In healthy carriers of human cytomegalovirus (HCMV), the virus-specific memory CD8+ T-cell population is often dominated by CD28− CD45RAhi cells that exhibit direct ex vivo cytotoxicity but whose capacity for proliferation and generation of further memory cells has been questioned. We show that when highly purified CD28− CD45RAhi CD8+ T cells are stimulated with viral peptide presented by autologous monocytes, the virus-specific T cells show early up-regulation of CD137 (4–1BB) and CD278 (ICOS), re-express CD28, and proliferate with similarly high cloning efficiency in limiting dilution analysis as CD28+ CD45ROhi cells or CD28− CD45ROhi cells. Using peptide-pulsed autologous fibroblasts transfected with individual costimulatory ligands as antigen presenting cells, we showed CD137L to be a key costimulatory ligand for proliferation of CD28− CD45RAhi CD8+ T cells and not CD80, CD86, or CD275 (ICOSL). Therefore, CD28− CD45RAhi CD8+ T cells were not terminally differentiated but required a specific costimulatory signal for proliferation.
Introduction
Understanding the heterogeneity of human antigen-experienced CD8+ T cells has advanced by studying combinations of different phenotypic cell surface molecules. Sallusto et al1 subdivided human antigen-experienced CD8+ T cells on the basis of chemokine receptor CCR7 expression into CCR7+ “central memory” cells capable of homing to secondary lymphoid organs and CCR7− “effector memory” cells. The low molecular weight isoform of the common leukocyte antigen CD45RO2 is expressed by antigen-experienced CD28+ CD45ROhi cells, most of which express CCR7, and by CD28−CD45ROhi cells, most of which lack CCR7. The high molecular weight isoform CD45RA is expressed by naive CD28+ CD45RAhi cells that express CCR7. CD28− CD45RAhi cells have been termed “effector memory RA” (TEMRA) or “revertant memory” cells because of their re-expression of CD45RA.3,–5 On the basis of expression of costimulatory molecules CD28 and CD27, human antigen-experienced CD8+ T cells have also been subdivided into early CD28+ CD27+ cells (most of which express CD45RO and CCR7), intermediate CD28− CD27+ and late CD28− CD27− cells (most of which lack CCR7). In association with different human persistent virus infections, the phenotypes of circulating virus-specific CD8+ T cells in long-term virus carriers vary according to the virus.6,7 It appears that the phenotype of virus-specific CD8+ T cells may be associated with the site(s) in which virus antigen is expressed. Thus, in carriers of Epstein-Barr virus (EBV), CD8+ T cells directed against the EBV latency-associated antigens expressed in infected B cells are predominantly CD28+ CD45ROhi cells, whereas CD8+ T cells directed against EBV lytic antigens expressed in pharyngeal epithelial cells are predominantly CD28− CD45RAhi cells.8
The β herpesvirus human cytomegalovirus (HCMV) establishes life-long infection with latency in cells of the myeloid lineage.9 The CD8+ T-cell response to HCMV in immunocompetent subjects is usually strong and can constitute up to 10% of an individual subject's antigen-experienced compartment.10 We have previously shown that during primary HCMV infection, the highly activated HCMV-specific CD8+ T cells in peripheral blood are predominantly CD28− CD45ROhi. During convalescence, circulating populations of HCMV-specific CD28+ CD45ROhi cells and CD28− CD45RAhi cells emerge and accumulate in the peripheral blood.4 In long-term virus carriers, the CD8+ T-cell response against a defined viral peptide is often dominated by a relatively small number of individual clones that have undergone massive expansion in vivo and that are maintained for years.5 Cells of a single expanded HCMV-specific clone are usually distributed in both CD28+ CD45ROhi cells and in CD28− CD45RAhi cells5 ; in many virus carriers, the response against a viral peptide is dominated by CD28− CD45RAhi cells.4,5 These HCMV-specific CD28− CD45RAhi T cells are heterogeneous for the expression of CD274 and as such can be viewed as a mixture of intermediate and late stage antigen experienced CD8+ T cells as defined by Appay et al.6
Antigen-experienced CD45RAhi CD8+ T cells have some characteristics of effector cells, including direct ex vivo antigen-specific cytotoxicity, intracellular perforin expression, and prompt secretion of interferon γ (IFNγ) after antigen recognition.11,–13 However, the capacity of antigen-experienced CD45RAhi CD8+ T cells to proliferate in vitro has been questioned by some investigators. When stimulated in vitro with monoclonal anti-CD3 antibody plus anti-CD28 antibody or allogeneic dendritic cells, CCR7− CD45RAhi CD8+ T cells showed reduced proliferation.14,15 In contrast, when stimulated in vitro with specific viral peptide presented by autologous peripheral blood mononuclear cells (PBMCs) in the presence of exogenous interleukin-2 (IL-2), highly purified CCR7− CD28− CD45RAhi CD8+ T cells or CCR7− CD27− CD45RAhi CD8+ T cells proliferated strongly.4,16 Because of their reduced proliferation under some but not all experimental conditions, our hypothesis was that CD28− CD45RAhi CD8+ T cells might have a strict requirement for specific costimulation after stimulation with antigen. The CD28 costimulation pathway was the first to be described and is considered to be the most important for activation of naive T cells.17 Several alternative costimulatory pathways belonging to the CD28/B7 family18 and TNF/TNF receptor (TNFR) family19 have subsequently been identified. Costimulation through CD137:CD137L has been demonstrated to promote proliferation of CD8+ CD28− memory T cells when administered with anti-CD3 stimulation20 and is an important costimulator for human anti-viral CD8 T cells,21 which is more potent than other costimulators such as OX40L and CD8022 ; so far, however, the costimulatory requirement(s) of antigen-specific CD28− CD45RAhi CD8+ T cells after antigen exposure remains unclear. This is an important question, bearing on whether the assumption of end-stage differentiation, and the designation of this population as “effector memory,” is fully justified. Therefore in highly purified populations of CD28− CD45RAhi CD8+ T cells, we analyzed the antigen-stimulated cloning efficiency, kinetics of costimulatory molecule expression, and proliferative response to antigen-expressing autologous fibroblasts that expressed individual costimulatory ligands.
Materials and methods
Ethical approval was obtained from the Addenbrookes National Health Service Hospital Trust institutional review board (Cambridge Research Ethics Committee) for this study. Informed consent was obtained from all recipients in accordance with the Declaration of Helsinki (LREC 97/092).
Donors
Ten healthy, HCMV-seropositive, long-term virus carriers were studied. Serostatus was determined with an IgG enzyme-linked immunosorbent assay (Trinity Biotech, Didcot, United Kingdom).
Peptides and MHC class I multimers
Peptides from the HCMV tegument protein pp65 (Proimmune, Oxford, United Kingdom) were used to stimulate virus-specific CD8+ T cells.23 NLVPMVATV presented by HLA-A2 and TPRVTGGGAM presented by HLA-B7 were diluted in unsupplemented RPMI 1640 medium (Sigma, Dorset, UK) and used at the dilutions indicated in the Figures. MHC class I multimers, either tetramers or pentamers, of NLVPMVATV plus HLA-A2 or TPRVTGGGAM plus HLA-B7 (from Proimmune or a kind gift of Prof Paul Klenerman, University of Oxford) were used to identify peptide-specific CD8+ T cells.
Cell purification
PBMC were prepared from fresh heparinized venous blood samples by density-gradient centrifugation (Lymphoprep; Nyegaard, Oslo, Norway). Cells were stained with allophycocyanin (APC)-tagged Alexa Fluor 750 (Invitrogen, Carlsbad, CA)–conjugated anti-CD8, fluorescein isothiocyanate (FITC)-conjugated anti-CD45RO and PE-conjugated anti-CD28 (Invitrogen) and sorted using a FACSVantage SE cell sorter (BD Biosciences, Oxford, United Kingdom) into subpopulations of CD28+ CD45ROhi, CD28− CD45ROhi, and CD28− CD45RO− (CD45RAhi) cells. In some experiments, CD8+ CD28− CD45RO− cells were depleted of CD27+ cells by restaining with phycoerythrin (PE)-conjugated anti-CD27 (eBioscience, San Diego, CA) and resorting. After sorting, the purity of cell populations was checked on a FACSCalibur flow cytometer (BD Biosciences); populations were consistently more than 99% pure. In some experiments, CD14+ monocytes were purified from PBMCs by directly conjugated anti-CD14 magnetic microbeads and magnetic cell separation (MACS) columns (both from Miltenyi Biotec, Surrey, United Kingdom) according to the manufacturer's instructions. Purity of the monocyte preparation was determined using a FITC-conjugated anti-CD14 monoclonal antibody (Invitrogen). In blocking antibody experiments, PBMCs were depleted of CD28+ cells by staining with PE-conjugated anti-CD28 and anti-CD16 followed by anti-PE magnetic microbeads and MACS columns.
Stimulation of CD8+ T cells with HCMV peptide
Sorted CD8+ T-cell populations were cultured in 96-well round-bottomed plates with irradiated autologous stimulator cells (50 000 monocytes or PBMCs per well or 5000 fibroblasts per well) that had been pulsed with viral peptide (40 μg/mL unless indicated) and repeatedly washed. Culture was in 100 μL/well RPMI 1640 medium supplemented with 2 mmol/L l-glutamine, 105 IU/L penicillin, 100 mg/L streptomycin, 5 IU/mL human recombinant IL-2 (National Institute for Biological Standards and Control, Potters Bar, United Kingdom), 10% fetal bovine serum certified (Invitrogen), plus either 10% human AB serum (HCMV seronegative; Blood Transfusion Service, Addenbrooke's Hospital, Cambridge, United Kingdom) or 10% autologous heat-inactivated serum. Cultures were incubated at 37°C in 5% CO2 and every 5 days, wells were refed with 50 μL of fresh medium containing supplements.
Limiting dilution analysis
The method was as published in detail previously.23 In brief, replicate microcultures (n = 27) of purified responder CD8+ T-cell populations were set up in 96-well round-bottomed plates in which the number of responder T cells per well was progressively reduced over an appropriate range of dilutions. Irradiated autologous peptide-pulsed PBMC (50 000 per well) were used as stimulator cells, in culture medium described in “Stimulation of CD8+ T cells with HCMV peptide.” On day 14, the cells in each well were mixed and divided into 3 aliquots, which were assayed for peptide-specific cytotoxicity in 4-hour 51Cr release assays against autologous or MHC mismatched lymphoblastoid B cells (4000 per well) pulsed with viral peptide or unpulsed. Analysis of cytotoxicity assays was as published previously.5 Aliquots of input CD8+ T-cell populations were stained with APC-conjugated peptide-MHC multimers (Proimmune) and anti-CD8 followed by flow cytometry, to quantify the input peptide-specific cells per well.
CD8+ cell phenotype analysis
After peptide stimulation, cells were removed from culture at intervals for flow cytometry. Cells were stained with peridinin chlorophyll α protein (PerCP)-conjugated anti-CD8, APC-conjugated peptide-major histocompatibility complex (MHC) multimers (Proimmune) to identify antigen-specific cells, and antibodies against CD28, CD45RA, CD45RO, and CD62L (from Invitrogen), inducible costimulator ICOS (CD278), CD27 (from eBiosciences), CD137 (4–1BB), CXCR3, CCR5 (from BD Biosciences), CCR7 (R&D Systems, Abingdon, United Kingdom) conjugated to FITC or PE, using FITC- or PE-conjugated isotype controls (Invitrogen, BD Biosciences, respectively). KLRG1-Alex488 was provided by Professor Hanspeter Pircher (University of Freiburg, Freiburg, Germany). Staining was carried out in 50 μL of PBS with 2% normal mouse serum, and cells were washed in PBS and resuspended in PBS with 2% paraformaldehyde before analysis using a FACSCalibur flow cytometer running Cellquest software (BD Biosciences). Data were analyzed using WinMDI 2.8 software (http://pingu.salk.edu/software.html).
Blocking antibody experiments
Unconjugated monoclonal antibodies against CD4, CD80, CD86 (from Caltag), CD275 (ICOSL) (from eBioscience), and CD137L (4–1BBL) (MBL, Nagoya, Japan) were dialyzed to remove sodium azide using Slide-a-Lyzer kits (Pierce, Rockford, IL) according to the manufacturer's instructions. In brief, a 3500 molecular weight cutoff cassette was prehydrated for 2 minutes in PBS, 0.5 mL of antibody was injected, and dialysis was performed for 12 hours at 4°C before the antibody was removed. Twenty milligrams of dialyzed antibody was added to cultures of peptide-pulsed PBMC, which were irradiated and plated at 20 000 cells per well, to which CD28−-depleted responder PBMCs 10 000 per well were added in supplemented medium. Responder cell proliferation was determined by cell counting.
Plasmids
CD137L (4–1BBL)-pcDNA324 was provided by Tania Watts (University of Toronto, Toronto, ON, Canada) and CD275 (ICOSL)-pcDNA3.1 by Lieping Chen (The Johns Hopkins School of Medicine, Baltimore, MD). CD80 pHR′CMV was constructed by cloning full-length CD80 into the pHR′CMV vector. CD86-pcDNA3.1 was provided by Jessica Boname and Paul Lehner (Cambridge Institute for Medical Research, Cambridge, United Kingdom).
Transfection of primary fibroblast lines
Autologous donor fibroblasts obtained by skin biopsy were maintained in Earle minimal essential medium (Sigma) plus 10% fetal calf serum. Fibroblasts were transfected using a Nucleofector kit (Amaxa, Cologne, Germany) according to the manufacturer's instructions. In brief, 106 trypsin-detached fibroblasts were combined with 5 μg of DNA in 100 μL of nucleofector solution and electroporated using nucleofector program U23. Cells were transferred to warm medium overnight. Cells were then detached using cell dissociation solution (Sigma) and pulsed with different concentrations of viral peptide, irradiated, washed, and plated as antigen-presenting cells at 5000 cells per well with purified CD28− CD45RAhi responder CD8+ T cells, 10 000 per well, in supplemented medium. Expression of costimulatory molecules on transfected fibroblasts was determined by flow cytometry using PE-conjugated anti-CD80, CD86, CD275, and CD137L (all BD Biosciences), compared with isotype controls. Control antigen-presenting cells were irradiated autologous peptide-pulsed monocytes. After 10 days in culture, cells in duplicate wells were counted, and the peptide-specific cells were quantified by staining with multimer and anti-CD8 followed by flow cytometry to determine the total number of antigen-specific cells per well.
Results
In response to peptide stimulation by autologous PBMC, HCMV-specific CD28− CD45RAhi CD8+ cells proliferated with high cloning efficiency similar to that of CD28+ CD45ROhi cells or CD28− CD45ROhi cells
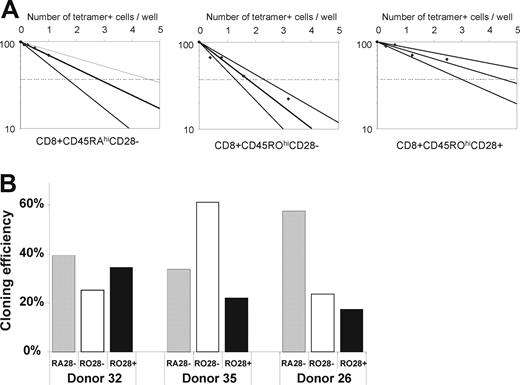
The cloning efficiency of highly purified CD28− CD45RAhi cells for proliferation and generation of cytotoxic daughter cells was assessed in limiting dilution analysis upon stimulation with irradiated autologous peptide-pulsed PBMC compared with CD28+ CD45ROhi cells and CD28− CD45ROhi cells of the same subject. The proportion of peptide-specific CD8+ T cells in each input responder population was first quantified by peptide-MHC class I multimer staining using HLA-A2-NLV-pp65 or HLA B7-TPR-pp65. The cloning efficiency was similar in each of the sorted subpopulations and was in the range of 20% to 60% (Figure 1), the same as the cloning efficiency previously reported for the CD8+ T-cell population as a whole when stimulated with irradiated peptide-pulsed autologous PBMC.25 In limiting-dilution assay plates at the lowest input cell dilutions, in which on average there was one multimer-positive cell per well at the start of culture, there were 88 000-380 000 antigen-specific cells per well; similar numbers of antigen-specific cells were observed in wells plated with CD28−CD45RAhi cells or CD28+ CD45ROhi cells. Without adjustment for cell death in vitro, this indicated that a single antigen-specific cell from each subpopulation had undergone at least 16 divisions. After further depletion of CD27+ cells, the cloning efficiency of the CD28− CD27− CD45RAhi subpopulation was closely similar to that of the CD28− CD45RAhi population (data not shown). The inhibitory Killer Cell Lectin-like receptor G1 (KLRG1) has previously been suggested to identify CD8+ T cells that have reduced proliferative response to PHA or anti-CD3 stimulation.26 In 2 HCMV-seropositive donors, almost all (> 95%) of the multimer-positive CD8+ T cells in PBMC expressed KLRG1 (data not shown). When stimulated with peptide antigen, these KLRG1+ virus-specific cells showed no apparent proliferation defect.
Cloning efficiency of sorted subpopulations of HCMV-specific CD8+ T cells in limiting dilution analysis. (A) Limiting dilution analysis of sorted CD28−CD45RO− cells, CD28−CD45ROhi cells, and CD28+ CD45ROhi cells (donor 35). The initial number of multimer-positive cells per well was calculated from the initial number of cells per well multiplied by the initial proportion of multimer-positive cells in each subpopulation determined by flow cytometry. (B) Cloning efficiency of each subpopulation in 3 healthy HCMV-seropositive donors; CD28−CD45RO− cells ( ), CD28−CD45ROhi cells (
), CD28−CD45ROhi cells ( ) and CD28+ CD45ROhi cells (
) and CD28+ CD45ROhi cells ( ).
).
Cloning efficiency of sorted subpopulations of HCMV-specific CD8+ T cells in limiting dilution analysis. (A) Limiting dilution analysis of sorted CD28−CD45RO− cells, CD28−CD45ROhi cells, and CD28+ CD45ROhi cells (donor 35). The initial number of multimer-positive cells per well was calculated from the initial number of cells per well multiplied by the initial proportion of multimer-positive cells in each subpopulation determined by flow cytometry. (B) Cloning efficiency of each subpopulation in 3 healthy HCMV-seropositive donors; CD28−CD45RO− cells ( ), CD28−CD45ROhi cells (
), CD28−CD45ROhi cells ( ) and CD28+ CD45ROhi cells (
) and CD28+ CD45ROhi cells ( ).
).
In response to peptide stimulation, virus-specific CD28− CD45RAhi cells up-regulated CD137 (4–1BB) and CD278 (ICOS) and up-regulated homing chemokine receptors
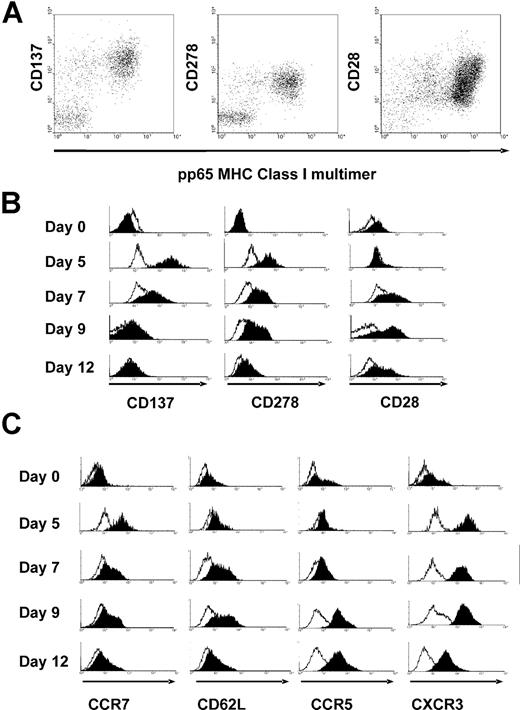
Cultures of CD28− CD45RAhi cells were harvested at intervals after stimulation with peptide-pulsed monocytes, and stained with multimers to identify antigen-specific cells and antibodies against the costimulatory molecules CD137 or CD278 or CD28 (Figure 2). Before stimulation, CD28− CD45RAhi cells did not express CD137 or CD278 or CD28. As early as day 2 in culture, CD137 was strongly expressed on a small percentage of the responder CD8+ T cells; simultaneous costaining with multimer was inconclusive due to TCR down-regulation after peptide stimulation (data not shown). By day 5 in culture, CD137 was strongly expressed on the antigen-specific cells but on few bystander CD8+ T cells (Figure 2A,B). CD137 expression was transient and was no longer present by day 12. By day 5 in culture, CD278 was also expressed on most of the antigen-specific cells but on few bystander CD8+ T cells. CD278 expression was also transient and was no longer present by day 12 (Figure 2A,B). By days 5 to 7, CD28 was transiently re-expressed on a proportion of the antigen-specific cells, albeit at a level 5- to 10-fold less than CD28 expressed on naive CD28+ CD45RAhi cells or CD28+ CD45ROhi cells (Figure 2A,B). By day 5 in culture, there was transient reexpression of CCR7 on most of the antigen-specific cells (Figure 2C) consistent with a previous study of CD27− CD45RAhi cells.27,28 By day 7 there was modest up-regulation of CD62L on a proportion of antigen-specific cells. Up-regulation of CCR5 expression on antigen-specific cells showed delayed kinetics with peak expression on day 9. Strong uniform up-regulation of CXCR3 on antigen-specific cells occurred by day 5 and was sustained to day 12 (Figure 2C). Both CCR5 and CXCR3 are expressed by effector T cells that infiltrate peripheral sites29 which suggests that activated CD28−CD45RAhi cells acquire the capacity to migrate to sites of HCMV reactivation.
Expression of costimulatory and homing molecules on antigen-specific CD28−CD45RAhi cells before and after stimulation with viral peptide. (A) Replicate cultures of sorted CD28−CD45RO− CD8+ T cells were stimulated with peptide-pulsed autologous monocytes and harvested at intervals for flow cytometry. Expression of CD137 (at day 5), CD278 (at day 5), and CD28 (at day 7) is shown against peptide-MHC Class I multimer, gated on CD8+ T cells. (B) Kinetics of expression of CD137, CD278, and CD28 after peptide stimulation (filled histograms) compared with isotype control (open histograms) gated on total input CD8+ cells (day 0) or gated on multimer-positive CD8+ T cells (days 5-12). (C) Kinetics of expression of CCR7, CD62L, CCR5, and CXCR3 after peptide stimulation gated on total input CD8+ cells (day 0) or gated on multimer-positive CD8+ T cells (days 5-12).
Expression of costimulatory and homing molecules on antigen-specific CD28−CD45RAhi cells before and after stimulation with viral peptide. (A) Replicate cultures of sorted CD28−CD45RO− CD8+ T cells were stimulated with peptide-pulsed autologous monocytes and harvested at intervals for flow cytometry. Expression of CD137 (at day 5), CD278 (at day 5), and CD28 (at day 7) is shown against peptide-MHC Class I multimer, gated on CD8+ T cells. (B) Kinetics of expression of CD137, CD278, and CD28 after peptide stimulation (filled histograms) compared with isotype control (open histograms) gated on total input CD8+ cells (day 0) or gated on multimer-positive CD8+ T cells (days 5-12). (C) Kinetics of expression of CCR7, CD62L, CCR5, and CXCR3 after peptide stimulation gated on total input CD8+ cells (day 0) or gated on multimer-positive CD8+ T cells (days 5-12).
CD137L provides costimulation for antigen-specific proliferation of CD28− CD45RAhi cells whereas CD80, CD86 and CD275 do not
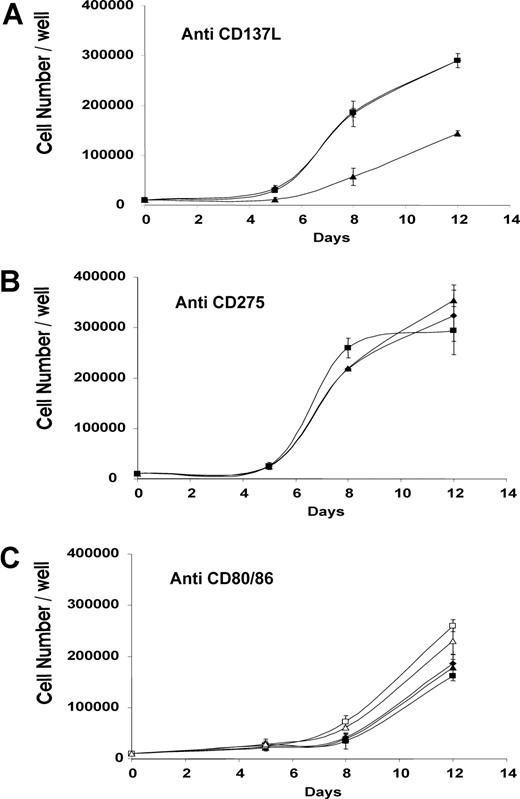
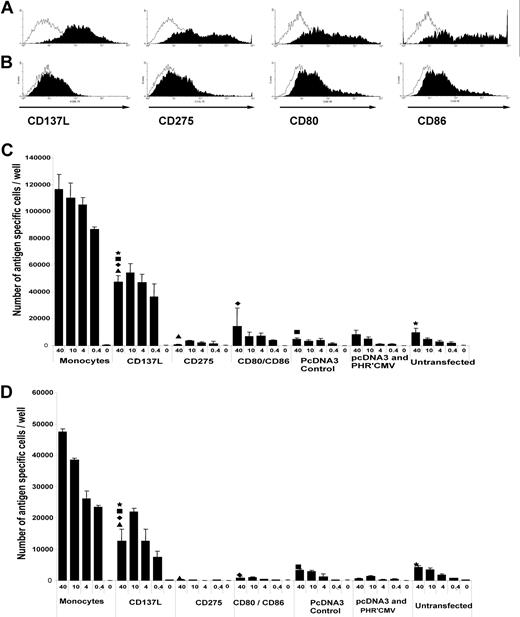
Monoclonal antibodies against individual costimulatory ligands on antigen-presenting cells were added to irradiated peptide-pulsed autologous PBMC before culture with purified CD28− responder cells. Addition of anti-CD137L reduced the antigen-stimulated proliferation of responder cells compared with control antibody (Figure 3A), whereas addition of anti-CD275 or anti-CD80 or anti-CD86 had no clear effect on antigen-stimulated proliferation (Figure 3B,C), the experiment was performed with three further donors with similar results; only anti-CD137L was able to block T-cell proliferation in the range of 40%-60%, depending on the donor. Autologous fibroblasts were transiently transfected to express individual costimulatory ligands, which was quantified by flow cytometry (Figure 4A,B). Untransfected fibroblasts expressed very low levels of each costimulatory ligand, whereas 50% to 80% of transfected fibroblasts expressed CD137L, CD275, CD80, or CD86. As artificial antigen-presenting cells, aliquots of transduced fibroblasts were pulsed with 4 dilutions (40-0.4 μg/ml) of viral peptide or unpulsed, and irradiated before culture with sorted CD28− CD45RAhi CD8+ T cells. In this model system, fibroblasts transfected with CD137L were consistently able to promote the antigen-specific proliferation even at low concentrations of viral peptide, whereas untransfected fibroblasts or fibroblasts transduced with CD275, CD80, or CD86 were unable to do so even at the highest peptide concentration (Figure 4C,D). The absolute magnitude of antigen-stimulated proliferation elicited by CD137L-transduced fibroblasts was approximately half that observed after stimulation with autologous monocytes.
Inhibition of peptide-stimulated proliferation of CD28− cells by anti-CD137L antibody. Replicate cultures of purified CD28−CD8+ T cells were stimulated with irradiated peptide-pulsed autologous PBMC in the presence or absence of antibody. Duplicate cultures were harvested at intervals and the total cell count per well determined. (A) ♦, no antibody; ■, control irrelevant antibody anti-CD4; ▴, anti-CD137L. (B) ♦, no antibody; ■, control irrelevant antibody anti-CD4; ▴, anti-CD275. (C) ♦, no antibody; ■, control irrelevant antibody anti-CD4; ▴, anti-CD80; □, anti-CD86; ▵, both anti-CD80 and anti-CD86. The experiment was performed on 4 independent donors, of which this is a representative experiment. The results from all 4 donors were antibodies similar to CD80, CD86, and ICOSL were unable to block proliferation, whereas anti-41BBL was (within the range 40%-60%, depending on donor). Anti-CD4 was an irrelevant antibody control in that this antibody is able to bind the antigen-presenting cells and demonstrates that steric hindrance by an irrelevant antibody was not able to block costimulation and T-cell proliferation. Anti-ICOSL, -CD80 and -CD86 antibodies were all the same isotype (IgG1) as the blocking 41BBL antibody and act as internal isotype controls. Error bars represent SD.
Inhibition of peptide-stimulated proliferation of CD28− cells by anti-CD137L antibody. Replicate cultures of purified CD28−CD8+ T cells were stimulated with irradiated peptide-pulsed autologous PBMC in the presence or absence of antibody. Duplicate cultures were harvested at intervals and the total cell count per well determined. (A) ♦, no antibody; ■, control irrelevant antibody anti-CD4; ▴, anti-CD137L. (B) ♦, no antibody; ■, control irrelevant antibody anti-CD4; ▴, anti-CD275. (C) ♦, no antibody; ■, control irrelevant antibody anti-CD4; ▴, anti-CD80; □, anti-CD86; ▵, both anti-CD80 and anti-CD86. The experiment was performed on 4 independent donors, of which this is a representative experiment. The results from all 4 donors were antibodies similar to CD80, CD86, and ICOSL were unable to block proliferation, whereas anti-41BBL was (within the range 40%-60%, depending on donor). Anti-CD4 was an irrelevant antibody control in that this antibody is able to bind the antigen-presenting cells and demonstrates that steric hindrance by an irrelevant antibody was not able to block costimulation and T-cell proliferation. Anti-ICOSL, -CD80 and -CD86 antibodies were all the same isotype (IgG1) as the blocking 41BBL antibody and act as internal isotype controls. Error bars represent SD.
Differential costimulation of peptide-stimulated proliferation of CD28−CD45RAhi cells by CD137L expressed on autologous fibroblasts. Replicate cultures of sorted CD28−CD45RO− CD8+ T cells were stimulated with autologous fibroblasts that had been transduced with individual costimulatory ligands and pulsed with dilutions of viral peptide (from 40 to 0.4 μg/mL) or unpulsed, or autologous monocytes pulsed with viral peptide or unpulsed. T cells were harvested at 10 days and the number of antigen-specific cells per well was calculated from the total cell count per well multiplied by the proportion of multimer-positive cells determined by flow cytometry. (A) Fibroblasts transfected with test construct stained with isotype control antibody (open histograms) or test antibody (filled histograms). (B) Fibroblasts transfected with control construct stained with isotype control antibody (open histograms) or test antibody (filled histograms). (C) Donor 28. (D) Donor 26. These experiments were performed twice on each donor, and the results in both cases confirm that on 41BBL transfection was able to support T-cell proliferation. At each peptide concentration, the number of antigen-specific cells per well stimulated by CD137L-transfected fibroblasts was significantly greater (P < .05 by Student t test) than stimulation with CD275-transfected fibroblasts or CD80/CD86-transfected fibroblasts. Error bars represent the standard deviation of the cell count from 3 independent wells at each peptide concentration multiplied by the proportion of antigen specific cells as determined by peptide-specific MHC class I multimers and flow cytometer.
Differential costimulation of peptide-stimulated proliferation of CD28−CD45RAhi cells by CD137L expressed on autologous fibroblasts. Replicate cultures of sorted CD28−CD45RO− CD8+ T cells were stimulated with autologous fibroblasts that had been transduced with individual costimulatory ligands and pulsed with dilutions of viral peptide (from 40 to 0.4 μg/mL) or unpulsed, or autologous monocytes pulsed with viral peptide or unpulsed. T cells were harvested at 10 days and the number of antigen-specific cells per well was calculated from the total cell count per well multiplied by the proportion of multimer-positive cells determined by flow cytometry. (A) Fibroblasts transfected with test construct stained with isotype control antibody (open histograms) or test antibody (filled histograms). (B) Fibroblasts transfected with control construct stained with isotype control antibody (open histograms) or test antibody (filled histograms). (C) Donor 28. (D) Donor 26. These experiments were performed twice on each donor, and the results in both cases confirm that on 41BBL transfection was able to support T-cell proliferation. At each peptide concentration, the number of antigen-specific cells per well stimulated by CD137L-transfected fibroblasts was significantly greater (P < .05 by Student t test) than stimulation with CD275-transfected fibroblasts or CD80/CD86-transfected fibroblasts. Error bars represent the standard deviation of the cell count from 3 independent wells at each peptide concentration multiplied by the proportion of antigen specific cells as determined by peptide-specific MHC class I multimers and flow cytometer.
Discussion
Here we showed that the cloning efficiency of CD28− CD45RAhi CD8+ T cells in limiting dilution analysis is similar to that of CD45ROhi CD8+ T cells. Therefore HCMV-specific CD28− CD45RAhi cells have no intrinsic proliferation defect when stimulated by antigen; previous studies that suggested impaired proliferative responses have underestimated their true proliferative capacity, probably through suboptimal provision of specific costimulation and/or exogenous IL-2. KLRG1 has been suggested to mark cells with a reduced proliferative capacity26 but was expressed by the overwhelming majority of multimer-positive cells in 2 subjects and therefore does not mark cells that are unable to proliferate. This suggests that appropriate costimulation may be able to “rescue” cells, which otherwise exhibit reduced proliferation. Provision of CD137L on autologous, peptide-pulsed, irradiated fibroblasts costimulates proliferation of CD28− CD45RAhi CD8+ T cells, whereas costimulation provided through CD278 or CD80/86 was unable to do so. This suggests a selective use of costimulatory pathways by the CD28− CD45RAhi CD8+ population. The reduced potency of fibroblasts compared with monocytes is not unexpected, given that fibroblasts may lack appropriate adhesion molecules and cytokine secretion as well as ligands for other additionally important costimulatory receptors.
Several studies in mice have suggested a role for CD137 in regulating memory T-cell responses. For influenza, the magnitude of the primary response is not altered in CD137L KO mice early but is lower later (21-38 days) with a defect in the secondary response.30 Both agonist monoclonal antibody to CD137 and transgenic CD137L expression have been reported to stimulate the proliferation of memory T cells in vivo,31 and IL-15 has been shown to up-regulate expression of CD137 and may thereby promote survival of memory T cells in the absence of antigen.32 CD137L has also been shown to be a potent costimulator of human virus specific CD8+ T cells.21,22
How may costimulation through CD137L influence the contribution of CD28− CD45RAhi CD8+ T cells to the control of virus infections? In the weeks after primary infection, virus-specific CD28−CD45ROhi cells become less numerous in peripheral blood, whereas virus-specific CD28− CD45RAhi CD8+ T cells become more abundant.4 CD28 expression can be modulated in vitro with its loss stimulated by culture in the presence of IL-2, IL-7, or IL-15 and type 1 IFN.33,34 Purified CD45RA− cells can re-express CD45RA during prolonged in vitro culture with appropriate cytokines (IL-7, IL-15)15 ; in the absence of antigen re-expression of CD45RA is related to the time since the last antigen stimulation.28 As shown using stable isotopic labeling techniques in vivo, circulating antigen-experienced CD45RAhi CD8+ T cells show reduced rates of proliferation and much-reduced clearance from the circulation compared with CD45ROhi cells, consistent with a long intermitotic interval.35 Together, these observations suggest that after exposure to viral antigen in vivo, activated CD28− CD45ROhi cells in the absence of antigen can, in an appropriate cytokine environment, revert to more quiescent CD28− CD45RAhi cells; the anatomical site(s) in vivo at which such reversion may occur are unclear but may include the peripheral blood and/or the bone marrow. The physiologic pathway(s) of recirculation of CD28− CD45RAhi cells are incompletely understood; it is unclear to what extent they migrate through the uninflamed mucosa of the lung, gastrointestinal or genital tract, or breast or through solid organs, such as liver or spleen. In the case of HCMV, reactivation of infectious virus from latently infected monocytes and dendritic cells is dependent upon activation/differentiation of the latently infected cell.9 Because monocytes and dendritic cells are very widely distributed and are recruited to sites of inflammation, HCMV reactivation could potentially occur at many different sites; in long-term carriers, known sites of virus shedding include the throat, the cervix, and breast milk. When virus-specific CD28− CD45RAhi CD8+ T cells encounter virus-infected cells, they can express immediate cytotoxic effector activity and secrete IFNγ,11,12,36 which enhances local antigen presentation and natural killer cell activation. We show that antigen stimulation also induces up-regulation of CD137; therefore, provided that there is local CD137L costimulation from the virus-infected cell or a bystander cell and there is sufficient IL-2, local proliferation of virus-specific CD28− CD45RAhi CD8+ T cells could follow. CD137L is expressed by monocytes and dendritic cells,37 and by human neurons and inflamed vessel walls.38,39 Utilization of different costimulatory ligands by T cells may also be influenced by HCMV itself, either in infected dendritic cells40 or in nearby cells by the action of virus-encoded IL-10.41
Antigen stimulation of virus-specific CD28− CD45RAhi cells induces down-regulation of CD45RA, up-regulation of CD45RO4 and transient re-expression of CCR7 that may enable virus-specific cells to re-circulate to lymph nodes, where (further) proliferation may take place. Subsequent time-dependent down-regulation of CCR7 and up-regulation of chemokine receptors, including CCR5 and CXCR3, might enable the daughter cells to migrate from lymph nodes and home to sites of inflammation where viral reactivation is occurring.
Several human costimulatory molecules and their ligands have been reported, but their relative importance for activation of human antigen-experienced T cells is incompletely understood. Here, we show for the first time that a well-characterized subpopulation of antigen-specific human CD8+ T cells has selective costimulatory ligand usage for proliferation. The cell types that express this costimulatory ligand CD137L, including monocytes and dendritic cells, may play an important role in the amplification of the virus-specific CD8+ T-cell response against HCMV reactivation. More generally, our data suggest that there is a greater functional plasticity among phenotypically defined subpopulations of memory T cells than is suggested by current classifications.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by a United Kingdom Medical Research Council Program Grant.
Authorship
Contribution: E.C.P.W. conceived experiments; performed research; collected, analyzed, and interpreted data; and drafted the manuscript. N.M. performed research and collected, analyzed and interpreted data. R.H. performed research and collected data. A.J.C. designed research and drafted the manuscript. J.G.P.S. designed research and drafted the manuscript. M.R.W. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Mark Wills, Department of Medicine, University of Cambridge Clinical School, Hills Rd, Cambridge, CB2 2QQ, UK; e-mail:mrw1004@cam.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal