Abstract
The lack of transplantable tumors has limited assessment of graft-versus-tumor effects following hematopoietic cell transplantation in clinically relevant large-animal models. We describe the derivation and characterization of porcine tumor cell lines with initial efforts of tumor transplantation using immunocompromised mice and highly inbred sublines of Massachusetts General Hospital major histocompatibility complex (MHC)–inbred miniature swine. Autopsies were performed routinely on swine that died unexpectedly or had suspicion of malignancy based on clinical symptoms or peripheral blood analysis. Tissue samples were obtained for pathology, phenotyped by flow cytometry, and placed in culture. Based on growth, lines were selected for passage into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice and miniature swine. Porcine tumor recipients were preconditioned with total body irradiation from 0 to 500 cGy or with a 30-day course of oral cyclosporine. We identified 19 cases of hematologic tumors. Nine distinct tumor cell lines were established from 8 of these cases, including 3 derived from highly inbred sublines. In vivo tumor growth and serial transfer were observed in immunocompromised mice for one tumor cell line and in miniature swine for 1 of 2 tumor cell lines expanded for this purpose. These results suggest the possibility of developing a transplantable tumor model in this large-animal system.
Introduction
Animal models of malignancy are valuable tools for studying cancer biology. Although rodent models have aided in elucidating mechanisms of oncogenesis and in developing treatment strategies, small-animal systems are limited in their ability to accurately reflect all aspects of the disease conditions associated with human cancers.1,2 Translation from these small-animal models toward the clinical setting is not always possible, as humans often have different responses and disease mechanisms.3,4 Since the life span of these animals is significantly shorter, it is also difficult to use them for longitudinal studies to investigate the efficacy and safety of new treatments.5,6 Large animals with greater genetic and physiologic similarities to humans can be used to bridge this translational gap.7 Such models of malignant diseases rely on spontaneous neoplasms occurring in certain breeds, such as canine melanoma, or on induced tumors resulting from exposure to a carcinogen.7-9 Virally induced tumor cells have also been used for evaluating imaging modalities and feasibility of interventional radiologic techniques in dogs, but these systems may not necessarily resemble in situ malignancies of those organs.10 The low incidence of disease and the length of time to disease onset limit practicality of these models for experimentation
Nonmyeloablative hematopoietic cell transplantation (HCT) protocols that achieve potent graft-versus-tumor (GVT) effects without graft-versus-host disease (GVHD) have been developed in rodent models.11-13 Successful translation of these protocols to the clinic has been limited, however, with GVHD remaining a major complication. Our laboratory has developed minimally toxic HCT protocols in miniature swine that achieve stable engraftment across major histocompatibility complex (MHC) barriers without GVHD.14 The lack of available transplantable tumor lines in miniature swine has so far prohibited the direct evaluation of antitumor effects of HCT and donor leukocyte infusion (DLI) in this clinically relevant large-animal model.
Recently, histocompatible sublines of SLAdd animals have been established that accept reciprocal skin grafts.15 Isolation of tumors and establishment of tumor cell lines from these animals should permit development of transplantable tumor models to assess GVT effects in miniature swine. Over the past 10 years, we have monitored our herd for the occurrence of malignancies, particularly hematologic cancers such as leukemias and lymphomas. From this surveillance, we now report our establishment of porcine tumor lines and our initial efforts of tumor transplantation.
Methods
Animals
Massachusetts General Hospital (MGH) partially inbred MHC-defined miniature swine have previously been described in detail.16,17 Three lines are homozygous for a different MHC haplotype (SLAa, SLAc, and SLAd), and 5 lines bear different intra-MHC recombinant haplotypes (SLAg, SLAh, SLAj, SLAk, and SLAl). Sublines of the SLAdd animals were established by sequential brother-sister matings to produce histocompatible lines with a coefficient of inbreeding of more than 94%. Reciprocal skin grafts within these sublines were accepted without immunosuppression.15 All animal care procedures were in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources.18 Protocols involving animals were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Detection and diagnosis
Animals were under continuous surveillance for the development of abnormal symptoms and physical findings. The preliminary screen for malignancy included review of clinical symptoms, physical exam, complete blood count (CBC), and white blood cell count (WBC) differential by manual count and flow cytometry. Based on this screen, animals suspected of malignancy were humanely euthanized and underwent an autopsy under aseptic conditions to obtain primary disease tissues. Animals that died unexpectedly were also subject to autopsy under aseptic conditions for tissue harvest and for diagnostic purposes. The diagnosis of malignancies was based on the clinical findings, history, physical examination, CBC, histology, and phenotypic analysis by surface staining and flow cytometry of peripheral blood cells.
Tissue and peripheral blood mononuclear cell harvest and processing
At the time of autopsy, tissue samples from lymphoid, hematopoietic, and any organ that appeared abnormal at autopsy were obtained in a sterile manner. Samples were then processed by mechanical maceration and subsequent filtering with Hanks buffered salt solution (HBSS) through a 40-μm cell strainer (BD Biosciences, Bedford, MA). Peripheral blood was processed for mononuclear cells (PBMCs) from heparinized whole blood diluted with HBSS containing Ca2+ and Mg2+. Cells were obtained by gradient centrifugation using lymphocyte separation medium (ICN Biomedicals, Aurora, OH) and lysis of red blood cells with ACK Lysing buffer (Cambrex BioScience, Walkersville, MD)
Histology and immunohistochemistry
At necropsy, any grossly abnormal tissue and suspicious lesions were sampled for histology and immunohistochemistry. For histology, tissues were fixed in paraformaldehyde and then stained using hematoxylin and eosin. For immunohistochemistry, tissues were embedded in Histopaque embedding matrix (Sigma-Aldrich, St Louis, MO) and frozen at −80°C. The following primary antibodies were used for immunohistochemistry: CD3 BB23-8E6 IgG119,20 ; CD16 G7 IgG121 ; and CD172a (porcine monocyte/granulocyte marker) 74-22-15 IgG1.22
Tissue culture
Cell suspensions obtained from processing were placed into culture with RPMI 1640 media supplemented with 12% fetal bovine serum (FBS), 10 mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), 1 mM glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 2 × 10−5 M 2-mercaptoethanol. Cultures are maintained at 37°C with 5% CO2. Establishment in culture was determined by continuous growth over a minimum period of 2 months. Once cell lines were established, each line was maintained with passage every 7 to 14 days. To identify growth parameters of the established cell lines under these conditions, cells were plated at an initial density of 104 cells/mL in a T75 flask (Corning, Corning, NY). Daily cell counts were obtained over a 2-week incubation period. Samples of cell lines were collected and frozen at −180°C in liquid nitrogen at a concentration of 107 cells/mL in cryoprotective medium (Cambrex BioScience) diluted 1:1 with FBS to a final concentration of 7.5% DMSO.
Flow cytometry
Peripheral blood samples obtained at the time of clinical symptoms were analyzed for a preliminary phenotype by flow cytometry. Cell suspensions from primary tissues including processed PBMCs, lymph node, liver, bone marrow, and spleen, as well as established tumor cell lines were also phenotyped using flow cytometry. Antibodies used included the following: CD1 76–7-4 IgG2a22 ; CD2 MSA-4 IgG2a23,24 ; CD3 898H2–6-15 IgG2a25 ; CD4 74–12-4 IgG2b22 ; CD5 BB6–9G12 IgG126 ; CD8 76–2-11 IgG2a27 ; CD9 1038H-4–6 IgM28 ; CD16 G7 IgG121 ; CD21 BB6–11C9 IgG129,30 ; CD25 231.3B2 IgG131 ; CD172a 74–22-15 IgG122 ; class II DR 40D IgG2b; anti-mu heavy chain 5C9 IgG132 ; and anti-kappa light chain K139 3E1 IgG2a.33 Flow cytometry was performed using a Becton Dickinson FACScan. Staining of cell suspensions was performed as previously described.34 Data were analyzed using Winlist list mode analysis software (Verity Software House, Topsham, ME).
In vivo transfer of tumors into NOD/SCID mice
In an attempt to select for variants with in vivo growth potential, cultured tumor cell lines were transplanted into NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME). Animals were 4 to 8 weeks of age and were housed in clean conditions. Animals were manually restrained and sterilely swabbed with a 70% alcohol solution at the site for intraperitoneal injection in the lower right abdominal quadrant. Sterile 23- to 27-gauge needles were used to deliver the sterile inoculum of tumor cells at a dose of ranging from 1 to 5 × 107 cells in a total volume of 0.2 mL. Tumor tissue was removed sterilely to obtain samples for cell culture, pathology, and surface phenotyping by flow cytometry. These tissue samples were also processed by mechanical maceration and subsequent filtering with HBSS through a 40-μm cell strainer (BD Biosciences). Cell suspensions were placed in tissue culture as described in “Tissue culture.”
In vivo transfer of tumors in miniature swine
Tumor cell lines established from highly inbred SLAdd animals were used for in vivo tumor transfer experiments using recipient animals also within this subgroup. Cells were injected into animals within histocompatible sublines, when possible, and also across sublines that share a common ancestor at the fifth generation of inbreeding. The degree of coancestry was calculated between the recipient and the animal from which the tumor cell line was derived. Recipient animals received tumor cell injections at doses from 107 to 108 cells/kg intravenously and/or subcutaneously at multiple injection sites and from 107 to 108 cells in up to 4 individual injection sites on the rear flanks of the animal, at up to 4 sites. Recipient animals ranged in age between 1.5 to 4 months of age and in weight between 5 kg to 20 kg in size. These animals were prepared with total body irradiation (TBI), a 30-day course of cyclosporine A (CyA), or no additional treatment. Animals receiving TBI prior to cellular injection were irradiated at day − 2 with 100, 300, or 500 cGy TBI. Animals treated with concurrent CyA received cyclosporine (Novartis Pharmaceuticals, East Hanover, NJ) orally beginning on day −1 and continuing for 30 days. Dosages were started at 15 mg/kg on day −1, and adjusted to maintain CyA whole blood levels between 400 to 800 ng/mL during the treatment period. Animals were monitored for tumor growth by palpation of injection sites. Blood samples from animals were analyzed for a complete blood count and for tumor growth after intravenous tumor administration by flow cytometry. If tumor growth was detected, animals were humanely killed. Tissues were sterilely harvested and processed for analysis and for culture as outlined in “Tissue culture” and “Flow cytometry.”
Results
Incidence of malignancies in MGH MHC-defined miniature swine
From surveillance of our herd, we identified 19 cases of malignancies. Ten of these cases were derived from animals with posttransplantation lymphoproliferative disease (PTLD), a complication of either experimental hematopoietic cell or solid organ transplantation protocols. The incidence of PTLD occurring in these protocols has been previously reported.35-37 Since no experimental hematopoietic cell transplant recipients from the inbred sublines were used, all PTLD cases occurred outside of the highly inbred SLAdd sublines of animals. The remaining 9 cases were spontaneously occurring malignancy. Of these cases, 5 occurred within highly inbred SLAdd histocompatible sublines. To determine the incidence of spontaneous, non–transplant-related malignancy, we used our animal database consisting of data collected over the last 10 years and evaluated the number of cases occurring in adult naive animals 24 months of age or older and unassigned to an experimental protocol. As most animals in our herd do not reach 24 months due to their use in experiments, only these animals were considered relevant for comparison. The overall incidence of spontaneous malignancy among susceptible animals (≥ 24 months of age) within these sublines was 2.5% (4/159), while disease incidence among all other haplotype lines was 1.3% (4/301), giving a P = .64 (χ2 = 0.216). A similar comparison of disease in incidence in older populations (≥ 36 months) revealed a statistically significant incidence of 10% (4/40) in the histocompatible sublines versus 1.3% (3/223), giving a P = .016 (χ2 = 5.763). However, it is uncertain whether this is an accurate reflection of disease incidence in our miniature swine as there are a limited number of animals, particularly for the SLAdd subline, that reach this age.
Diagnosed malignancies observed in miniature swine
The age, haplotype, and diagnosed malignancy of animals that developed disease are compiled in Table 1. After the fifth generation (G5) of brother-sister mating, 3 parallel sublines with common ancestry at G5 were set up for further inbreeding. For the purposes of this study, we will refer to these distinct sublines as DD1, DD2, and DD3. For disease occurring in highly inbred SLAdd animals, the subline to which each animal belonged is also noted in Table 1. Of the 19 observed cases of malignancy, 6 cases were diagnosed as leukemia, 11 were lymphomas, and 1 was a nonidentified carcinoma with lung involvement. All cases of leukemia occurred in adult animals ranging from 30 to 76 months at time of disease diagnosis. Three cases occurred in inbred SLAdd animals (P.S.C., Raimon Duran-Struuck, Shannon G. Moran, K.J.W., D.P.L., Michael Duggan, J.S.A., R.A.B., S.L.H., Susan V. Westmoreland, D.H.S., and C.A.H., manuscript in preparation). Only one case of lymphoma was a spontaneous neoplasm, which occurred in an inbred SLAdd adult animal that was 38 months old. The remaining 10 cases of lymphoma were due to PTLD.35-37 These cases occurred in adolescent animals between 3 to 7 months of age.
Summary of tumors identified and harvested from MHC-defined miniature swine
| Animal . | Haplotype . | Age, mo . | Clinical disease diagnosis . | Phenotype . |
|---|---|---|---|---|
| 12933 | Inbred DD0 (G5) | 44 | Myelomonocytic leukemia | Myeloid |
| 13556 | Inbred DD1 (G6) | 23 | Carcinoma | N/A |
| 14037 | Inbred DD2 (G7) | 38 | Lymphoma (spontaneous) | B cell |
| 14736 | Inbred DD2 (G8) | 38 | Leukemia | Myeloid |
| 15433 | Inbred DD3 (G9) | 36 | Leukemia | Myeloid |
| 11985 | JJ | 76 | Leukemia | Myeloid |
| 13271 | AD | 3 | Lymphoma (PTLD) | B cell |
| 13381 | DD | 39 | Leukemia | Undifferentiated |
| 13026 | DD | 50 | Leukemia | Myeloid |
| 14806 | AD | 7 | Lymphoma (PTLD) | B cell |
| 15005 | CC | 3 | Lymphoma (PTLD) | B cell |
| 15568 | AD | 3 | Lymphoma (PTLD) | B cell |
| 15399 | CC | 5 | Lymphoma (PTLD) | B cell |
| 15446 | CC | 6 | Lymphoma (PTLD) | B cell |
| 15549 | HH | 30 | Leukemia | Myeloid |
| 16556 | AD | 4 | Lymphoma (PTLD) | B cell |
| 17016 | AD | 4 | Lymphoma (PTLD) | B cell |
| 17018 | AD | 4 | Lymphoma (PTLD) | B cell |
| 17102 | AD | 4 | Lymphoma (PTLD) | B cell |
| Animal . | Haplotype . | Age, mo . | Clinical disease diagnosis . | Phenotype . |
|---|---|---|---|---|
| 12933 | Inbred DD0 (G5) | 44 | Myelomonocytic leukemia | Myeloid |
| 13556 | Inbred DD1 (G6) | 23 | Carcinoma | N/A |
| 14037 | Inbred DD2 (G7) | 38 | Lymphoma (spontaneous) | B cell |
| 14736 | Inbred DD2 (G8) | 38 | Leukemia | Myeloid |
| 15433 | Inbred DD3 (G9) | 36 | Leukemia | Myeloid |
| 11985 | JJ | 76 | Leukemia | Myeloid |
| 13271 | AD | 3 | Lymphoma (PTLD) | B cell |
| 13381 | DD | 39 | Leukemia | Undifferentiated |
| 13026 | DD | 50 | Leukemia | Myeloid |
| 14806 | AD | 7 | Lymphoma (PTLD) | B cell |
| 15005 | CC | 3 | Lymphoma (PTLD) | B cell |
| 15568 | AD | 3 | Lymphoma (PTLD) | B cell |
| 15399 | CC | 5 | Lymphoma (PTLD) | B cell |
| 15446 | CC | 6 | Lymphoma (PTLD) | B cell |
| 15549 | HH | 30 | Leukemia | Myeloid |
| 16556 | AD | 4 | Lymphoma (PTLD) | B cell |
| 17016 | AD | 4 | Lymphoma (PTLD) | B cell |
| 17018 | AD | 4 | Lymphoma (PTLD) | B cell |
| 17102 | AD | 4 | Lymphoma (PTLD) | B cell |
N/A indicates diagnosis made based on histologic findings: cellular material not available for phenotyping by fluorescence-activated cell sorting.
Clinical presentation and laboratory findings of animals at initial presentation
Animals that developed malignancies, whether spontaneously occurring or secondary to transplantation, demonstrated similar clinical presentations. On initial presentation, animals exhibited lethargy, pallor, decreased appetite, and weight loss. Laboratory analysis of animals was significant for elevated WBCs. Animals with lymphoma or PTLD had elevated WBC greater than 30 × 109/L (30 000 cells/μL), while those eventually diagnosed with leukemia typically had counts greater than 50 × 109/L (50 000 cells/μL). The peripheral blood of most animals with spontaneous lymphoma or PTLD showed elevated B-cell counts greater than 2000 cells/μL, with the presence of enlarged blastlike cells in the peripheral blood. By similar analysis of animals with leukemia, flow cytometry analysis was significant for the presence of enlarged blastlike cells in the peripheral blood. These cells were determined to be myeloid in origin based on positive surface staining of markers CD16 and CD172a. The animal that developed a carcinoma demonstrated similar clinical symptoms including fever, decreased appetite, and weight loss. This malignancy was histologically diagnosed as a nonspecific carcinoma obtained from the lung at necropsy.
Gross anatomic and histologic findings of primary tumors
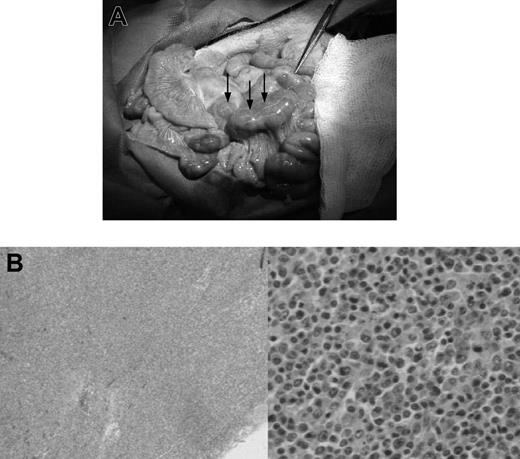
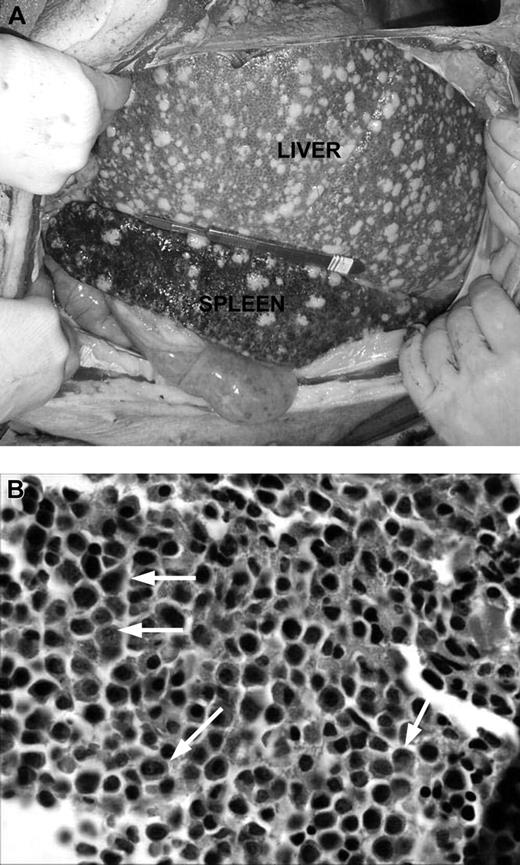
In animals with lymphoma or PTLD, mild hepatosplenomegaly and diffuse lymphadenopathy that included prominent enlargement of mesenteric lymph node chain were observed at autopsy (Figure 1A). This lymphadenopathy, in some cases, also included significantly enlarged lymph nodes in the neck and inguinal regions of the animal. Lymphoid tissues had histologic evidence of atypical cells and destruction of normal architecture (Figure 1B). Animals with leukemia had less significant lymphadenopathy and greater hepatosplenomegaly, with firmer and paler organs (Figure 2A). Lesions 0.5 to 1 cm in diameter were commonly found on the liver of animals with leukemia. Pleural effusions and ascites were not uncommon in these animals. There was also histologic evidence of diffuse infiltration consisting of large, pleiomorphic malignant cells containing heterochromatic nuclei. These cells were present throughout all vascularized tissues such as the kidney and the lung, and they were also prominent in the bone marrow (Figure 2B).
Gross pathologic and histologic findings of lymphoma (PTLD) in miniature swine. (A) Animals that developed lymphomas or PTLD typically had pronounced lymphadenopathy, as represented by animal 17018. Image was acquired using a Kodak camera (Eastman Kodak, Rochester, NY) model Easyshare Z740 with a 45.5-mm to 55-mm lens adapter. No further image processing was done. (B) Lymph node tissue harvested from these animals demonstrated destruction of normal architecture and predominance of abnormal cells as represented by animal 13271. Slides were viewed with an Olympus BX51 compound microscope (Olympus America, Melville, NY) of sections stained with hematoxylin and eosin (H&E; Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics, Fair Lawn, NJ; Eosin-Y, Richard-Allan Scientific, Kalamazoo, MI) using a lens at 40× (left) and 400× (right). Images were acquired using an Olympus digital microscope camera (Olympus America) model Q-Color 3, and were processed with Adobe Photoshop CS version 8 software (Adobe Systems, San Jose, CA).
Gross pathologic and histologic findings of lymphoma (PTLD) in miniature swine. (A) Animals that developed lymphomas or PTLD typically had pronounced lymphadenopathy, as represented by animal 17018. Image was acquired using a Kodak camera (Eastman Kodak, Rochester, NY) model Easyshare Z740 with a 45.5-mm to 55-mm lens adapter. No further image processing was done. (B) Lymph node tissue harvested from these animals demonstrated destruction of normal architecture and predominance of abnormal cells as represented by animal 13271. Slides were viewed with an Olympus BX51 compound microscope (Olympus America, Melville, NY) of sections stained with hematoxylin and eosin (H&E; Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics, Fair Lawn, NJ; Eosin-Y, Richard-Allan Scientific, Kalamazoo, MI) using a lens at 40× (left) and 400× (right). Images were acquired using an Olympus digital microscope camera (Olympus America) model Q-Color 3, and were processed with Adobe Photoshop CS version 8 software (Adobe Systems, San Jose, CA).
Gross pathologic and histologic findings of leukemia in miniature swine. (A) The most consistent findings of animals with leukemias were enlarged liver and spleen, which on palpation were firm and pale in color with visible lesions, as shown by animal 15549. Image was acquired using a Kodak camera (Eastman Kodak) model Easyshare Z740 with a 45.5-mm to 55-mm lens adapter. No further image processing was done. (B) Bone marrow from these animals was predominantly populated with abnormal cells, as represented by tissue from animal 14736. Slide was viewed with an Olympus BX51 compound microscope (Olympus America) of sections stained with H&E (Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics; Eosin-Y, Richard-Allan Scientific) using a lens at 400×. Image was acquired using an Olympus digital microscope camera (Olympus America) model Q-Color 3, and was processed with Adobe Photoshop CS version 8 software (Adobe Systems).
Gross pathologic and histologic findings of leukemia in miniature swine. (A) The most consistent findings of animals with leukemias were enlarged liver and spleen, which on palpation were firm and pale in color with visible lesions, as shown by animal 15549. Image was acquired using a Kodak camera (Eastman Kodak) model Easyshare Z740 with a 45.5-mm to 55-mm lens adapter. No further image processing was done. (B) Bone marrow from these animals was predominantly populated with abnormal cells, as represented by tissue from animal 14736. Slide was viewed with an Olympus BX51 compound microscope (Olympus America) of sections stained with H&E (Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics; Eosin-Y, Richard-Allan Scientific) using a lens at 400×. Image was acquired using an Olympus digital microscope camera (Olympus America) model Q-Color 3, and was processed with Adobe Photoshop CS version 8 software (Adobe Systems).
Establishment of tumor cell lines
Of the 19 cases of malignancy, tissues from 8 animals grew into tumor cell lines. Since 2 phenotypically distinct cell lines were derived from lymph node (LCL-17016L) and PBMCs (LCL-17016P) from animal 17016, these 8 cases allowed for the establishment of 9 distinct tumor cell lines. Characteristics of each line, including cell surface markers and doubling time, are summarized in Table 2, while in vitro growth patterns are shown in Figure 3. All established tumor cell lines express surface MHC class I (dull) and MHC class II in addition to the pertinent markers listed in the last column of Table 2. Each of the 5 lymphoma lines was established after obtaining primary tumors from involved lymph nodes and/or processed PBMCs and placing these cells in culture. Tumor lines were considered established once they were able to be passaged continuously in culture for a minimum period of 2 months prior to freezing down for storage. The leukemia lines were established from cultures of either processed bone marrow or processed PBMCs. Leukemia cell lines had positive staining for both myeloid markers CD16 and CD172a, which, similar to pattern of cell surface markers on the primary tumors, originally identified in diseased animals. Cell lines derived from lymphoma or PTLD lacked CD3 and CD5 found on T cells and myeloid markers such as CD16 and CD172a. One line, LCL-17018, gained expression of myeloid marker CD172a after in vitro culture. With this one exception, the pattern of cell surface markers was similar to the primary tissues from which the lines were derived.
Summary of established tumor cell lines
| Tumor line . | Doubling time, d . | Growth constant, k . | Pertinent markers* . |
|---|---|---|---|
| MML-12933 | 2.36 | 0.2931 | CD16, CD25, CD172a |
| LCL-13271 | 0.984 | 0.7042 | CD2, CD25, anti-mu heavy chain |
| ML-13381 | 1.65 | 0.4192 | CD25, CD172a, anti-mu heavy chain, anti-kappa light chain |
| CML-14736 | 2.295 | 0.302 | CD9, CD 16, CD25, CD172a |
| CML-15433 | 2.25 | 0.3078 | CD25, CD172a, anti-kappa light chain |
| LCL-15446 | 1.57 | 0.4416 | CD25, CD172a |
| LCL-17016L† | 1.99 | 0.3487 | CD25, anti-kappa light chain |
| LCL-17016P† | 0.52 | 1.324 | CD2, CD25, CD172a |
| LCL-17018 | 0.76 | 0.9128 | CD2, CD25, CD172a, anti-kappa light chain |
| Tumor line . | Doubling time, d . | Growth constant, k . | Pertinent markers* . |
|---|---|---|---|
| MML-12933 | 2.36 | 0.2931 | CD16, CD25, CD172a |
| LCL-13271 | 0.984 | 0.7042 | CD2, CD25, anti-mu heavy chain |
| ML-13381 | 1.65 | 0.4192 | CD25, CD172a, anti-mu heavy chain, anti-kappa light chain |
| CML-14736 | 2.295 | 0.302 | CD9, CD 16, CD25, CD172a |
| CML-15433 | 2.25 | 0.3078 | CD25, CD172a, anti-kappa light chain |
| LCL-15446 | 1.57 | 0.4416 | CD25, CD172a |
| LCL-17016L† | 1.99 | 0.3487 | CD25, anti-kappa light chain |
| LCL-17016P† | 0.52 | 1.324 | CD2, CD25, CD172a |
| LCL-17018 | 0.76 | 0.9128 | CD2, CD25, CD172a, anti-kappa light chain |
A total of 9 porcine tumor cell lines have been established from our MHC-defined miniature swine. Of these lines, 3 are derived from animals that developed spontaneous myeloid leukemias, while the remaining 6 lines were established from animals with lymphoma or posttransplantation lymphoproliferative disease (PTLD).
Growth constant, k, equals ln 2/T, where T is doubling time in days.
Full panel includes the following markers: negative IgG2a, MHC I, MHC II-DR, MHC II-DQ, CD1, CD2, CD3, CD4, CD5, CD8, CD9, CD16, CD21, CD25, CD172a, anti-mu heavy chain, and anti-kappa light chain. All tumor lines were found to be positive for MH.
LCL-17016L and LCL-17016P were both established from the same animal, but from different primary tissues. After growth in vitro, the cells derived from these tissues had different growth rates and surface marker patterns.
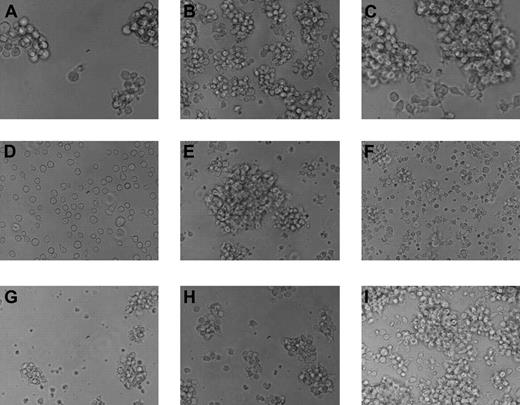
In vitro growth of tumor cell lines. The 9 tumor cell lines display various patterns of growth and sizes in vitro. Most preferentially grow in clusters, although one line, 14736, grows as a single-cell suspension. (A) MML-12933; (B) LCL-13271; (C) ML-13381; (D) CML-14736; (E) CML-15433; (F) LCL-15446; (G) LCL-17016L; (H) LCL-17016P; (I) LCL-17018. Slides were viewed with a Nikon Eclipse TE2000-U microscope (Nikon Instruments, Melville, NY) using Nikon Plan Fluor lenses at 40× in a cell culture media. Images were acquired using a NIkon Eclipse TE2000-U camera (Nikon Instruments) and were processed with Adobe Photoshop Elements version 3.0 software (Adobe Systems).
In vitro growth of tumor cell lines. The 9 tumor cell lines display various patterns of growth and sizes in vitro. Most preferentially grow in clusters, although one line, 14736, grows as a single-cell suspension. (A) MML-12933; (B) LCL-13271; (C) ML-13381; (D) CML-14736; (E) CML-15433; (F) LCL-15446; (G) LCL-17016L; (H) LCL-17016P; (I) LCL-17018. Slides were viewed with a Nikon Eclipse TE2000-U microscope (Nikon Instruments, Melville, NY) using Nikon Plan Fluor lenses at 40× in a cell culture media. Images were acquired using a NIkon Eclipse TE2000-U camera (Nikon Instruments) and were processed with Adobe Photoshop Elements version 3.0 software (Adobe Systems).
In vivo tumor growth after transfer into immunocompromised mice
To select variants with better in vivo growth potential, 3 tumor cell lines were initially tested for in vivo growth after transfer into NOD/SCID mice. The 3 tumor cell lines tested included LCL-13271, MML-12933, and CML-14736. Results of these experiments are summarized in Table 3. Of these lines, only LCL-13271 demonstrated growth in vivo after intraperitoneal injection into mice (Figure 4). This particular cell line was isolated from a SLAad animal, 13271, that developed recipient-derived PTLD after a hematopoietic cell transplantation.35 Similar to the phenotype of some human lymphoma cell lines,38 LCL-13271 had a stable phenotype after 72 passages based on continued expression of CD2 and CD25 while lacking other T-cell markers such as CD3 and CD5 and lacking myeloid markers such as CD16 and CD172.
Summary of in vivo tumor transfer in NOD/SCID mice and in histocompatible miniature swine
| Cell line/recipient animal species . | Outcome . |
|---|---|
| LCL-13271, NOD/SCID mice | Successful growth with serial transfer |
| MML-12933 | |
| NOD/SCID mice | No growth |
| Histocompatible miniature swine | No growth |
| CML-14736 | |
| NOD/SCID mice | No growth |
| Histocompatible miniature swine | Successful growth with serial transfer with irradiation before treatment |
| Cell line/recipient animal species . | Outcome . |
|---|---|
| LCL-13271, NOD/SCID mice | Successful growth with serial transfer |
| MML-12933 | |
| NOD/SCID mice | No growth |
| Histocompatible miniature swine | No growth |
| CML-14736 | |
| NOD/SCID mice | No growth |
| Histocompatible miniature swine | Successful growth with serial transfer with irradiation before treatment |
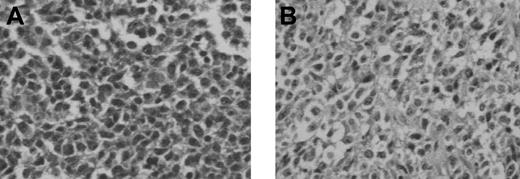
Histologic findings after in vivo transfer of LCL-13271 tumor cells in NOD/SCID mice. LCL-13271 injected intraperitoneally into NOD/SCID mice with tumor growth at 2 months in primary and secondary recipients. Abdominal tumor mass from NOD/SCID primary (A) and secondary (B) recipients of LCL-13271. Histologic findings were similar in morphology compared with the primary tumor (Huang et al35 ). Slides were viewed with an Olympus BX40 microscope (Olympus America) of sections stained with H&E medium (Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics; Eosin-Y, Richard-Allan Scientific) using a lens at 400×. Images were acquired using a Hitachi charge-coupled device color camera (Hitachi Kokusai Electric America, Woodbury, NY) model HV-C20 3-CCD, and were processed with ACDSee version 4.0 software (ACD Systems International, Victoria, BC).
Histologic findings after in vivo transfer of LCL-13271 tumor cells in NOD/SCID mice. LCL-13271 injected intraperitoneally into NOD/SCID mice with tumor growth at 2 months in primary and secondary recipients. Abdominal tumor mass from NOD/SCID primary (A) and secondary (B) recipients of LCL-13271. Histologic findings were similar in morphology compared with the primary tumor (Huang et al35 ). Slides were viewed with an Olympus BX40 microscope (Olympus America) of sections stained with H&E medium (Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics; Eosin-Y, Richard-Allan Scientific) using a lens at 400×. Images were acquired using a Hitachi charge-coupled device color camera (Hitachi Kokusai Electric America, Woodbury, NY) model HV-C20 3-CCD, and were processed with ACDSee version 4.0 software (ACD Systems International, Victoria, BC).
LCL-13271 cells were initially injected intraperitoneally into 4 NOD/SCID mice at a dose of 1.5 × 107 cells. After 2 months, animals had palpable masses that were harvested and evaluated by culture and histology. Abnormal cells with pleiomorphism and heterochromatic nuclei were found and were similar to what was seen in the primary tumor (Figure 4). Cell suspensions from these tissues were confirmed as LCL-13271 in origin based on expression of CD2 and on the lack of other T-cell and myeloid markers. For secondary transfer, these cells were then injected into 3 additional NOD/SCID mice at a dose of 5 × 107 cells, and these animals also grew palpable tumors. Tissues were harvested, processed, cultured, and frozen.
In vivo tumor growth after serial transfer in miniature swine
Our initial efforts of in vivo experiments in miniature swine focused on tumors and recipient animals from within the highly inbred histocompatible subline of SLAdd miniature swine. Although LCL-13271 demonstrated growth in immunocompromised mice, we did not test this particular line in miniature swine initially since it was derived from a SLAad animal. Two established cell lines from SLAdd inbred animals—CML-14736 and MML-12933—were expanded in culture and transplanted into miniature swine (Table 3). Of these 2 lines, only CML-14736 demonstrated in vivo growth after intravenous and subcutaneous injection into irradiated miniature swine (Figure 5). This particular line was established from the bone marrow cells from animal 14736 that was diagnosed with chronic myeloid leukemia (CML). CML-14736 has been maintained in culture for more than 20 passages and maintains the phenotype of the original tumor with surface expression of myeloid markers CD16 and CD172a and with a lack of T-cell markers CD3 and CD5.
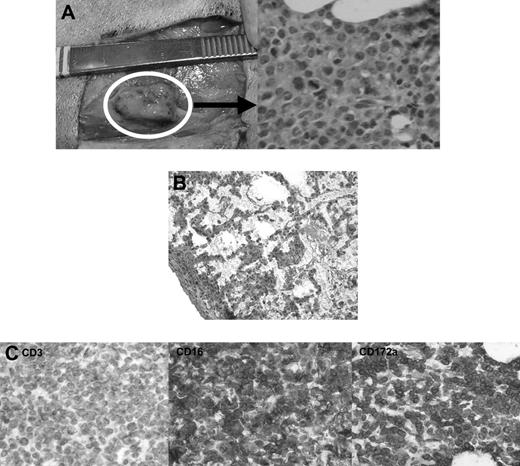
Histologic findings after in vivo transfer of CML-14736 tumor cells into miniature swine. CML-14736 grew after in vivo transfer to histocompatible miniature swine pretreated with TBI. Tumor growth was found at the subcutaneous injection sites (A) and in the lungs after intravenous administration (B). Immunohistochemistry of the subcutaneous injection site tissue negative staining for CD3, but positive staining for CD16 and CD172a, which was consistent with the surface phenotype of the primary tumor and cultured cells (C). Slides were veiwed with a Nikon Eclipse E800 microscope (Nikon Instruments) of sections stained with H&E (Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics; Eosin-Y, Richard-Allan Scientific) using Nikon Plan Fluor lenses at 400× (A right; C left, middle, right) and 200× (B). Images were acquired using Nikon HRD060-NIK 0.6X optical coupler diagnostic instruments (Nikon Instruments) connected to a computer with SPOT-diagnostic instruments and was processed with SPOT Advanced or Basic version 3.5.6 software for Windows (Diagnostic Instruments, Sterling Heights, MI).
Histologic findings after in vivo transfer of CML-14736 tumor cells into miniature swine. CML-14736 grew after in vivo transfer to histocompatible miniature swine pretreated with TBI. Tumor growth was found at the subcutaneous injection sites (A) and in the lungs after intravenous administration (B). Immunohistochemistry of the subcutaneous injection site tissue negative staining for CD3, but positive staining for CD16 and CD172a, which was consistent with the surface phenotype of the primary tumor and cultured cells (C). Slides were veiwed with a Nikon Eclipse E800 microscope (Nikon Instruments) of sections stained with H&E (Hematoxylin Gill's Formulation no. 2, Fisher Diagnostics; Eosin-Y, Richard-Allan Scientific) using Nikon Plan Fluor lenses at 400× (A right; C left, middle, right) and 200× (B). Images were acquired using Nikon HRD060-NIK 0.6X optical coupler diagnostic instruments (Nikon Instruments) connected to a computer with SPOT-diagnostic instruments and was processed with SPOT Advanced or Basic version 3.5.6 software for Windows (Diagnostic Instruments, Sterling Heights, MI).
CML-14736 did not grow in animals that received only CyA or were pretreated with 100 cGy or less TBI. Pretreatment with 500 cGy TBI allowed for growth after both intravenous and subcutaneous tumor administration, while 300 cGy TBI was sufficient for growth following subcutaneous growth (Table 4). All tumor growth occurred within 2 weeks after injection. Animals that grew tumors after receiving subcutaneous injections developed palpable masses at the injection site (Figure 5A). In one case of tumor growth following intravenous administration, abnormal malignant cells could be detected in the lung (Figure 5B). Tissue sampled from these masses revealed abnormal cells with pleiomorphism and heterochromatic nuclei, suggestive of a neoplastic process, and also appeared similar in morphology to cells found in the primary bone marrow tissue of animal no. 14736. These cells seemed to be actively dividing based on the presence of mitotic figures. There was a lack of a cellular infiltrate, suggesting that the recipient did not mount an immune response to these cells. Immunohistochemistry of these tissues revealed positive staining for myeloid markers CD16 and CD172a, with negative staining for T-cell marker CD3 (Figure 5C). These findings were consistent with the phenotype of cultured CML-14736 cells. Cell suspensions were made from these lesions and had a phenotype identical to the CML-14736 cultured cells. Secondary transfer by subcutaneous injection of these cells into an animal pretreated with 300 cGy TBI led to growth of a palpable mass injection site.
Summary of outcomes after in vivo transfer of CML-14736 into histocompatible miniature swine
| Animal . | Age, mo . | Coancestry . | Pretreatment . | Immunosuppression . | Tumor source . | Outcome . |
|---|---|---|---|---|---|---|
| 16354 (DD2, 10) | 3 | 0.94 | None | None | Cultured cells: IV, SC | No growth |
| 16354 (DD2, 10) | 3 | 0.94 | None | None | Primary tissue: SC | No growth |
| 16524 (DD4, G9) | 1.5 | 0.87 | 500 cGy TBI | None | Cultured cells: IV, SC | Tumor growth at SC sites and in lung |
| 16523 (DD4, G9) | 1.5 | 0.87 | 300 cGy TBI | None | Cultured cells: IV, SC | Tumor growth at SC sites only |
| 16526 (DD4, G9) | 3 | 0.87 | 300 cGy TBI | None | Cultured cells: IV, SC | Tumor growth at SC sites only |
| 16526 (DD4, G9) | 3 | 0.87 | 300 cGy TBI | None | Passaged cells: SC | Tumor growth |
| 16527 (DD4, G9) | 4 | 0.87 | 100 cGy TBI | None | Cultured cells: IV, SC | No growth |
| 16527 (DD4, G9) | 4 | 0.87 | 100 cGy TBI | None | Passaged cells: SC | No growth |
| 16525 (DD4, G9) | 2 | 0.87 | None | CyA (30 d) | Cultured cells: IV, SC | No growth |
| 16525 (DD4, G9) | 2 | 0.87 | None | CyA (30 d) | Passaged cells: SC | No growth |
| Animal . | Age, mo . | Coancestry . | Pretreatment . | Immunosuppression . | Tumor source . | Outcome . |
|---|---|---|---|---|---|---|
| 16354 (DD2, 10) | 3 | 0.94 | None | None | Cultured cells: IV, SC | No growth |
| 16354 (DD2, 10) | 3 | 0.94 | None | None | Primary tissue: SC | No growth |
| 16524 (DD4, G9) | 1.5 | 0.87 | 500 cGy TBI | None | Cultured cells: IV, SC | Tumor growth at SC sites and in lung |
| 16523 (DD4, G9) | 1.5 | 0.87 | 300 cGy TBI | None | Cultured cells: IV, SC | Tumor growth at SC sites only |
| 16526 (DD4, G9) | 3 | 0.87 | 300 cGy TBI | None | Cultured cells: IV, SC | Tumor growth at SC sites only |
| 16526 (DD4, G9) | 3 | 0.87 | 300 cGy TBI | None | Passaged cells: SC | Tumor growth |
| 16527 (DD4, G9) | 4 | 0.87 | 100 cGy TBI | None | Cultured cells: IV, SC | No growth |
| 16527 (DD4, G9) | 4 | 0.87 | 100 cGy TBI | None | Passaged cells: SC | No growth |
| 16525 (DD4, G9) | 2 | 0.87 | None | CyA (30 d) | Cultured cells: IV, SC | No growth |
| 16525 (DD4, G9) | 2 | 0.87 | None | CyA (30 d) | Passaged cells: SC | No growth |
IV indicates intravenous; and SC, subcutaenous.
Discussion
We have now identified and diagnosed 19 cases of malignancies in miniature swine, including 5 cases within animals used to develop histocompatible sublines. With the exception of one case, all were hematologic malignancies, either leukemias or lymphomas. The cases of lymphoma and leukemia were similar to human disease (P.S.C., Raimon Duran-Struuck, Shannon G. Moran, K.J.W., D.P.L., Michael Duggan, J.S.A., R.A.B., S.L.H., Susan V. Westmoreland, D.H.S., and C.A.H., manuscript in preparation).35,36 The incidence of malignancy after transplantation is high and restricted to certain haplotypes due to experimental protocols, and these results have been previously reported.35-37 Overall incidence of spontaneous malignancy in our herd is low, but appears to be slightly increased in the highly inbred SLAdd sublines. However, this difference is not statistically significant when considering all animals 24 months of age or older and may reflect our increased awareness and lower threshold for suspicion of malignancy. There may also be a predilection for disease within this group, therefore suggesting a possible genetic etiology.
These malignancies have allowed us to successfully establish 9 tumor cell lines, including 3 lines derived from highly inbred SLAdd animals. Our experience with tumor cell lines from these cases of malignancy is similar compared with the published experience of human tumor lines. Human cell lines are typically considered established after 6 months of continuous growth and demonstrate immunophenotypic markers similar to their primary tumors.39-41 For these porcine tumors, a minimum period of 2 months appeared to be sufficient to allow for in vitro tumor cell growth and establishment of a line. Although this is shorter than the time to establish human tumor lines, frozen aliquots of our porcine tumors from this stage in vitro could be thawed and be easily grown continuously in culture.
Similar to human tumors, porcine tumor cell lines could not be derived from all cases of malignancy. In published reports, success rates for establishment of cell lines derived from human primary leukemia and lymphomas range between 10% to 20%. Growing new tumor lines from hematologic malignancies is largely an unpredictable random process, though several reasons have been suggested to explain the failure of in vitro culturing.39-41 One explanation is the removal of these neoplastic cells from original environment. It has been suggested that this change may alter factors contributing to their growth, such as support from hematopoietic growth factors. In vitro cultures may lack these elements and lead to the death of neoplastic cells, while promoting overgrowth of nonneoplastic cells. This overgrowth of other types of cells was observed in our lymphoma cultures and may have prevented establishment of a cultured line. The removal from their native environments may also alter beneficial interactions, such as with supporting cells such as fibroblasts. In porcine leukemia lines, growth of a fibroblast-like layer was observed in cultured primary tissue and may have contributed to the establishment of some lines.
The tissue source of primary tumor cells may influence the culture of leukemia and lymphoma lines.40 Most human lines have reportedly been derived from ascites, processed peripheral blood, or bone marrow samples, rather than from solid tissues such as lymph nodes. This may partly be due to the limited access of these tissues in the clinic. The differences in culturing tissues may also be due to the clinical status of the patient at the time tumor tissue is obtained. For example, leukemia cell lines were more likely to be established when tissues were obtained from CML patients during a blast crisis when the tumor burden is greater and there is decreased immune surveillance.40 Our relative success in culturing porcine leukemia lines may be attributed to harvesting tissues during a blast crisis when clinical symptoms were prominent and prompted euthanizing the animal. In these instances, cell suspensions of processed blood and bone marrow may have been more homogeneous for abnormal cells. For tissues that did not grow into tumor lines, primary cell suspensions may have been heterogeneous containing both abnormal neoplastic cells and reactive T cells. This heterogeneity would reflect a lower tumor burden and possibly greater immune competence of the host animal.
Established porcine tumor cell lines have multiple applications, including a range of in vitro uses in investigating oncogenesis and in the development of biologic assays and tools.2,42,43 One particular application of interest of these lines is the development of preclinical large-animal transplantable tumor models using histocompatible miniature swine. Tumor transplantation in syngeneic rodent systems has been widely used for modeling cancers.44,45 Initial attempts of tumor transplantation using our cell lines in histocompatible miniature swine led to successful growth in cases where recipients were pretreated with irradiation. However, we have not yet achieved tumor growth without immunosuppression. One possible explanation is the pattern of surface marker expression. Markers such as MHC antigens can lead to rejection by the host immune system. Although this risk is minimized by matching of major and many minor histocompatibility loci, there may be other minor or unknown antigens present that can still be recognized as foreign by the host immune system.46-49 Conversely, a lack of antigens or surface markers may also contribute, as markers necessary for in vivo growth of our porcine tumor lines may be lost by in vitro culturing. With some murine tumors, the loss of surface sialoglycoproteins abolished the ability to transplant tumor cell lines that could previously be transferred within and across strains.50-52 Based on literature in murine systems, such changes in surface markers may be overcome by immediate direct, serial transfer of tumor cells between animals, while genetic differences may be circumvented using conditions favoring tumor growth, such as the dose of cells given to the host animal or the environment into which the cells are injected.40,47,48,53-57
Overall, our results indicate the feasibility of a large-animal transplantable tumor model using cells derived from spontaneous or nongenetically induced hematologic malignancies. Further optimization is necessary to achieve tumor growth in host animals without any preconditioning or concurrent therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by research funding from the following grants: JDRF 1–2005-72, American Cancer Society RSG-03–227-01-LIB, and NIH/NCI P01 CA111519–01A2.
We gratefully appreciate the contributions of Frank Dor, MD, Andrew Cameron, MD, PhD, Robert Cina, MD, Yasushi Fuchimoto, MD, and Zachary Gleit, MD, for aiding in the surveillance and identification of experimental animals with malignancy. We also thank Susan Westmoreland, DVM, for her expertise in veterinary pathology and Elena Rakhlin, MD, for her assistance in the harvesting of primary tissues of affected animals, as well as Henry Winn, PhD, and John Hanekamp, MD, PhD, for their critical review of this paper. We appreciate Maria Doherty and Annette Sugrue for their assistance in the preparation of this paper.
National Institutes of Health
Authorship
Contribution: P.S.C., Y.-G.Y., D.H.S., and C.A.H. designed research; P.S.C., D.P.L., K.J.W., H.C.R., J.G.G., R.C.C., I.M.M., J.S.A., R.A.B., S.L.H., and A.S. performed research and collected data; P.S.C. and C.A.H. analyzed data; P.S.C., D.H.S., and C.A.H. prepared the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christene A. Huang, Transplantation Biology Research Center, Massachusetts General Hospital, 149-9019 13th St, Boston, MA 02129; e-mail: huangc@helix.mgh.harvard.edu.