Abstract
Background: Imatinib (IM) is the drug of choice for the treatment of CML, where it was shown that a complete cytogenetic response (CCgR) and a major molecular response (MMolR) at 12 months (mos) were the best surrogate markers of progression-free survival (PFS) (O’Brien et al, NEJM, 2003; Hughes et al, NEJM 2003). The issue of the MolR is particularly sensitive, because the degree of the MolR may fluctuate over time and among different labs, also for methodological reasons (Hughes et al, Blood 2006).
Aim: To evaluate the prognostic value of the MolR based on a retrospective analysis of the patients (pts) who achieved a CCgR with IM 400 mg daily.
Patients and Methods: 130 pts who achieved a CCgR for more than 1 year (confirmed CCgR) and were tested for MolR at least 3 times after achieving the CCgR, were analyzed. Cytogenetic response was assessed every 6 mos by conventional analysis of at least 20 marrow cell metaphases. MolR was assessed every 3 mos by RTQ-PCR (TaqMan) on peripheral blood buffy coat cells and was defined as MMolR when BCR-ABL:ABL<0.05%, corresponding to 0.1% on the International Scale.
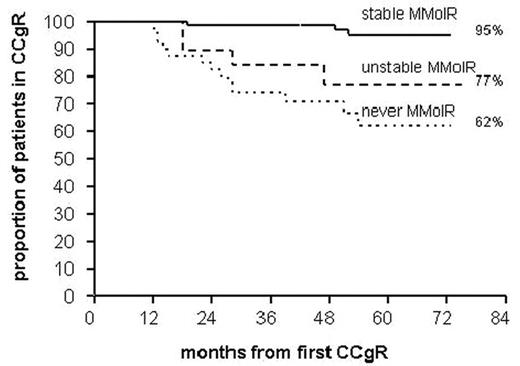
Results: In 71/130 pts (55%) MolR was always major (stable MMolR). In 19/130 (15%) pts MolR was sometimes major and sometimes less than major (unstable MMolR). In 40/130 pts (30%) MolR was never major (never MMolR). The proportion of pts remaining in CCgR after 5 years was calculated by the Kaplan-Meyer method (Fig. 1) and was 95% for pts in stable MMolR, 77% for pts with unstable MMolR and 62% for pts without a MMolR during follow-up (p<0.0001, log-rank test).
Conclusions: These data confirm that achieving a MMolR is prognostically important but point out that the prognostic value of achieving a MMolR is greater if the response is confirmed, and introduce the concept of sustained Mol response, alerting from relying on single determinations.
Aknowledgements: The study was supported by the Italian Association for Cancer Research (A.I.R.C.), by European LeukemiaNet founds, and by Associazione Italiana contro le Leucemie (AIL) grants. Fig 1. CCgR duration according to RQ-PCR categories.
Author notes
Disclosure:Consultancy: Novartis, Wyeth, Bristol-Myers Squibb. Research Funding: Novartis, Whyet, Bristol-Meyers Squibb, Roche, Johnson & Johnson, Schering-Plough. Honoraria Information: Novartis, Whyet, Bristol-Meyers Squibb, Roche, Johnson & Johnson, Schering-Plough. Paid Export Testimony Information: Novartis, Wyeth, Bristol-Myers Squibb, Roche, Johnson & Johnson. Membership Information: Novartis, Wyeth, Bristol-Myers Squibb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal