Abstract
Erythropoietic protoporphyria (EPP) results from deficiency of ferrochelatase (FECH). Accumulation of protoporphyrin IX causes life-long acute photosensitivity. Microcytic anemia occurs in 20% to 60% of patients. We investigated 178 patients with dominant EPP confirmed by molecular analysis. Erythropoiesis was impaired in all patients; all had a downward shift in hemoglobin (Hb), and the mean decreased in males by 12 g/L (1.2 g/dL). By World Health Organization criteria, 48% of women and 33% of men were anemic. Iron stores, assessed by serum ferritin (sFn), were decreased by two-thirds, but normal serum soluble transferrin receptor-1 and iron concentrations suggested that erythropoiesis was not limited by iron supply. FECH deficiency in EPP appears to lead to a steady state in which decreased erythropoiesis is matched by reduced iron absorption and supply. This response may in part be mediated by protoporphyrin, but we found no correlation between erythrocyte protoporphyrin and Hb, sFn, total iron-binding capacity, or transferrin saturation.
Introduction
Erythropoietic protoporphyria (EPP, MIM 177000) is an inherited disorder caused by partial deficiency of ferrochelatase (FECH; EC 4.99.1.1) that catalyzes the chelation of ferrous iron by protoporphyrin IX. FECH deficiency leads to accumulation of protoporphyrin in normoblasts, erythrocytes, plasma, skin, and liver, causing lifelong acute photosensitivity and, in approximately 2% of patients, severe liver disease.1,2
Microcytic anemia occurs in 20% to 60% of patients.3-6 In contrast to other inherited disorders of erythroid heme biosynthesis,7 the anemia is not dyserythropoietic, there is no iron overload, and there is evidence for iron deficiency5,6,8,9 without iron loss.9 A mouse model of EPP, the homozygous Fechm1Pas mutant, develops a similar microcytic anemia.10,11 Although it is probable that the anemia of EPP reflects limitation of heme formation by FECH deficiency, its incidence, mechanism, and relationship to disordered iron metabolism remain unclear.
Patients and methods
Patients and control subjects
Blood samples were obtained from 210 patients with EPP during a cross-sectional study of EPP in the United Kingdom.12 One hundred ninety-two patients had one FECH mutation with 1 or 2 FECH IVS3–48C alleles and were classified as dominant EPP (dEPP)13 ; 14 of these were excluded because they had diseases likely to affect iron metabolism. Ethical approval was obtained from the North West Multicentre Research Ethics Committee and 84 local research ethics committees. Informed consent was obtained in accordance with the Declaration of Helsinki from all patients or their parents.
Hematologic and biochemical measurements
All analyses were carried out in the same laboratory. Serum iron (sFe),14 total iron-binding capacity (TIBC),14 serum ferritin (sFn, Elecsys 2010; Roche Diagnostics, Indianapolis, IN), soluble transferrin receptor-1 (sTfR; R&D Systems, Abingdon, United Kingdom), and erythrocyte protoporphyrin15 were determined as described. Other measurements were by standard automated methods. Data obtained previously for 611 male first-time blood donors were used for comparisons.16
Statistical methods
Results were expressed as mean plus or minus a standard deviation (SD) for normally distributed data and median and range for data (sFn, protoporphyrin) with a log-normal distribution. Differences between quantitative variables were assessed by the Mann-Whitney test. Spearman rank correlation (rs) was used to test the significance of relationships between pairs of variables and the chi-square test for differences between proportions.
Results and discussion
Red cell indices
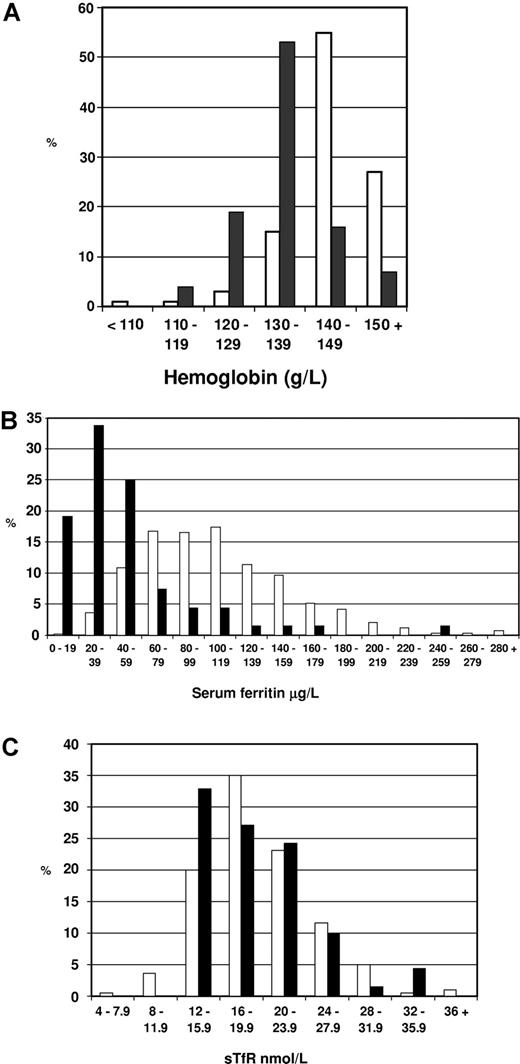
By World Health Organization criteria, 73 (41%; 95% confidence interval: 34%-48%) of our patients with dEPP were anemic. All had a mild microcytic, hypochromic anemia; 48% of females and 33% of males were affected. The anemic patients did not form a separate subgroup. In both sexes, hemoglobin (Hb; females: 119 ± 10 g/L [11.9 ± 1.0 g/dL]; males: 133 ± 10 g/L [13.3 ± 1.0 g/dL]), mean cell volume (MCV), and mean corpuscular hemoglobin (MCH) were normally distributed with a shift in their means toward lower values (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article); the mean Hb for males was 12 g/L (1.2 g/L) lower than in the general population (Figure 1A). A similar shift has been noted in Dutch EPP patients.19 This downward shift in Hb leads to some patients falling within the definition of anemia; part of the wide variation in reported incidences can be explained by use of different definitions.2-6,9 Erythrocyte protoporphyrin concentrations (females: 21.9 μM [range, 4.1-75.3 μM]; males: 25.5 μM [range, 8.9-77.3 μM]) showed no correlation with Hb.
Hemoglobin, serum ferritin, and serum soluble transferrin receptor-1 concentrations in male patients with dominant EPP. (A). Hemoglobin concentrations in 66 male patients with dEPP aged 16 years or older (■) and in a sample of 5206 men aged 16 years or older from the English population17 (□). (B) Serum ferritin concentrations in 66 male patients with dEPP aged 16 years or older (■) and in 612 male first-time blood donors from south Wales aged 17 to 62 years16 (□). (C) Serum soluble transferrin receptor-1 concentrations in 61 male patients with dEPP aged 16 years or older (18.6 ± 5.1 nM) (■) and in 225 hematologically healthy male and female subjects from the United States aged 17 to 97 years18 (□) assayed using the same method.
Hemoglobin, serum ferritin, and serum soluble transferrin receptor-1 concentrations in male patients with dominant EPP. (A). Hemoglobin concentrations in 66 male patients with dEPP aged 16 years or older (■) and in a sample of 5206 men aged 16 years or older from the English population17 (□). (B) Serum ferritin concentrations in 66 male patients with dEPP aged 16 years or older (■) and in 612 male first-time blood donors from south Wales aged 17 to 62 years16 (□). (C) Serum soluble transferrin receptor-1 concentrations in 61 male patients with dEPP aged 16 years or older (18.6 ± 5.1 nM) (■) and in 225 hematologically healthy male and female subjects from the United States aged 17 to 97 years18 (□) assayed using the same method.
FECH activity in dEPP is approximately 35% of normal.13 Our data show that this decrease is sufficient to produce in all patients a mild defect of erythropoiesis that impairs hemoglobinization. Defective erythropoiesis persists throughout life and our findings (Table S1 legend) and previous reports8,20 suggest that it may not be corrected by oral iron unless there is evidence of coexisting iron loss.
Iron status
Both sexes showed evidence of iron depletion (Table S2). Differences in sFn and transferrin saturation (TS) between women and men suggested that more of the former had iron depletion due to iron loss in addition to abnormalities caused by EPP. Therefore, we restricted detailed analysis of iron indices to the 67 male patients (Hb, 135 ± 9 g/L [13.5 ± 0.9 g/dL]) older than 15 years who had never received iron supplements.
The main abnormality was a marked shift in sFn toward lower values (Figure 1B; Table 1); sFn correlated with Hb (rs = 0.415; P < .001). Because protoporphyrin is hepatotoxic and accumulates in the liver in EPP, and liver cell damage may increase sFn, we assessed liver cell function by measuring liver enzymes. One or more of these was increased in 17 (25%) patients; sFn correlated with γ-glutamyl transpeptidase (rs = 0.507; P < .001) and alanine aminotransferase (rs = 0.392; P < .001) but not with aspartate aminotransferase. Since sFn correlates with mobilizable iron stores,21 the downward shift in sFn by approximately two-thirds (Figure 1B; Table 1) suggests that iron stores in dEPP are decreased to a similar extent or a little more if the effect of liver dysfunction is taken into account. Turnbull et al8 found that storage iron, determined by venesection, was less than 250 mg in 3 patients; otherwise, quantitative measurements of tissue iron have not been reported in EPP. However, in contrast to iron deficiency due to iron loss, stainable iron is present in erythroblasts.9
Comparison of indicators of iron status in male patients with dEPP and male first-time blood donors
| Serum iron indices . | EPP patients, n = 67 . | First-time blood donors, n = 611 . |
|---|---|---|
| sFe, μM | 15.1 ± 6.6* | 16.7 ± 6.0 |
| TIBC, μM | 63.0 ± 6.9† | 54.5 ± 10.0 |
| TS, % | 23.9 ± 10.3† | 31.1 ± 10.9 |
| sFn, μg/L | 37 (10-119)† | 101 (35-220) |
| Serum iron indices . | EPP patients, n = 67 . | First-time blood donors, n = 611 . |
|---|---|---|
| sFe, μM | 15.1 ± 6.6* | 16.7 ± 6.0 |
| TIBC, μM | 63.0 ± 6.9† | 54.5 ± 10.0 |
| TS, % | 23.9 ± 10.3† | 31.1 ± 10.9 |
| sFn, μg/L | 37 (10-119)† | 101 (35-220) |
Values are means plus or minus SD, except for sFn, which are medians and 95% ranges. EPP patients are males aged 16 to 77 years who have never been prescribed iron supplements. Blood donors are male first-time donors aged 17 to 62 years from South Wales15 ; samples for analysis were obtained prior to first donation. Only TIBC showed any correlation with sFn (rs = −0.412, P < .001).
P value is not significant.
P < .001 compared with donors.
In homozygous Fechm1Pas mice, total body iron is normal but iron is redistributed from peripheral tissues to an enlarged hematopoietic spleen.11 Although these mice have liver disease, lower FECH activity and more severe anemia,11 it seems unlikely that FECH deficiency limits erythropoiesis and disturbs iron metabolism by different mechanisms in the 2 species. The anomalous observation in EPP of accumulation of iron in erythroblasts9 suggests that there may also be redistribution of iron stores toward the site of erythropoiesis in EPP. Thus, in both species, FECH deficiency appears to provoke a response that leads to accumulation of protoporphyrin IX but prevents accumulation of the other, more toxic, substrate iron.
A second notable feature of iron depletion in dEPP is our finding that sFe (Table 1) and sTfR (18.6 ± 5.1 nM; Figure 1C) concentrations are normal. The normal sTfR in our patients is consistent with the degree of depletion of iron stores indicated by sFn and, together with the normal sFe, suggests that erythropoiesis is not limited by iron supply.22 This indicates that the reduction in iron stores has not led to iron-deficient erythropoiesis. Furthermore, the rate of erythropoiesis is not increased as this would also increase sTfR levels. These findings suggest FECH deficiency in dEPP leads to the establishment of a steady state in which iron absorption and supply is diminished but matches the requirement for reduced erythropoiesis.
The mechanism of these changes in iron metabolism has not been established. Iron metabolism is also altered in griseofulvin-induced protoporphyria.23 Because serum transferrin is increased in Fechm1Pas BALB/c mice and correlates with erythrocyte protoporphyrin concentration, it has been suggested that protoporphyrin may act as a signal to increase hepatic transferrin synthesis when iron supply to erythroid cells is insufficient and thus modulate iron metabolism.11 We found only a slight increase in TIBC (Table 1) and no correlation with erythrocyte protoporphyrin. Alternatively, FECH deficiency within enterocytes might affect duodenal iron transport by altering enterocyte mitochondrial iron status.24
Finally, measurement of sTfR, in addition to sFn, may help to distinguish those patients in whom the anemia of EPP is exacerbated by iron loss and who might benefit from iron replacement.25
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the British Skin Foundation and the Royal College of Physicians (Lewis Thomas Gibbon Jenkins of Britton Ferry Memorial Trust) and the Royal Gwent Hospital Dermatology Research Fund.
We thank all the physicians who helped with this study and allowed access to their patients; Dr Sharon D. Whatley for molecular analyses; Richard Ellis, Ms Nicola Mason, and Ms Jacqueline Woolf for expert laboratory assistance; and Ms Sonia van Lierop for secretarial assistance.
Authorship
Contribution: S.A.H. collected the clinical data and patient samples; M.W. and M.N.B. supervised laboratory analyses; A.V.A. supervised patient contact and clinical aspects; G.H.E. and M.W. wrote the paper; and all authors participated in the design of the research and checked the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. N. Badminton, Department of Medical Biochemistry and Immunology, Cardiff University, Heath Park, Cardiff CF14 4XN, United Kingdom; e-mail: badmintonmn@cardiff.ac.uk.