Abstract
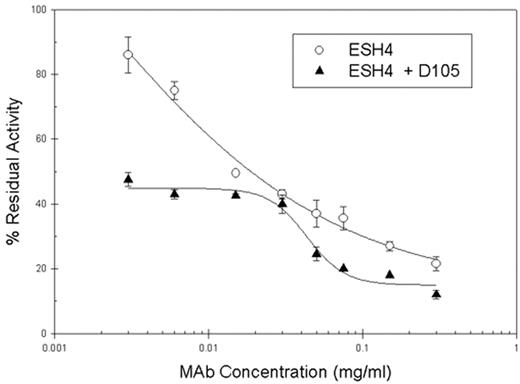
We recently identified 5 distinct structural classes of anti-human factor VIII (fVIII) C2 domain monoclonal antibodies (MAbs) in a murine model of hemophilia A. Three classes, Groups A, B, and C, consist of MAbs with non-overlapping epitopes. Group AB MAbs overlap epitopes of Group A and B MAbs, and Group BC MAbs overlap epitopes of Group B and C MAbs. Concentration dependent binding of biotinylated Group A MAbs to fVIII bound to a microtiter plate was studied in the presence and absence of Group BC MAbs by ELISA. Surprisingly the binding of all 3 Group A antibodies tested, ESH4, 3E6, and I54, was increased in the presence of Group BC antibodies. Group A antibodies block the binding of fVIII to von Willebrand factor (VWF) and phospholipid. Group BC antibodies inhibit the proteolytic activation of fVIII but do not block the binding of fVIII to VWF and phospholipid. To establish functional relevance, the effect of Group BC MAbs on the ability of Group A MAbs to block fVIII binding to VWF was determined by ELISA. Group A MAbs were serially diluted into fixed concentrations of Group BC MAbs and then incubated with fVIII in solution for 2 hours. The fVIII - antibody mixtures were added to microtiter wells coated with VWF, and fVIII binding to VWF was measured. Group BC MAbs increased the ability of ESH4 and 3E6 to inhibit the binding of fVIII to VWF, consistent with the observation that the binding of Group A and Group BC antibodies is positively cooperative. We previously have shown that antibody-mediated inhibition of fVIII binding to VWF is correlated with inhibition of fVIII binding to phospholipid and with fVIII inhibitor titers. Although most Group BC MAbs have high inhibitory titers against fVIII, Group BC MAb D105 is only minimally inhibitory at 0.8 Bethesda units/mg IgG. Because D105 increased the binding of ESH4 to fVIII like other Group BC MAbs, the effect of D105 on the inhibitor titer of ESH4 was investigated using the Bethesda assay. The inhibition of fVIII was significantly increased by the presence of D105 (Fig. 1). This study identifies increased binding of Group A MAbs to fVIII in the presence of Group BC MAbs using 3 different assays. Our results suggest that the cooperative interaction of fVIII antibody epitopes affects the overall inhibitor patient phenotype and that inhibitors with very low inhibitor titers can significantly increase the inhibitory effects of other anti-fVIII antibodies.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal