Abstract
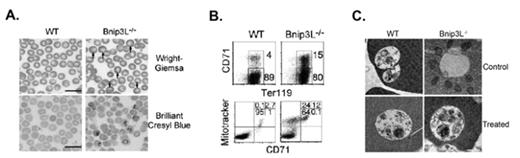
Prevention of apoptosis is a well-recognized mechanism essential for productive early erythropoiesis and preventing anemia; however, the importance of pro-apoptotic processes for productive terminal erythropoiesis is not defined. Erythroid cells undergo enucleation and the removal of organelles during terminal differentiation. Although autophagy has been shown to mediate the elimination of organelles for erythroid maturation, the molecular mechanisms underlying this process remain unknown. Here we report a novel role for Bnip3L, a pro-apoptotic Bcl-2 family member, in the regulation of erythroid maturation through autophagy. Bnip3L−/− mice developed hemolytic anemia and expansion of erythroid precursors (Fig.1A). Moreover, erythrocytes in the peripheral blood of Bnip3L−/− mice exhibited mitochondrial retention associated with reduced lifespan (Fig. 1B). While the clearance of ribosomes proceeded normally in the absence of Bnip3L, the entry of mitochondria into autophagosomes was defective (Fig. 1C). Overexpression of Bnip3L led to the loss of mitochondrial membrane potential (ΔΨm). Further, disrupting Δ Ψ m with an uncoupling chemical restored the sequestration of mitochondria into autophagosomes in Bnip3L−/− erythrocytes (Fig. 1C). Our study indicates that Bnip3L-dependent loss of Δ Ψ m is important for targeting the mitochondria into autophagosomes for degradation during erythroid maturation, and interference with this function impairs terminal erythroid maturation and results in hemolysis. These data underlie the importance of pro-apoptotic mechanisms for productive terminal erythropoiesis through autophagy.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal