels, Belgium) encodes the HTLV-1 Tax transcriptional activator protein from a cytomegalovirus (CMV) promoter. Cells were transfected with pCRLuc and pCMVTax1 or pCMV empty vector using GeneJammer reagent (Stratagene, La Jolla, CA) following the manufacturer's protocol. Different amounts and ratios of effector and reporter plasmids were tested in preliminary assays: 1 μg pCRLuc and 25 ng pCMVTax1 were found to be optimal. After transfection, cells were grown at 37°C for 18 hours in the presence of different concentrations of valproate (VPA; 0, 1, 5 mM) and luciferase enzyme activity was measured using the dual-luciferase assay system (Promega). Data (in mean light arbitrary units ± standard deviations) are derived from 3 independent experiments.
Jurkat T lymphocytes were cultivated in OptiMEM (Invitrogen, Frederick, MD) supplemented with 20% heat-inactivated FCS, 2 mM l-glutamine, 100 U penicillin, and 100 μg streptomycin per milliliter. Cells (4 × 106) were transfected with 2 μg pCRLuc reporter in the presence of 10 ng plasmid pCMVTax1 or control pCMV. Transfected cells were cultivated for 24 hours with VPA (0, 1, 5 mM) and luciferase activity was measured.
Analysis of apoptosis by flow cytometry
Peripheral blood mononuclear cells from HAM/TSP or ATL patients were isolated by Ficoll density gradient centrifugation (GE Healthcare, Little Chalfont, United Kingdom) and either frozen in liquid nitrogen or directly cultivated in RPMI medium (data of Figure 2) containing 15% (vol/vol) fetal bovine serum, 2 mM l-glutamine, 100 U penicillin, and 100 μg streptomycin per milliliter (Eurobio, Paris, France). Preliminary experiments with frozen or fresh cells fixed the optimal times of culture (up to 96 hours) and concentrations of VPA (in a range of 0 to 10 mM). After culture, cells were labeled with either a CD4- or a CD8-specific antibody conjugated to allophycocyanin (APC; BD Biosciences, San Jose, CA). Apoptotic cells were identified after staining with fluorescein-isothiocyanate (FITC)–labeled annexin V (annexin) and propidium iodide (PI; BD Biosciences). Ten thousand events of dual- or triple-labeled sample were collected by a flow cytometer (FACSCalibur; BD Biosciences) using the FSC/SSC, FL1 (annexin), FL2 (PI), and FL4 (APC) detectors. Proportions of live and apoptotic cells were determined using Cellquest software (Becton Dickinson Immunocytometry Systems, San Jose, CA) after appropriate compensation. Apoptotic rates were expressed as mean plus or minus standard deviation and comparisons made by Wilcoxon signed rank test at 95% confidence. Similar cultures and assays were also performed with the HTLV-1–infected MT2 cells (ECACC 93121518).
Western blot analysis for histone H3 acetylation and p19 viral core protein ELISA
Primary PBMCs freshly isolated from 3 HAM/TSP patients and 3 controls were cultivated with an optimal concentration of 2 mM VPA for 48 hours. Corresponding cell culture supernatants were collected, and the viral core 19 protein was analyzed by enzyme-linked immunosorbent assay (ELISA, Retrotek; Zeptometrix, Buffalo, NY) according to the manufacturer's protocol. Absolute concentrations of p19 were determined with a standard purified antigen dilution curve. Cells were then fractionated and analyzed by flow cytometry to determine apoptotic rates (as described in the previous paragraph) or by Western blot to evaluate the levels of histone H3 acetylation. After 3 washes in phosphate-buffered saline, cells were lysed in Tris 20 mM (pH 7.5), 0.1% Triton X-100, and a cocktail of antiproteases (Complete; Roche, Indianapolis, IN). Protein (10 μg) was migrated on a denaturing sodium dodecylsulfate polyacrylamide gel and transferred onto PVDF membranes. After saturation of the filters in Tris-buffered saline containing 1% blocking solution (Roche), proteins were revealed with anti–acetylated histone H3 (Upstate, Lake Placid, NY) or antiactin (Sigma) antibodies in association with a goat antirabbit alkaline phosphatase conjugate (Dako Cytomation, Carpinteria, CA). After incubation with chemiluminescent substrate (Perkin Elmer, Waltham, MA), immunoblots were exposed to autoradiographic films (GE Healthcare) or scanned by the Kodak Digital Science image station type 440 (Rochester, NY). After background subtraction, a ratio was calculated between the light intensities (in arbitrary units) generated by acetylated histone H3 and the signal corresponding to actin used to normalize protein levels.
VPA trial in HAM/TSP patients
The study was approved by the Local and Regional Research Ethics Committee and all procedures were carried out in accordance with the Declaration of Helsinki. After having obtained written consent, 20 patients suffering from HAM/TSP were recruited according to World Health Organization criteria25 from a cohort at the “Centre Hospitalier Universitaire” of the Neurology department in Fort-de-France (Martinique). All patients presented with long-term mobility impairment with a DSS score (expanded disability status scale) of between 5 and 7.5. VPA was administrated orally at a maximal dose of 20 mg/kg/day. Serum concentrations of VPA were measured at day 15 by the EMIT method (SYVA reagents; Dade-Behring, Düdingen, Switzerland) with a Cobas Mira analyzer (Roche). Regular clinical evaluation included determination of the DSS score as well as a more sensitive estimation of mobility based on the duration of an 8-meter walk, the shortest time of 2 tests being considered.
Measurement of HTLV-1 proviral load in PBMCs
HTLV-1 proviral load in PBMCs was quantified in the HAM/TSP patients and asymptomatic carriers. PBMCs were isolated from EDTA-containing blood by density gradient centrifugation and cryopreserved until use. Genomic DNA was extracted using a spin column DNA extraction system (GFXtm PCR DNA; GE Healthcare). HTLV-1 proviral load was quantified using a real-time TaqMan polymerase chain reaction (PCR) method, as described previously.26 Briefly, SK110/SK111 primers were used to amplify a 186-bp fragment of the pol gene, and the dual-labeled TaqMan probe (5′FAM and 3′TAMRA) was located at 4829 to 4858 bp of the HTLV-1 reference sequence (HTLVATK). Albumin DNA was quantified in parallel to determine the input cell number and was used as an endogenous reference to avoid the variations due to differences in the PBMC count or DNA extraction. Standard curves were generated using 10-fold serial dilutions of a double standard plasmid (pcHTLV-ALB) containing one copy of the target regions of both the HTLV-1 pol gene and the cellular albumin gene. The HTLV-1–infected human lymphocyte line MT2 was used as a control for quantification. All samples (standard curve dilutions, the MT2 control sample, and the patient samples) were run in duplicate for both HTLV-1 and albumin DNA quantification. The value for the HTLV-1 proviral load was reported as the [(HTLV-1 average copy number)/(albumin average copy number)] × 2 × 103 and expressed as the number of HTLV-1 copies per 103 (or 106) cells.
Results
Inhibitors of histone deacetylase (HDAC) efficiently activate gene transcription by modulating the levels of acetylation of histone and nonhistone proteins.27 Apart from efficacy, a prerequisite for clinical use of this type of molecule would be a good tolerability and bioavailability. In contrast to other HDAC inhibitors, VPA has demonstrated good safety profile for 35 years of use as long-term therapy for epileptic disorders. VPA is very well tolerated at millimolar concentrations and, with a serum half-life of 9 to 18 hours, has acceptable pharmacokinetic properties.28 For these reasons, VPA was selected as a tool to activate gene transcription.
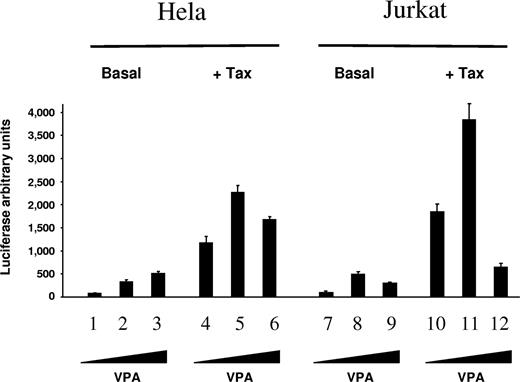
First, the potency of VPA to initiate viral expression was evaluated using an HTLV-1 promoter-luciferase reporter assay. Viral promoter activity was found to be increased in HeLa and Jurkat cell lines following stimulation with different concentrations of VPA both in the absence or presence of ectopically expressed Tax transcriptional activator (Figure 1: basal and + Tax, respectively). Real time reverse-transcription (RT)–PCR amplifications demonstrated that VPA activates transcription of the luciferase gene (data not shown). These results thus demonstrate that VPA efficiently activates HTLV-1 promoter-driven transcription.
VPA activates luciferase expression directed by the viral LTR promoter. HeLa cells (lanes 1-6) and Jurkat T lymphocytes (lanes 7-12) were transfected with 1 μg pCRLuc reporter, which contains the HTLV-1 long terminal repeat (LTR) cloned upstream of the luciferase gene, in the absence (Basal, lanes 1-3 and 7-9) or in the presence (+ Tax, lanes 4-6 and 10-12) of 10 ng plasmid pCMVTax encoding the viral transactivator protein Tax-1. Transfected cells were cultivated for 18 hours without (lanes 1, 4, 7 and 10) or with (1 mM and 5 mM on lanes 2, 5, 8, and 11 and lanes 3, 6, 9, and 12, respectively) VPA, and luciferase activity was determined. The data (in light arbitrary units ± standard deviation) result from 3 independent experiments.
VPA activates luciferase expression directed by the viral LTR promoter. HeLa cells (lanes 1-6) and Jurkat T lymphocytes (lanes 7-12) were transfected with 1 μg pCRLuc reporter, which contains the HTLV-1 long terminal repeat (LTR) cloned upstream of the luciferase gene, in the absence (Basal, lanes 1-3 and 7-9) or in the presence (+ Tax, lanes 4-6 and 10-12) of 10 ng plasmid pCMVTax encoding the viral transactivator protein Tax-1. Transfected cells were cultivated for 18 hours without (lanes 1, 4, 7 and 10) or with (1 mM and 5 mM on lanes 2, 5, 8, and 11 and lanes 3, 6, 9, and 12, respectively) VPA, and luciferase activity was determined. The data (in light arbitrary units ± standard deviation) result from 3 independent experiments.
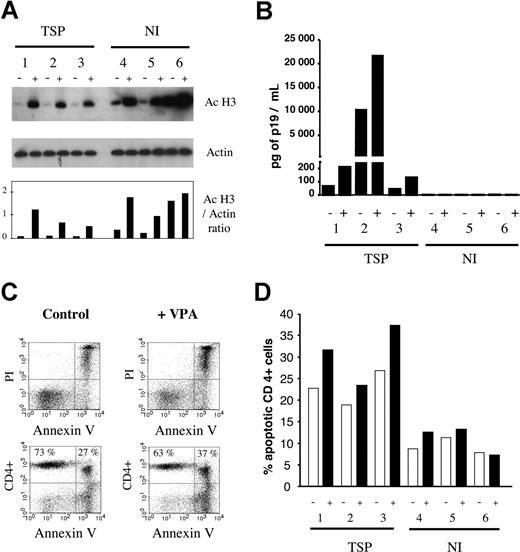
Since HDAC inhibitors are known to induce apoptosis, initial time course and dose response experiments were performed in an HTLV-1–infected cell line (MT2) as well as in primary PBMCs isolated from HAM/TSP or ATL patients (data not shown). A VPA concentration of 2 mM for 48 hours, found to induce moderate levels of apoptosis in HAM/TSP PBMCs, was selected and added to primary cell cultures derived from 3 HAM/TSP patients and 3 noninfected controls. VPA induced hyperacetylation of histone H3 tails, used as an indirect marker of increased general acetylation within these cells (Figure 2A). In parallel, expression of the HTLV-1 core protein p19 was increased in the corresponding supernatant (Figure 2B). Moreover, CD4+ T lymphocytes underwent moderate rates of apoptosis (Figure 2C,D). In other words, under hyperacetylated conditions, VPA concomitantly stimulated both viral expression and apoptosis.
VPA induces hyperacetylation, triggers viral expression, and is proapoptotic for primary cells freshly isolated from HTLV-1–infected patients with tropical spastic paraparesis. (A) Histone H3 is hyperacetylated in the presence of VPA. Peripheral blood mononuclear cells were isolated from 3 HTLV-infected, untreated HAM/TSP patients (1-3) and 3 noninfected (NI) healthy controls (4-6) and directly cultivated (ie, without cryopreservation) for 48 hours in the absence (−) or the presence (+) of 2 mM VPA. Cell lysate protein (10 μg) was analyzed by Western blot using an antibody specific for the acetylated forms of histone H3 (Ac H3) or, as a control for normalization of the protein levels, an antiactin antiserum (actin). After incubation with the appropriate alkaline phosphatase–linked conjugates, the blots were revealed by chemiluminescence and autoradiography (top 2 panels). After quantification of the luminescence signals with a luminometer and subtraction of the background, a ratio between the mean intensities generated by each antibody was calculated (Ac H3/actin ratio). (B) VPA activates expression of the viral core protein p19 in the supernatant of these primary T-lymphocyte cultures. The culture supernatants in the absence (−) or the presence (+) of VPA were collected, and expression of the viral p19 core protein was quantified using a Zeptometrix ELISA. Absolute concentrations of p19 (in pg per mL) were determined by normalization of the absorbance values with a standard curve. (C) The CD4+ T lymphocytes undergo increased apoptosis in the presence of VPA compared with the controls. After culture, cells were labeled with a CD4-specific antibody conjugated to allophycocyanin (APC). Apoptotic cells were identified after staining with fluorescein-isothiocyanate (FITC)–labeled annexin V (annexin) and propidium iodide (PI). Ten thousand events per thrice-labeled sample were collected by flow cytometry and analyzed with the Cellquest software. The dot plots illustrate annexin/PI (upper panels) and CD4/annexin (bottom panels) labeling of cells isolated from a HAM/TSP patient and cultivated in the absence (control) or the presence (+VPA) of VPA. The percentages of CD4+ annexin− and CD4+ annexin+ are indicated in the upper quadrants. (D) The percentages of apoptotic CD4+ cells were measured in samples from the 3 HAM/TSP patients (1-3) and 3 noninfected controls (4-6) cultivated without (−) or with (+) 2 mM VPA. Note that all experiments illustrated in this figure were performed in parallel on freshly isolated noncryopreserved cells.
VPA induces hyperacetylation, triggers viral expression, and is proapoptotic for primary cells freshly isolated from HTLV-1–infected patients with tropical spastic paraparesis. (A) Histone H3 is hyperacetylated in the presence of VPA. Peripheral blood mononuclear cells were isolated from 3 HTLV-infected, untreated HAM/TSP patients (1-3) and 3 noninfected (NI) healthy controls (4-6) and directly cultivated (ie, without cryopreservation) for 48 hours in the absence (−) or the presence (+) of 2 mM VPA. Cell lysate protein (10 μg) was analyzed by Western blot using an antibody specific for the acetylated forms of histone H3 (Ac H3) or, as a control for normalization of the protein levels, an antiactin antiserum (actin). After incubation with the appropriate alkaline phosphatase–linked conjugates, the blots were revealed by chemiluminescence and autoradiography (top 2 panels). After quantification of the luminescence signals with a luminometer and subtraction of the background, a ratio between the mean intensities generated by each antibody was calculated (Ac H3/actin ratio). (B) VPA activates expression of the viral core protein p19 in the supernatant of these primary T-lymphocyte cultures. The culture supernatants in the absence (−) or the presence (+) of VPA were collected, and expression of the viral p19 core protein was quantified using a Zeptometrix ELISA. Absolute concentrations of p19 (in pg per mL) were determined by normalization of the absorbance values with a standard curve. (C) The CD4+ T lymphocytes undergo increased apoptosis in the presence of VPA compared with the controls. After culture, cells were labeled with a CD4-specific antibody conjugated to allophycocyanin (APC). Apoptotic cells were identified after staining with fluorescein-isothiocyanate (FITC)–labeled annexin V (annexin) and propidium iodide (PI). Ten thousand events per thrice-labeled sample were collected by flow cytometry and analyzed with the Cellquest software. The dot plots illustrate annexin/PI (upper panels) and CD4/annexin (bottom panels) labeling of cells isolated from a HAM/TSP patient and cultivated in the absence (control) or the presence (+VPA) of VPA. The percentages of CD4+ annexin− and CD4+ annexin+ are indicated in the upper quadrants. (D) The percentages of apoptotic CD4+ cells were measured in samples from the 3 HAM/TSP patients (1-3) and 3 noninfected controls (4-6) cultivated without (−) or with (+) 2 mM VPA. Note that all experiments illustrated in this figure were performed in parallel on freshly isolated noncryopreserved cells.
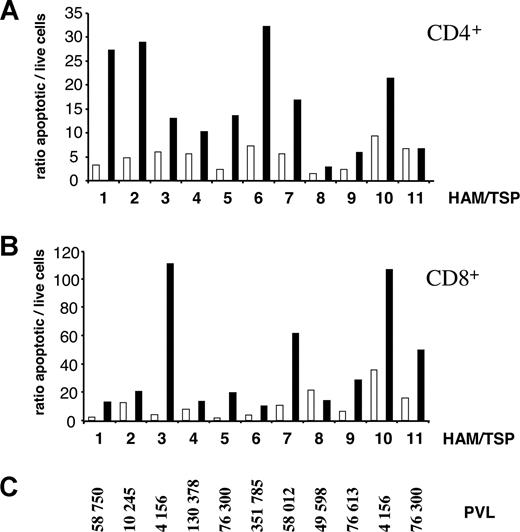
To assess the proapoptotic potency of VPA in CD4+ cells, the main provirus reservoir, as well as in CD8+ T lymphocytes, which are thought to be major contributors of collateral damage leading to inflammation, levels of apoptosis were evaluated concurrently in PBMCs from 11 HAM/TSP patients (Figure 3A,B) harboring different PVL levels (Figure 3C). Mean apoptotic rates measured in the CD4+ T-cell population increased in the presence of VPA (16.3 compared with 5.0 in controls; Figure 3A, Table 1); this difference was statistically highly significant (*** Wilcoxon 2-tailed signed rank test, P = .002; Table 1). In the same samples, CD8+ T lymphocytes also underwent significant apoptosis in the presence of VPA (40.7 versus 11.2 for controls; ** Wilcoxon 2-tailed signed rank test, P = .007; Table 1). Of note, VPA was also proapoptotic in the non–T-cell population as well as to lymphocytes isolated from healthy individuals (B and NI, respectively; Table 1).
Valproate is proapoptotic for CD4+ and CD8+ cells isolated from HAM/TSP patients. Cryopreserved peripheral blood mononuclear cells isolated from 11 HTLV-infected HAM/TSP patients (as indicated on the x-axis) were cultivated for 48 hours in the absence (open bars) or the presence (filled bars) of 2 mM VPA. After culture, apoptotic cells were identified by flow cytometry based on CD4/annexin/PI (A) or CD8/annexin/PI (B) triple labeling, and a ratio between apoptotic and live cells was calculated. (C) PVL of the 11 HAM/TSP subjects determined by real-time PCR (in number of HTLV copies per 106 PBMCs).
Valproate is proapoptotic for CD4+ and CD8+ cells isolated from HAM/TSP patients. Cryopreserved peripheral blood mononuclear cells isolated from 11 HTLV-infected HAM/TSP patients (as indicated on the x-axis) were cultivated for 48 hours in the absence (open bars) or the presence (filled bars) of 2 mM VPA. After culture, apoptotic cells were identified by flow cytometry based on CD4/annexin/PI (A) or CD8/annexin/PI (B) triple labeling, and a ratio between apoptotic and live cells was calculated. (C) PVL of the 11 HAM/TSP subjects determined by real-time PCR (in number of HTLV copies per 106 PBMCs).
Apoptotic rates from B cells and PBMCs
| . | CD4 . | CD8 . | B . | NI . |
|---|---|---|---|---|
| Control | 5.0 (± 2.4)*** | 11.2 (± 10.1)** | 4.2 (± 3.4)* | 1.1 (± 0.4)** |
| VPA | 16.3 (± 10.0)*** | 40.7 (± 37.2)** | 10.9 (± 13.0)* | 1.6 (± 0.5)** |
| . | CD4 . | CD8 . | B . | NI . |
|---|---|---|---|---|
| Control | 5.0 (± 2.4)*** | 11.2 (± 10.1)** | 4.2 (± 3.4)* | 1.1 (± 0.4)** |
| VPA | 16.3 (± 10.0)*** | 40.7 (± 37.2)** | 10.9 (± 13.0)* | 1.6 (± 0.5)** |
The mean apoptotic rates (± standard deviation) were calculated from the data of Figure 3A (CD4+) and 3B (CD8+), from the B-cell population (B) and from noninfected (NI) PBMCs. The data were statistically compared with the Wilcoxon 2-tailed signed rank test. Note that, in contrast to the results of Figure 2, the data presented here were obtained with cryopreserved cells.
** and *** indicate levels of statistical significance.
Thus the ex vivo data provide compelling evidence that VPA activates viral expression and triggers apoptosis of T lymphocytes isolated from HAM/TSP patients. If our working model is valid, these mechanisms would allow the elimination of many infected cells in vivo. To assess this concept, we conducted an exploratory trial with standard doses of VPA orally administered to 4 HAM/TSP patients at late clinical stages (DSS score of 5-7.5). Serum concentrations of VPA reached steady-state levels of 45 to 93 μg/mL a few days after initiation of treatment (Table 2). Except for HAM-2 who showed a brief increase in the DSS score at day 30 (which prompted therapy interruption), no major alteration of clinical symptoms was observed during the 2-month trial period. A more sensitive parameter of ambulation, the time required to walk 8 meters, worsened transiently at day 15 most likely due to a reduction in spasticity.
Effect of VPA on HAM/TSP patients
| Patient code . | Age, y . | Duration of TSP, y . | Duration of VPA treatment, d . | Seric concentration of VPA at day 15, μg/mL . | DSS score (at day) . | 8 meters walking time (at day) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 30 . | 60 . | 0 . | 15 . | 60 . | |||||
| HAM-1 | 58 | 7 | 21 | 58.2 | 5.0 | 5.0 | 5.0 | 12 | 15 | 12 |
| HAM-2 | 70 | 11 | 30 | 45.9 | 6.5 | 7.0 | 6.5 | 60 | 106 | 69 |
| HAM-3 | 55 | 19 | 60 | 93.1 | 6.5 | 6.5 | 6.5 | 19 | 24 | 21 |
| HAM-4 | 75 | 11 | 60 | 71.4 | 7.5 | 7.5 | 7.5 | ND | ND | ND |
| Patient code . | Age, y . | Duration of TSP, y . | Duration of VPA treatment, d . | Seric concentration of VPA at day 15, μg/mL . | DSS score (at day) . | 8 meters walking time (at day) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | 30 . | 60 . | 0 . | 15 . | 60 . | |||||
| HAM-1 | 58 | 7 | 21 | 58.2 | 5.0 | 5.0 | 5.0 | 12 | 15 | 12 |
| HAM-2 | 70 | 11 | 30 | 45.9 | 6.5 | 7.0 | 6.5 | 60 | 106 | 69 |
| HAM-3 | 55 | 19 | 60 | 93.1 | 6.5 | 6.5 | 6.5 | 19 | 24 | 21 |
| HAM-4 | 75 | 11 | 60 | 71.4 | 7.5 | 7.5 | 7.5 | ND | ND | ND |
VPA was taken orally by 4 volunteers with tropical spastic paraparesis at a dose of 20 mg/kg of weight per day for 3 (HAM-1), 4 (HAM-2), or 8 (HAM-3 and HAM-4) weeks. The steady-state seric concentrations of VPA in micrograms per milliliter were measured at day 15. All HAM/TSP patients had experienced mobility impairments for 7 to 19 years, and, as indicated, their DSS scores (expanded disability status scale) were between 5 and 7.5. A more sensitive estimation of mobility was calculated based on the time (in seconds) required to walk more than 8 meters. ND indicates not determined.
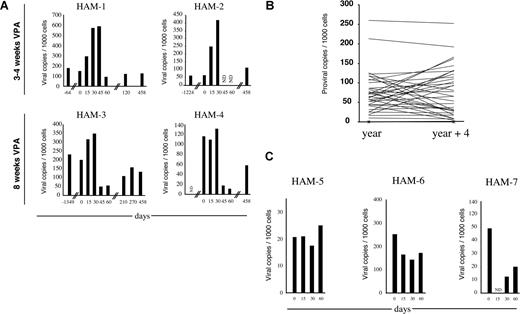
PVL is reported to be very stable in HAM/TSP patients19-21,29 ; in contrast we observed a transient increase in the PVL in all patients independent of the duration of VPA treatment (3-4 or 8 weeks; Figure 4A). However, around day 45, PVL collapsed, typically when the VPA treatment had been maintained for 2 months (HAM 3-4, Figure 4A). The increase in PVL with VPA from day 0 to 30, the decrease from day 30 to 60, and the decrease from day 0 to 60 were all significantly greater than normal fluctuations seen in PVL in 36 untreated HTLV-1–infected subjects (P = .042, P = .009, P = .027, respectively, Wilcoxon Mann-Whitney 2-tailed test; Figure 4B). This did not appear related to unusually unstable PVL in the subjects treated since the difference in PVL observed in 3 of the subjects prior to treatment with VPA (Figure 4A) was well within normal ranges (P = .564, Wilcoxon Mann-Whitney 2-tailed test). Furthermore, treatment of 3 HAM/TSP patients with reverse-transcriptase inhibitors (AZT + 3TC; Figure 4C) did not result in a decreased PVL between day 0 and day 30 that was significantly different from normal fluctuations (P = .16, Wilcoxon Mann-Whitney 2-tailed test). Finally, when VPA therapy was interrupted, PVL gradually increased but remained lower than the initial level for patients HAM-3 and HAM-4 who had been treated for 8 weeks (compare PVL at days 0 and 458 on Figure 4A).
Pilot study of valproate-based therapy in HAM/TSP patients. (A) The PVLs were determined by real-time PCR at the indicated days of the valproate treatment. The PVLs are represented in number of copies per 1000 cells. ND indicates not determined. (B) The PVLs at 4-year intervals were determined by real-time PCR in 36 HAM/TSP patients. The PVLs are represented in number of copies per 1000 cells. (C) Three HAM/TSP patients (HAM-5, HAM-6, and HAM-7) were treated with AZT + 3TC for 2 months. The PVLs were determined by real-time PCR at the indicated days and are represented in number of copies per 1000 cells.
Pilot study of valproate-based therapy in HAM/TSP patients. (A) The PVLs were determined by real-time PCR at the indicated days of the valproate treatment. The PVLs are represented in number of copies per 1000 cells. ND indicates not determined. (B) The PVLs at 4-year intervals were determined by real-time PCR in 36 HAM/TSP patients. The PVLs are represented in number of copies per 1000 cells. (C) Three HAM/TSP patients (HAM-5, HAM-6, and HAM-7) were treated with AZT + 3TC for 2 months. The PVLs were determined by real-time PCR at the indicated days and are represented in number of copies per 1000 cells.
To further broaden the observations of this pilot study, a series of 16 HAM/TSP subjects volunteered for a 3-month treatment with VPA. During this period, the proviral loads decreased in all patients by 2.3- to 89.3-fold (mean, 24-fold; Table 3). There was a significant drop in PVL from month 0 to month 3 (P < .001, Wilcoxon 2-tailed signed rank test). The drop in PVL on valproate treatment in the 16 patients is considerably greater than normal load fluctuations being greater than the change in PVL in the patients prior to treatment (P < .001, Wilcoxon 2-tailed signed rank test) and greater than the change in proviral load seen in the control set of 36 untreated patients (P < .001, Wilcoxon Mann-Whitney 2-tailed test). Concomitantly to the reduction in the PVLs, the numbers of infected cells, as evaluated by flow cytometry of CD4+ spontaneously expressing the Tax viral protein, decreased over the 3-month period of the trial (data not shown).
Proviral leads in 16 HAM/TSP patients treated with VPA
| Patient no. . | Proviral loads, no. of copies per 103 cells . | Fold reduction of the proviral loads at 3 mo . | |
|---|---|---|---|
| At d 0 . | At 3 mo . | ||
| 1 | 8.310 | 0.795 | 10.5 |
| 2 | 45.697 | 19.406 | 2.4 |
| 3 | 23.850 | 5.617 | 4.2 |
| 4 | 63.243 | 8.389 | 7.5 |
| 5 | 65.822 | 29.227 | 2.3 |
| 6 | 83.633 | 17.862 | 4.7 |
| 7 | 51.266 | 11.098 | 4.6 |
| 8 | 67.920 | 3.277 | 20.7 |
| 9 | 87.322 | 7.793 | 11.2 |
| 10 | 23.946 | 0.517 | 46.3 |
| 11 | 45.997 | 7.009 | 6.6 |
| 12 | 80.104 | 0.897 | 89.3 |
| 13 | 13.614 | 0.189 | 72.0 |
| 14 | 84.948 | 1.518 | 56.0 |
| 15 | 52.010 | 1.482 | 35.1 |
| 16 | 33.081 | 2.560 | 12.9 |
| Patient no. . | Proviral loads, no. of copies per 103 cells . | Fold reduction of the proviral loads at 3 mo . | |
|---|---|---|---|
| At d 0 . | At 3 mo . | ||
| 1 | 8.310 | 0.795 | 10.5 |
| 2 | 45.697 | 19.406 | 2.4 |
| 3 | 23.850 | 5.617 | 4.2 |
| 4 | 63.243 | 8.389 | 7.5 |
| 5 | 65.822 | 29.227 | 2.3 |
| 6 | 83.633 | 17.862 | 4.7 |
| 7 | 51.266 | 11.098 | 4.6 |
| 8 | 67.920 | 3.277 | 20.7 |
| 9 | 87.322 | 7.793 | 11.2 |
| 10 | 23.946 | 0.517 | 46.3 |
| 11 | 45.997 | 7.009 | 6.6 |
| 12 | 80.104 | 0.897 | 89.3 |
| 13 | 13.614 | 0.189 | 72.0 |
| 14 | 84.948 | 1.518 | 56.0 |
| 15 | 52.010 | 1.482 | 35.1 |
| 16 | 33.081 | 2.560 | 12.9 |
The mean fold reduction is 24-fold.
These data thus clearly demonstrate that VPA leads to depletion of HTLV-1–infected cells in vivo.
Discussion
Our findings show that treatment of HAM/TSP patients with a standard clinical dose of VPA produces a substantial decline in the PVL. Contrasting with current unsatisfactory therapies based on inhibition of inflammation, cell proliferation, or viral replication, we demonstrate the efficacy of a novel approach aimed, paradoxically, at activating viral gene expression to expose virus-positive cells to the host immune response. The basic principle of this approach is to temporarily force the latent virus out of hiding to displace the dynamic equilibrium modulating its long-term persistence and thereby decrease the PVL. For the first time, we provide evidence that VPA leads to depletion of HTLV-1–infected cells in vivo, most probably via activation of viral expression and subsequent destruction by the host immune system.
Triggering expression of viral proteins and more particularly Tax could, in principle, induce proliferation of the infected cell and indirectly increase the proviral loads. Besides, VPA at a concentration of 5 mM has recently been shown to interfere with CD8+ cell-mediated lysis of Tax-expressing cells ex vivo,30 suggesting that the efficiency of CTL surveillance of HTLV-1 might be reduced in vivo. The net outcome of these 2 processes (immune control and Tax-induced cell proliferation) could not be accurately predicted from cell culture assays. Our present data demonstrate that VPA at a pharmacologically relevant dose of approximately 1 to 3 mM effectively decreases the proviral loads in all HTLV-1–infected subjects. It is noteworthy that the PVL transiently increased in some patients (Figure 4), possibly indicating an imbalance between clonal expansion and immune surveillance in favor of the former mechanism.
Since a close relationship exists between increased PVL and clinical deterioration, a transient burst in viral replication and/or expression might temporarily exacerbate inflammation. Although long-term VPA treatment is possible, adverse effects potentially occurring during treatment (HAM-2 at day 30 [Table 2]; S.O., ongoing clinical trial, begun May 2006) could benefit from combination therapy with molecules reducing inflammation (prednisolone), viral replication (AZT + 3TC), or cell proliferation (IFN-β1). However, we think that VPA treatment alone would be particularly useful before irreversible CNS damage occurs, although HDAC inhibitors may harbor interesting neurologic properties.31 The idea is to avoid high levels of PVL in the 3% to 5% of infected patients who are evolving toward HAM/TSP. Importantly, long-term treatment (over years) with pharmacologically relevant doses of VPA as low as 20 mg/kg/day is possible, and this approach might thus also have biologic effects directly on HAM/TSP. In this context, we should mention that VPA reduced spasticity in all patients shortly after initiation of their treatment. Our present report does not stress the long-term clinical outcome of VPA treatment in HAM/TSP. Whether the elimination of the silent population of virus will affect the course of disease is currently unknown. However, since there is a clear relationship between the evolution of the proviral loads and occurrence of HAM/TSP,18 the idea is to restrict the number of infected cells to reduce the probability of disease onset.
Although we favor an immunologic mechanism for the action of VPA, an alternative but not exclusive paradigm would be that induction of histone acetylation results in transcriptional activation of critical nonviral genes. In this context, a selective proapoptotic effect of VPA against tumor cells has recently been associated with death receptor signaling.32,33 If virus independent, VPA therapy might also be effective in other Th1 cytokine–driven inflammatory, demyelinating, neurodegenerative CNS diseases such as multiple sclerosis (MS). Indeed, recent evidence in an animal model of MS (experimental autoimmune encephalomyelitis) demonstrated that another HDAC inhibitor, trichostatin A, reduced chemotactic, pro-Th1, and proproliferative mRNAs concomitantly with clinical improvement in mice.34
In summary, we propose a novel therapeutic approach to HAM/TSP that significantly decreased proviral load and reduced muscle spasticity in all subjects. This is a proof-of-concept study for gene activation therapy for HAM/TSP using the HDAC inhibitor VPA that should spur phase 1 double-blind placebo-controlled crossover trials. After several decades of clinical use for epilepsy, this novel application for this old drug in a neurodegenerative disease further broadens its potential therapeutic use as already proposed in cancer.11,28,35
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Sixth Research Framework Program of the European Union, Project INCA (LSHC-CT-2005–018704), the “Université des Antilles et de la Guyane” (EA 2434), the “Délégation Régionale de l'Institut National de la Santé et de la Recherche Médicale” (INSERM), the “Fonds National de la Science du Ministère de l'Education Nationale, de l'Enseignement et de la Recherche,” the Belgian Foundation against Cancer, the “Fonds national de la recherche scientifique” (FNRS), the Bekales Foundation, and the Leverhulme Trust. N. Gillet (Télévie fellow) and L.W. (Research Director) are members of the FNRS. N. Grandvaux was the recipient of a FRSQ (Fonds de la recherche en Santé du Québec) chercheur-boursier.
We thank Patrice Urbain, Régine Marlin, Stéphanie Olière, and Aurélie Chalon for experimental help. We are also grateful to Charles Bangham and Derek Macallan for careful reading of the paper and constructive comments. Finally, we thank Françoise Bex (Brussels, Belgium), Ralph Grassmann (Erlangen, Germany), and Eric Wattel (Lyon, France) for providing plasmid vectors.
Authorship
Contribution: A.L. and L.W. designed research and wrote the paper; N. Gillet, N. Grandvaux, O.V., G.B., and M.C.B. performed experiments; S.O., A.S., and D.S. performed the clinical trials; B.A. performed statistical analyses; J.H., A.B., and R.C. participated in analyses. R.C. and L.W. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luc Willems, Molecular and cellular biology, National Fund for Scientific Research (FNRS at FUSAG), 13 avenue Maréchal Juin, 5030 Gembloux, Belgium; e-mail:willems.l@fsagx.ac.be.