Abstract
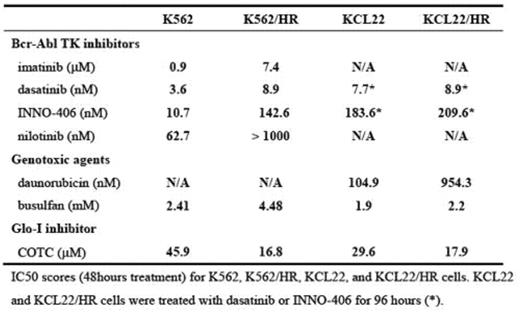
Growth under hypoxic conditions can lead to resistance to a variety of cell death stimuli in cancer cells. Bone marrow (BM) has less vasculature than other organs, and the rapid growth of leukemic cells in BM frequently outpaces the oxygen supply. Thus, adaptation to hypoxia may be critical to the survival and proliferation of leukemic cells. However, the role of hypoxia in the treatment of leukemia has received little attention. We hypothesized that leukemic cells acquire drug resistance by adapting to hypoxia in the BM, and that identifying the underlying molecular mechanisms for this adaptation would reveal new approaches to the development of new therapeutic targets in drug- resistant leukemia cells. To test this hypothesis, we cultured five chronic myelogenous leukemia (CML)-derived cell lines (BV173, K562, KCL22, KT-1, and MYL) continuously in 1.0% oxygen. Among them, hypoxia resistant (HR) subclones of K562 and KCL22 cells (K562/HR and KCL22/HR) were selected under 1.0% oxygen conditions, and these cells were able to grow for several months and to be suspended in RPMI1640 plus 10% FCS without any additional nutrients, although their growth rate was slower than parental cells in 20% oxygen. Both K562/HR and KCL22/HR cells produced less ATP (78% and 92%, respectively), perhaps due to impairment of the oxygen-dependent energy production pathway, and HR cells showed higher expression and enhanced kinetics of glyoxalase-I (Glo-I), a detoxification kinase of methylglyoxal (a cytotoxic byproduct of glycolysis), compared to parental cells. Glo-I has previously been implicated in drug resistance in cancer, including leukemia. Autophosphorylation of Bcr-Abl was reduced in HR cell lines, while the phosphorylation status of the downstream signalling molecules Akt and Stat5 was unchanged, which suggests that there are alternative, hypoxia-specific activators of these pathways. K562/HR and KCL22/HR cells were less sensitive to chemotherapeutic agents such as the Bcr-Abl tyrosine kinase (TK) inhibitors imatinib, dasatinib, nilotinib, and INNO-406, and the alkylating agents daunorucbicin and busulfan. In contrast, K562/HR and KCL22/HR cells were more sensitive to cell death induced by a Glo-I inhibitor, COTC, compared to parental cells. These results indicated that Glo-I plays an important role in survival under hypoxic conditions, and that Glo- I may be an alternative molecular target in CML cells that have acquired resistance to conventional chemotherapeutics through adaptating to hypoxia.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal