Abstract
Both G-CSF and GM-CSF (alone or in combination) may be used for mobilization of hematopoietic progenitor cells in patients undergoing autologous stem cell transplantation (ASCT). It has been suggested that GM-CSF use during mobilization may impact graft composition and therefore clinical outcomes.
METHODS: We prospectively evaluated patients ≤ 70 years old with relapsed CD20+ NHL who were candidates for ASCT. Additional eligibility criteria included adequate marrow and organ function. Patients with history of pelvic radiation, > 3 prior chemoregimens or > 6 cycles of fludarabine chemotherapy were excluded. Patients recieved chemotherapy with ifosfamide 3.33 g/m2 daily × 3 days, etoposide 150 mg/m2 × 6 doses and rituximab (375 mg/m2 on day 1 and 1 g/m2 on day 8). Using a Bayesian adaptive randomization based on treatment outcomes, patient’s were randomized to receive G-CSF 12 μg/kg/d (Group G) or G-CSF 12 μg/kg/d plus GM-CSF 500 μg/d (Group G/GM). We assumed that the success rate for each treatment arm had a β prior distribution with mean 0.90 and variance 0.03. Cytokines started 24 hours after completion of chemotherapy and continued until completion of apheresis.
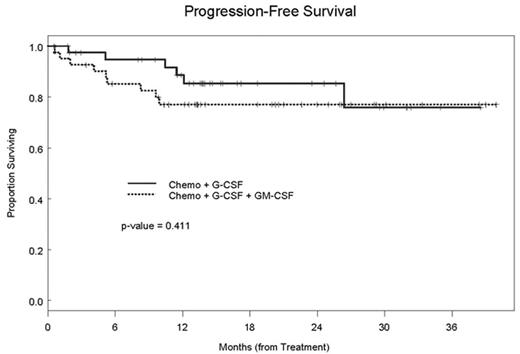
RESULTS: Forty-three patients were randomized to Group G and 41 patients to Group G/GM. In each arm 1 patient withdrew consent after randomization. Baseline characteristics were similar in the 2 groups (Table 1). Both regimens were equally well tolerated. Data are presented as intent to treat analysis. Thirty-nine patients (90.7%) in Group G and 35 patients (85.4%) in Group G/GM collected ≥ 4 × 106 CD34+ cells/kg. The probability that Group G has a higher success rate than Group G/GM is 0.778. The median CD34+ cell dose collected was 10.3 × 106/kg (range, 0.1–59) and 7.5 × 106/kg (range, 0.7–73) in Groups G and G/GM respectively (P=NS). A median of 2 apheresis procedures were required in both arms. Seventy-three patients have undergone ASCT. After a median follow up time of 14.5 months (range, 0.6–38.5) in Group G and 14.0 months (range, 1.1–39.9) in Group G/GM, the 3 year PFS is 75% (95% CI 57.9–99.4) and 77% (95% CI 65–91.5) respectively (P=0.41).
CONCLUSION: Our study does not support the hypothesis that using G-CSF plus GM-CSF versus G-CSF alone for progenitor cell mobilization alters graft composition in a way that impacts clinical outcomes after ASCT for NHLs.
Baseline Patient Characteristics
| *Missing data 1 patient | ||
| G-CSF, N (%) | G-CSF + GM-CSF, N (%) | |
| 43 (51) | 41 (49) | |
| AGE | ||
| <39 | 4 (9.3) | 3 (7.3) |
| 40–59 | 29 (57.4) | 26 (63.5) |
| >59 | 10 (23.2) | 12 (29.3) |
| GENDER (Male/Female) | 29/14 (67.4/32.6) | 24/17 (58.5/41.5) |
| HISTOLOGY | ||
| Low grade | 4 (9.3) | 7 (17.1) |
| Intermediate grade | 39 (90.7) | 34 (82.9) |
| ANN ARBOR STAGE >I | 18 (41.9) | 18 (43.9) |
| LDH>Normal* | 15 (34.9) | 11 (27.5) |
| *Missing data 1 patient | ||
| G-CSF, N (%) | G-CSF + GM-CSF, N (%) | |
| 43 (51) | 41 (49) | |
| AGE | ||
| <39 | 4 (9.3) | 3 (7.3) |
| 40–59 | 29 (57.4) | 26 (63.5) |
| >59 | 10 (23.2) | 12 (29.3) |
| GENDER (Male/Female) | 29/14 (67.4/32.6) | 24/17 (58.5/41.5) |
| HISTOLOGY | ||
| Low grade | 4 (9.3) | 7 (17.1) |
| Intermediate grade | 39 (90.7) | 34 (82.9) |
| ANN ARBOR STAGE >I | 18 (41.9) | 18 (43.9) |
| LDH>Normal* | 15 (34.9) | 11 (27.5) |
Figure
Author notes
Disclosure:Research Funding: This study was supported by a research grant from Bayer HealthCare (previously Berlex Laboratories).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal