Abstract
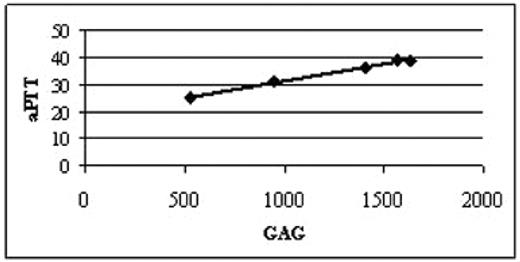
MPS IH (Hurler syndrome) is caused by severe mutations in a-L-iduronidase gene, leading to accumulation of complex sugars, termed glycosaminoglycans (GAG), in tissues. The disorder is lethal unless treated with hematopoietic stem cell transplantation (HSCT). When compared to other recipients of HSCT, MPS I patients are well-known to have an increased incidence of bleeding complications in peri-transplant period. To investigate whether this bleeding propensity is related to the enzyme deficiency and GAG accumulation we evaluated five MPS IH patients (2 females and 3 males, mean age: 1.1 years) prior to any therapy. We observed that three of the five patients had an elevated activated partial thromboplastin time (aPTT, normal range 22–35 seconds). As no heparin was used in any of these patients, we reasoned that the accumulation of heparan sulfate, a hallmark of MPS IH, may serve as a heparin-like anticoagulant. Should this assumption be correct, higher levels of GAG would be expected in the three patients with the elevated aPTT. This, in fact, was what we have observed (Figure), suggesting that dose - effect relationship exists between whole body GAG load (assessed as milligrams of urinary GAG normalized to grams of creatinine; x-axis) and level of anticoagulation (aPTT in seconds; y-axis).
In this context, it is important to note that the patient with the second highest value of urinary GAG and aPTT experienced pulmonary bleeding on day 12 after HSCT requiring intubation and ventilatory support for 25 days. We hypothesized that endogenous GAG heparan sulfate exerts its antithrombotic effect through antithrombin III-mediated inhibition of factor Xa, as in clinically used low molecular weight heparinoid, danaparoid. To test this hypothesis, we have assessed anti-Xa levels in mice with a-L-iduronidase deficiency, an animal model of MPS IH. As expected, the anti-Xa level were not significantly different in wild type C57Bl/6 mice (N=3) and heterozygous mice (N=6): 0.11±0.005 IU/cc plasma versus 0.10±0.004 IU/cc plasma (p=0.2). Anti-Xa values of MPSI mutant mice (N=14), however, were significantly elevated when compared to the age-matched sex-matched wild type controls (0.19±0.05 IU/cc plasma versus 0.11±0.005 IU/cc plasma; p<0.001). In addition, we have observed that GAG anticoagulant activity was neutralized by addition of the heparinase enzyme (MPS I, N=3, 0.16±0.02 IU/cc plasma versus MPS I + heparinase, N =3, 0.10±0.002 IU/cc plasma; p<0.01). In conclusion, the current study links GAG produced in excess as a result of a-L-iduronidase deficiency and a hemostatic defect in MPS I mice and humans. The implications of the current study are that an impaired hemostasis may exist in untreated patients with MPS IH, and that MPS IH patients with high urinary GAG are likely to experience bleeding complications. This information may be useful in identifying MPS IH patients at higher risk for bleeding, especially in the peri-transplant period, when GAG levels have not yet normalized and additional, HSCT-related factors (e.g., thrombocytopenia and hepatic dysfunction affecting clotting factors) contribute to the high risk of bleeding.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal