Abstract
Background: A previous CALGB trial (
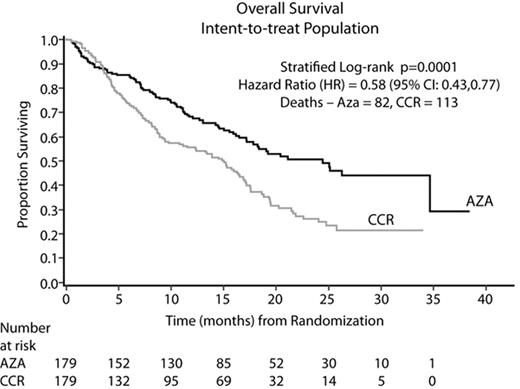
Results: In all, 358 pts (70% male), were randomized at 79 sites to AZA (N=179) or CCR (N=179): BSC only (N=105, 59%), LDAC (N=49, 27%), or Std CT (N=25, 14%). Median age was 69 yrs (38–88) and per treatment (TX): AZA (69 yrs); BSC only (70 yrs); LDAC (71 yrs); and Std CT (65 yrs). The AZA and CCR groups were comparable for baseline (BL) parameters. At BL, 95% of pts were higher risk: RAEB (58%), RAEB-T/WHO AML (34%), CMML (3%), and other (5%). By IPSS, 87% were higher risk: Int-2 (40%), High (47%), and 13% indeterminate/other. AZA was administered for a median of 9 cycles; LDAC for 4 cycles. Median followup for the OS analysis was 21.1 months (mo). AZA demonstrated statistically superior OS vs CCR (stratified log-rank p=0.0001). AZA showed a median Kaplan-Meier (KM) OS time of 24.4 mo vs 15 mo with CCR (Figure). The hazard ratio (HR, Cox Model) was 0.58 (95% CI: 0.43, 0.77) for a 74% OS improvement. At 2 yrs, there was a 2-fold OS advantage: AZA (51%) vs CCR (26%), 95% CI: 13, 36%, p<0.0001. Differences in OS KM medians (HR; log-rank p) between AZA and BSC, LDAC, and Std CT, respectively, were 12.9 mo (0.55; p=0.0003), 9.1 mo (0.60; p=0.016), and 8.7 mo (0.69; p=0.19). Median OS per IPSS cytogenetic subgroup showed similar results (Table). The 1, 2, and 3-mo survival rates did not differ between AZA and BSC only (p>0.20). AZA was well tolerated with safety data consistent with previous reports.
Conclusion: These data confirm and extend previous CALGB findings. This AZA trial is the first MDS clinical study to demonstrate a significant OS advantage, thus altering the natural disease course. AZA should now be considered first-line therapy for higher-risk MDS pts.
OS Analyses per IPSS Cytogenetic Group
| Group . | % (n/N) Pts . | AZA Median (Months) . | CCR Median (Months) . | HR (95%CI) . | Log-rank p . |
|---|---|---|---|---|---|
| Good | 46 (166/358) | Not reached | 17.1 | 0.61 (0.39, 0.96) | 0.030 |
| Intermediate | 21 (76/358) | 26.3 | 17.0 | 0.43 (0.21, 0.88) | 0.017 |
| Poor | 28 (100/358) | 17.2 | 6.0 | 0.52 (0.32, 0.87) | 0.011 |
| Group . | % (n/N) Pts . | AZA Median (Months) . | CCR Median (Months) . | HR (95%CI) . | Log-rank p . |
|---|---|---|---|---|---|
| Good | 46 (166/358) | Not reached | 17.1 | 0.61 (0.39, 0.96) | 0.030 |
| Intermediate | 21 (76/358) | 26.3 | 17.0 | 0.43 (0.21, 0.88) | 0.017 |
| Poor | 28 (100/358) | 17.2 | 6.0 | 0.52 (0.32, 0.87) | 0.011 |
Figure
Author notes
Disclosure:Employment: Dr Jay Backstrom, CL Beach, Linda Zimmerman, and David McKenzie are employees of Pharmion Corporation. Consultancy: Drs Bennett, Byrd, List, Giagounidis, Gore, and Mufti. Ownership Interests:; Dr Byrd, Dr Giagounidis, Dr Backstrom, CL Beach, Linda Zimmerman, and David McKenzie. Research Funding: Drs. Fenaux, Seymour, Silverman, List, and Hellstrom-Lindberg. Honoraria Information: Drs. Fenaux, Seymour, Gore, Mufti, List, Santini, Silverman, and Hellstrom-Lindberg. Membership Information: Drs Seymour, Hellstrom-Lindberg, and Gore. Off Label Use: The abstract discusses results of a long term randomized, multicenter, controlled survival trial of azacitidine in the label-approved population of MDS patients. However, the current labeling for azacitidine does not include an effect on survival.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal