Posttransplantation lymphoproliferative disorder (PTLD) is a serious complication of solid organ transplantation. Hepatitis C virus (HCV) infection has been linked to increased risk of lymphoma among immunocompetent individuals. We therefore investigated the association between HCV infection and PTLD in a retrospective cohort study of all individuals in the United States who received their first solid organ transplant from 1994 to 2005 (N = 210 763) using Scientific Registry of Transplant Recipients data. During follow-up, 1630 patients with PTLD were diagnosed. HCV prevalence at transplantation was 11.3%. HCV infection did not increase PTLD risk in the total cohort (Cox regression model, hazard ratio [HR] = 0.84; 95% confidence interval [CI] 0.68-1.05), even after adjustment for type of organ transplanted, indication for transplantation, degree of HLA mismatch, donor type, or use of immunosuppression medications. Additional analyses also revealed no association by PTLD subtype (defined by site, pathology, cell type, and tumor Epstein-Barr virus [EBV] status). HCV infection did increase PTLD risk among the 2.8% of patients (N = 5959) who were not reported to have received immunosuppression maintenance medications prior to hospital discharge (HR = 3.09; 95% CI, 1.14-8.42; P interaction = .007). Our findings suggest that HCV is not a major risk factor for PTLD, which is consistent with the model in which an intact immune system is necessary for development of HCV-related lymphoproliferation.
Introduction
Posttransplantation lymphoproliferative disorder (PTLD) is one of the most serious complications of immunosuppression in patients undergoing solid organ transplantation, with mortality rates that often exceed 50%.1,2 PTLD comprises a heterogeneous group of diseases characterized by the proliferation of lymphocytes. The major categories of PTLD include polymorphic hyperplasia/polymorphic PTLD, monomorphic PTLD, and other lymphoid malignancies, including multiple myeloma and Hodgkin lymphoma.3 PTLD is observed most frequently in the first year following transplantation. However, as the prognosis improves for individuals receiving solid organ transplants, the development of PTLD late after transplantation is increasingly recognized. Whereas early PTLD is commonly Epstein-Barr virus (EBV) positive and involves the transplanted organ, late PTLD is more commonly EBV− and involves various nodal and extranodal sites, suggesting that these may be distinct diseases and that there could be alternative pathways to lymphomagenesis following organ transplantation.1,4
Risk of PTLD varies substantially among the types of solid organ transplants, with the highest risk among heart, lung, and small-bowel transplant recipients (cumulative incidence, 5%-20%), and the lowest risk among kidney transplant recipients (1%-3%).1,2,5 This variability in risk has been proposed to reflect differences in the types and amounts of immunosuppressive therapy that these patients receive, the major risk factor for PTLD. Other possible risk factors for PTLD include young age, male sex, white race, EBV seroconversion following transplantation, and cytomegalovirus infection.1,2,5
At present, there is emerging evidence to support that hepatitis C virus (HCV) infection, which causes chronic liver infection, may be of importance in lymphomagenesis. HCV is a well-established risk factor for the lymphoproliferative syndrome type II mixed cryoglobulinemia.6 HCV infection has also been established recently as a possible risk factor for non-Hodgkin lymphoma (NHL) in the general population, particularly for B-cell lymphomas.7,,,,,–13 These lymphoproliferative conditions are thought to arise due to chronic stimulation of the immune system by HCV.6,14 Although there are a number of case reports of PTLD occurring in patients positive for HCV, few studies have investigated the relationship between HCV and PTLD systematically, and the results are conflicting.15,,,–19 Because of the inconsistencies in the limited literature on this topic, we conducted a large, population-based cohort study of solid organ transplant recipients using registry data to examine the association between HCV and PTLD. Our study included all individuals in the United States (N = 210 763) who received their first solid organ transplant between 1994, the approximate time when HCV testing of recipients became routine, and 2005.
In the past 15 years, the number of solid organ transplants in the United States has almost doubled, the number and types of available immunosuppressive therapeutic drugs have increased, and transplantation approaches have evolved substantially.20,–22 The prevalence of HCV in the United States is highest among middle-aged adults, in whom solid organ transplantation is most frequent, and HCV-associated cirrhosis is the most common reason for liver transplantation.23,–25 Thus, there is a pressing need to elucidate the relationship between HCV and subsequent risk of developing PTLD.
Patients and methods
Data source and study population
We conducted a retrospective cohort study of solid organ transplant recipients in the United States using data from the Scientific Registry of Transplant Recipients (SRTR), as submitted by the Organ Procurement and Transplantation Network (OPTN; http://www.optn.org). All transplantation centers and organ procurement organizations in the United States submit data to OPTN, which maintains a database that includes both baseline and follow-up information regarding every organ donation and transplantation that has occurred in the United States since 1986. The SRTR database includes baseline demographic and clinical data provided at the time of registration and transplantation, and follow-up data provided at 6 and 12 months following transplantation and annually thereafter.
The study cohort included all individuals in the United States who received their first solid organ transplant between January 1, 1994, the approximate time when HCV testing of recipients became routine, and April 1, 2005, the most recent time for which data were available. Patients were followed from the date of first transplantation until the earliest of the following events: PTLD diagnosis, graft failure, retransplantation, death from any cause, or loss to follow-up. Baseline and follow-up records were combined for individuals receiving simultaneous transplants of different organs. After excluding individuals with fewer than 30 days of follow-up (N = 29 437), the study cohort included 210 763 solid organ transplant recipients.
This research was exempted from review by the Office of Human Subjects Research of the National Cancer Institute because we analyzed anonymized data.
Exposure assessment
For each solid organ transplant recipient, data were obtained from the SRTR baseline files regarding demographic characteristics (sex, age, race/ethnicity, and highest level of education obtained); type of organ transplanted; number of HLA mismatches at the A, B, and DR loci; indication for transplantation; and results of an enzyme immunoassay test for serum HCV antibodies. Data regarding graft status (functioning or failed), acute rejection episodes, and results from any subsequent HCV antibody screening tests were obtained from the SRTR follow-up files. Finally, data regarding immunosuppression medications used prior to hospital discharge to induce and maintain immunosuppression and to treat early episodes of acute graft rejection were obtained from the SRTR immunosuppression files.
We used the baseline enzyme immunoassay serum screening test for HCV antibodies as the primary measure of HCV infection. A recombinant immunoblot assay (RIBA) test can be used to confirm the presence of HCV antibodies, indicating resolved or persistent infection, whereas an HCV RNA test is necessary to confirm persistent HCV infection. While only 9406 (45.7%) individuals with a positive HCV enzyme immunoassay screening test had informative results on confirmatory RIBA and/or HCV RNA tests, HCV infection was confirmed by these tests in 8094 (86.1%) of these individuals, supporting our use of the enzyme immunoassay to indicate infection. Furthermore, of the 5679 individuals with a positive HCV enzyme immunoassay screening test and HCV RNA results, HCV RNA was detected in 4891 (86.1%), indicating that most recipients with a positive baseline enzyme immunoassay serum screening test had persistent HCV infection. The rate of persistence was higher among liver transplant recipients (HCV RNA detected in 4061 of 4475 patients; 90.7%) than non–liver transplant recipients (HCV RNA detected in 830 of 1204; 68.9%).
In analyses where we considered HCV status as a time-dependent covariate, individuals who were antibody-positive at the time of transplantation were considered to be positive throughout the follow-up period. Individuals who were antibody-negative at the time of transplantation were considered to be negative throughout the follow-up period unless they had an antibody-positive result, at which time they were considered positive for the remainder of the follow-up period. The HCV status was considered to be unknown for individuals who did not have HCV antibody screening results recorded at the time of transplantation; the HCV status of these individuals remained unknown throughout the follow-up period until they had an informative (positive or negative) result. It is uncommon for organ donors to be HCV+, and data on donor HCV status were largely incomplete (N = 275 positive, N = 45 560 negative, N = 164 838 unknown); thus, we excluded these data from our analysis.
A total HLA mismatch score was created by summing the number of mismatches at the A, B, and DR loci (each locus was scored from 0-2, for a total HLA mismatch score ranging from 0-6). Reason for transplantation was categorized according to the broad OPTN categories. For liver transplant recipients, these categories included acute hepatic necrosis, noncholestatic cirrhosis, cholestatic liver disease/cirrhosis, biliary atresia, and metabolic diseases. For kidney and/or pancreas transplant recipients, these categories included glomerular diseases, diabetes, polycystic kidneys, hypertensive nephrosclerosis, vascular disease, congenital/rare familial/metabolic disorders, and tubular and interstitial diseases. For heart and/or lung transplant recipients, these categories included cardiomyopathy, coronary artery disease, congenital heart disease, valvular heart disease, congenital lung disease, emphysema/chronic obstructive pulmonary disease, cystic fibrosis, idiopathic pulmonary fibrosis, primary pulmonary hypertension, and alpha-1-antitrypsin deficiency. For all transplant recipients, additional categories included malignant neoplasms and other or unknown reason for transplantation.
Medication data included information on the use (yes/no) of specific medications prior to hospital discharge for induction and initial maintenance of immunosuppression and to treat early rejection episodes. However, detailed information on dosage was not available. For this analysis we created both broad medication use categories (yes/no use of any induction medication, any maintenance medication, or any antirejection medication) and detailed medication use categories. For induction medications, the detailed categories were defined as yes/no use of polyclonal antibodies or other. For maintenance medications, the detailed categories were defined as yes/no use of steroids, antimetabolites, calcineurin inhibitor, or target-of-rapamycin (TOR) inhibitors. For antirejection medications, the detailed categories were defined as yes/no use of polyclonal antibodies or other.
PTLD ascertainment
We identified patients with PTLD using data from the SRTR follow-up files, derived from the forms filled out at follow-up visits at 6 and 12 months following transplantation and annually thereafter. During each follow-up visit, healthcare providers were required to report any diagnosis of posttransplantation malignancy. If a patient was diagnosed with a de novo lymphoproliferative disease or lymphoma, additional data on diagnosis date, pathology, cell type, and site were collected.
For this analysis, patients with PTLD included all individuals with a diagnosis of de novo lymphoproliferative disease or lymphoma. We categorized the PTLD according to site (liver or nonliver; within or outside the allograft, defined separately for each type of organ transplanted), pathology (polymorphic, monomorphic, or other/unknown), cell type (B-cell, T-cell, or other/unknown), and tumor EBV status (positive, negative, or unknown). PTLD pathology refers to morphologic classification of tumor cell size and shape as heterogeneous (“polymorphic hyperplasia and polymorphic PTLD”) or homogeneous (“monomorphic PTLD”). Both disease entities are thought to be monoclonal. The “other/unknown” PTLD pathology category included recipients diagnosed with other lymphoid malignancies such as multiple myeloma and Hodgkin lymphoma, and recipients with no information on PTLD pathology.
Although the method of PTLD ascertainment remained the same throughout the follow-up period, reporting methods for follow-up data changed from a paper-based to a web-based system in 1999. In conjunction with this change, we observed an increase in PTLD incidence in 1999, which we addressed in our statistical analysis.
Statistical analysis
We estimated the relative risk of PTLD with hazard ratios (HRs) and 95% confidence intervals (CIs) derived from Cox regression models, using time since transplantation as the time metric and the Breslow method for handling ties.26 HCV antibody status was considered in 3 levels (positive, negative, and unknown), and individuals who were HCV antibody–negative at the time of transplantation were used as the referent group. All models were adjusted for basic demographic characteristics of transplant recipients, including age, sex, race/ethnicity, and education (Table 1; categories are listed). Additional adjustment for other candidate confounders (Table 2) did not result in a material change (> 10%) in the relative risk estimate for HCV; these variables were therefore excluded from the final models. We used a competing risks model to estimate the relative risk of PTLD subtypes as defined by site, pathology, cell type, and EBV status.27 We used time-dependent covariates to estimate the relative risk of PTLD associated with HCV infection by time since transplantation (< 1 year, 1-4 years, or 5 or more years following transplantation).27,28
Risk factors for PTLD and HCV prevalence by demographic group among 210 763 solid organ transplant recipients
| . | Patients with PTLD, no. . | PTLD HR (95% CI)* . | HCV prevalence, %† . |
|---|---|---|---|
| Total | 1630 | — | 11.3 |
| Sex | |||
| Female | 606 | 1.00 (referent) | 8.2 |
| Male | 1024 | 1.08 (0.98-1.19) | 13.3 |
| Age, y | |||
| 0-20 | 479 | 6.80 (5.45-8.48) | 1.6 |
| 21-30 | 125 | 1.77 (1.36-2.31) | 2.6 |
| 31-35 | 76 | 1.30 (0.96-1.76) | 4.7 |
| 36-40 | 94 | 1.25 (0.94-1.67) | 10.0 |
| 41-45 | 94 | 1.00 (referent) | 17.8 |
| 46-50 | 182 | 1.65 (1.29-2.12) | 20.6 |
| 51-55 | 181 | 1.69 (1.31-2.16) | 16.2 |
| 56-60 | 181 | 1.93 (1.50-2.47) | 10.2 |
| 61-65 | 144 | 2.10 (1.62-2.72) | 9.0 |
| > 65 | 74 | 1.79 (1.32-2.43) | 8.2 |
| Race | |||
| White, non-Hispanic | 1294 | 1.00 (referent) | 10.9 |
| Black, non-Hispanic | 145 | 0.46 (0.39-0.55) | 12.2 |
| Hispanic | 124 | 0.63 (0.52-0.76) | 13.3 |
| Asian | 46 | 0.63 (0.47-0.85) | 9.0 |
| Other/unknown | 21 | 1.09 (0.71-1.67) | 8.2 |
| Education | |||
| 0 to 8 y | 34 | 1.00 (referent) | 11.5 |
| 9 to 12 y | 333 | 1.24 (0.87-1.76) | 13.6 |
| Some college | 186 | 1.28 (0.89-1.85) | 12.2 |
| College graduate | 215 | 1.64 (1.14-2.35) | 9.0 |
| Unknown | 365 | 1.18 (0.83-1.67) | 12.8 |
| Recipients younger than 22 y | 497 | 4.99 (3.52-7.06) | 1.6 |
| Organ transplant | |||
| Heart and/or lung | 513 | 2.95 (2.63-3.31) | 1.8 |
| Kidney and/or pancreas | 698 | 1.00 (referent) | 4.9 |
| Liver | 382 | 1.73 (1.53-1.96) | 40.2 |
| Other | 37 | 4.13 (2.97-5.75) | 26.2 |
| . | Patients with PTLD, no. . | PTLD HR (95% CI)* . | HCV prevalence, %† . |
|---|---|---|---|
| Total | 1630 | — | 11.3 |
| Sex | |||
| Female | 606 | 1.00 (referent) | 8.2 |
| Male | 1024 | 1.08 (0.98-1.19) | 13.3 |
| Age, y | |||
| 0-20 | 479 | 6.80 (5.45-8.48) | 1.6 |
| 21-30 | 125 | 1.77 (1.36-2.31) | 2.6 |
| 31-35 | 76 | 1.30 (0.96-1.76) | 4.7 |
| 36-40 | 94 | 1.25 (0.94-1.67) | 10.0 |
| 41-45 | 94 | 1.00 (referent) | 17.8 |
| 46-50 | 182 | 1.65 (1.29-2.12) | 20.6 |
| 51-55 | 181 | 1.69 (1.31-2.16) | 16.2 |
| 56-60 | 181 | 1.93 (1.50-2.47) | 10.2 |
| 61-65 | 144 | 2.10 (1.62-2.72) | 9.0 |
| > 65 | 74 | 1.79 (1.32-2.43) | 8.2 |
| Race | |||
| White, non-Hispanic | 1294 | 1.00 (referent) | 10.9 |
| Black, non-Hispanic | 145 | 0.46 (0.39-0.55) | 12.2 |
| Hispanic | 124 | 0.63 (0.52-0.76) | 13.3 |
| Asian | 46 | 0.63 (0.47-0.85) | 9.0 |
| Other/unknown | 21 | 1.09 (0.71-1.67) | 8.2 |
| Education | |||
| 0 to 8 y | 34 | 1.00 (referent) | 11.5 |
| 9 to 12 y | 333 | 1.24 (0.87-1.76) | 13.6 |
| Some college | 186 | 1.28 (0.89-1.85) | 12.2 |
| College graduate | 215 | 1.64 (1.14-2.35) | 9.0 |
| Unknown | 365 | 1.18 (0.83-1.67) | 12.8 |
| Recipients younger than 22 y | 497 | 4.99 (3.52-7.06) | 1.6 |
| Organ transplant | |||
| Heart and/or lung | 513 | 2.95 (2.63-3.31) | 1.8 |
| Kidney and/or pancreas | 698 | 1.00 (referent) | 4.9 |
| Liver | 382 | 1.73 (1.53-1.96) | 40.2 |
| Other | 37 | 4.13 (2.97-5.75) | 26.2 |
— indicates not applicable.
PTLD HRs are unadjusted.
HCV prevalence among 182 121 recipients with known HCV status at the time of transplantation; HCV status was unknown for an additional 28 642 (13.6%) of all recipients.
PTLD risk associated with HCV status at the time of transplantation among 210 763 solid organ transplant recipients
| . | HCV+ . | HCV status unknown . |
|---|---|---|
| Unadjusted | 0.63 (0.51-0.78) | 0.88 (0.76-1.02) |
| Adjusted for age, sex, race/ethnicity, and education | 0.84 (0.68-1.05) | 0.84 (0.73-0.98) |
| Additional adjustments | ||
| Organ type (HL, KP, liver, other) | 0.78 (0.62-0.97) | 0.81 (0.70-0.94) |
| Indication for transplantation | 0.83 (0.65-1.05) | 0.84 (0.72-0.97) |
| Degree of HLA mismatch (score: 0-6) | 0.75 (0.61-0.94) | 0.81 (0.70-0.94) |
| Donor type (cadaveric/living) | 0.78 (0.63-0.97) | 0.82 (0.71-0.95) |
| Use of any induction medication | 0.85 (0.68-1.05) | 0.86 (0.74-1.00) |
| Use of specified induction medications: polyclonal antibodies or other | 0.85 (0.69-1.06) | 0.86 (0.74-1.00) |
| Use of any maintenance medication | 0.85 (0.68-1.05) | 0.86 (0.74-1.00) |
| Use of specified maintenance medications: steroids, antimetabolites, calcineurin inhibitor, or TOR inhibitors | 0.85 (0.69-1.06) | 0.85 (0.73-0.99) |
| Use of any antirejection medication | 0.84 (0.68-1.04) | 0.84 (0.73-0.98) |
| Use of specified antirejection medications: polyclonal antibodies or other | 0.84 (0.68-1.04) | 0.84 (0.73-0.98) |
| Use of any induction, maintenance, and antirejection immunosuppresion medications | 0.84 (0.68-1.05) | 0.87 (0.75-1.01) |
| Use of specified induction, maintenance, and antirejection immunosuppression medications | 0.85 (0.69-1.06) | 0.87 (0.75-1.01) |
| . | HCV+ . | HCV status unknown . |
|---|---|---|
| Unadjusted | 0.63 (0.51-0.78) | 0.88 (0.76-1.02) |
| Adjusted for age, sex, race/ethnicity, and education | 0.84 (0.68-1.05) | 0.84 (0.73-0.98) |
| Additional adjustments | ||
| Organ type (HL, KP, liver, other) | 0.78 (0.62-0.97) | 0.81 (0.70-0.94) |
| Indication for transplantation | 0.83 (0.65-1.05) | 0.84 (0.72-0.97) |
| Degree of HLA mismatch (score: 0-6) | 0.75 (0.61-0.94) | 0.81 (0.70-0.94) |
| Donor type (cadaveric/living) | 0.78 (0.63-0.97) | 0.82 (0.71-0.95) |
| Use of any induction medication | 0.85 (0.68-1.05) | 0.86 (0.74-1.00) |
| Use of specified induction medications: polyclonal antibodies or other | 0.85 (0.69-1.06) | 0.86 (0.74-1.00) |
| Use of any maintenance medication | 0.85 (0.68-1.05) | 0.86 (0.74-1.00) |
| Use of specified maintenance medications: steroids, antimetabolites, calcineurin inhibitor, or TOR inhibitors | 0.85 (0.69-1.06) | 0.85 (0.73-0.99) |
| Use of any antirejection medication | 0.84 (0.68-1.04) | 0.84 (0.73-0.98) |
| Use of specified antirejection medications: polyclonal antibodies or other | 0.84 (0.68-1.04) | 0.84 (0.73-0.98) |
| Use of any induction, maintenance, and antirejection immunosuppresion medications | 0.84 (0.68-1.05) | 0.87 (0.75-1.01) |
| Use of specified induction, maintenance, and antirejection immunosuppression medications | 0.85 (0.69-1.06) | 0.87 (0.75-1.01) |
Data are HR (95% CI). HCV− recipients were used as the referent group in all analyses.
HL indicates heart and/or lung; and KP, kidney and/or pancreas.
We conducted 3 sensitivity analyses. First, we estimated PTLD risk for individuals with confirmed HCV infection (enzyme immunoassay–positive and RIBA and/or HCV RNA–positive) and for individuals with confirmed persistent HCV infection (enzyme immunoassay–positive and HCV RNA–positive). Second, to investigate whether the change in the method of PTLD ascertainment in 1999 affected the risk estimate for HCV, we included a time-dependent covariate in the model (1994-1998 vs 1999-2005). Finally, we incorporated results from additional HCV antibody screening tests conducted during the follow-up period by including HCV status as a time-dependent covariate.
Modification of the effect of HCV antibody status on PTLD risk by selected demographic characteristics (age, sex, race/ethnicity) and clinical factors (type of organ transplanted, HLA mismatch level, use of medications for induction and maintenance of immunosuppression or for antirejection) was evaluated under the multiplicative model by including an interaction term in the Cox regression models. SAS, version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
Results
The 210 763 solid organ transplant recipients in the study cohort accrued 780 628.6 person-years at risk during the follow-up period (mean follow-up, 3.7 years; range, 31 days-11.7 years). The patients within the cohort were predominantly male (61%) and white (66%), with a mean (± standard deviation) age at the time of transplantation of 45.1 (± 15.9) years. Most patients received a kidney and/or pancreas transplant (N = 135 020; 64.1%), whereas comparatively fewer patients received a liver (N = 42 825; 20.3%), heart and/or lung (N = 30 666; 14.5%), or other organ transplant (N = 2252; 1.1%).
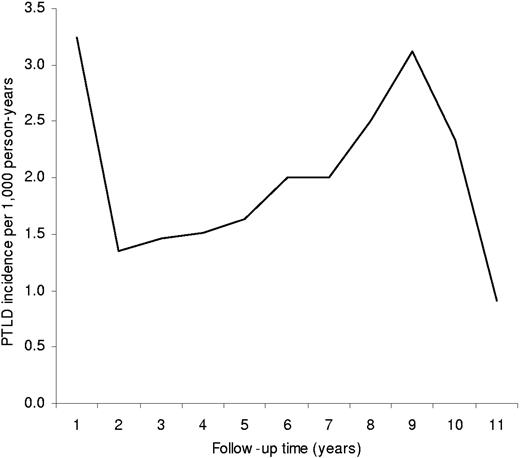
During the follow-up period, 1630 patients with PTLD were diagnosed among the 210 763 solid organ transplant recipients (incidence, 2.1 patients per 1000 person-years). PTLD incidence by time since transplantation followed a U-shaped pattern, with the highest incidence observed in the first year following transplantation (3.2 patients per 1000 person-years), substantially lower incidence 2 to 5 years following transplantation (1.4-1.6 patients per 1000 person-years), and higher incidence observed again 6 or more years following transplantation (2.0-3.1 patients per 1000 person-years) (Figure 1). PTLD risk was positively associated with male sex, age younger than 21 years, white race/ethnicity, and higher education level (Table 1). PTLD risk was lower among kidney and/or pancreas recipients compared with heart and/or lung, liver, or other solid organ recipients.
Incidence of PTLD by time since transplantation among 210 763 solid organ transplant recipients.
Incidence of PTLD by time since transplantation among 210 763 solid organ transplant recipients.
A total of 182 121 (86.4%) recipients had serologic testing for HCV status at the time of transplantation, among whom 11.3% were seropositive (20 598 HCV+ patients; Table 1). Compared with HCV− patients, HCV+ patients were more likely to be male, aged 41 to 50 years, and Hispanic or black, and less likely to be college graduates. HCV prevalence was substantially higher among liver recipients (40.2%) than among kidney and/or pancreas (4.9%), heart and/or lung (1.8%), or other solid organ (26.2%) recipients. HCV prevalence at the time of transplantation increased during the study period from 8.5% in 1994 to 15.2% in 2005 (data not shown).
Compared with HCV− patients at the time of transplantation, HCV+ patients actually appeared to be at reduced risk of PTLD (HR = 0.63; 95% CI, 0.51-0.78; Table 2). However, after adjustment for demographic factors, including age, sex, race/ethnicity, and education, the inverse association was attenuated and no longer statistically significant (HR = 0.84; 95% CI, 0.68-1.05). Additional adjustment for type of organ transplanted, indication for transplantation, degree of HLA mismatch, donor type, or use of medications at baseline to induce and maintain immunosuppression or to treat early episodes of acute graft rejection did not materially alter the risk estimate. Among 8094 individuals with confirmed HCV infection (enzyme immunoassay–positive and RIBA and/or HCV RNA–positive), HCV was not related to PTLD risk (HR = 0.99; 95% CI, 0.72-1.36; compared with HCV− patients). Similarly, among 4891 individuals with confirmed persistent HCV infection (enzyme immunoassay–positive and HCV RNA–positive), HCV also was not related to PTLD risk (HR = 1.06; 95% CI, 0.68-1.65).
Inclusion in the model of a time-dependent covariate controlling for the year of diagnosis (1994-1998 versus 1999-2005) also did not materially alter the HCV relative risk estimate (controlling for year of diagnosis: HR = 0.81; 95% CI, 0.65-1.01). During follow-up, HCV status changed for 2.6% of patients. Of the 161 523 patients who were HCV− at transplantation, 1096 (0.7%) became positive during follow-up, consistent with acute HCV infection. Of the 28 642 patients with unknown HCV status at transplantation, 3553 (12.4%) tested HCV− and 801 (2.7%) tested HCV+ during follow-up. Inclusion in the model of a time-dependent covariate incorporating results from additional HCV antibody tests during follow-up lowered the relative risk estimate slightly (HR = 0.74; 95% CI, 0.66-0.82), due to the low incidence of PTLD (0.3 patients per 1000 person-years) among individuals with acute HCV infection.
Analyses by PTLD subtype demonstrated similar HCV relative risk estimates by PTLD site (liver or nonliver; within or outside the allograft), pathology (polymorphic, monomorphic or other/unknown), cell type (B-cell, T-cell, or other/unknown), and tumor EBV status (Table 3). Similar relative risk estimates were also observed for PTLD diagnosed in less than 1 year, 1 to 4 years, or 5 or more years following transplantation (Table 3). Higher relative risks were observed for individuals receiving heart and/or lung (HR = 1.53; 95% CI, 0.79-2.96) or “other” solid organs (HR = 1.84; 95% CI, 0.52-6.53) compared with individuals receiving kidney and/or pancreas (HR = 0.55; 95% CI, 0.30-1.01) or liver transplants (HR = 0.83; 95% CI, 0.61-1.13), although this heterogeneity was not statistically significant (P interaction = .07) (Table 3).
PTLD risk associated with HCV status at the time of transplantation among 210 763 solid organ transplant recipients by PTLD subtype and type of organ transplanted
| . | Patients with PTLD, no. . | HCV+, HR (95% CI)* . | HCV status unknown, HR (95% CI)* . |
|---|---|---|---|
| Total | 1630 | 0.84 (0.68-1.05) | 0.84 (0.73-0.98) |
| By PTLD site | |||
| Liver | 208 | 1.06 (0.62-1.82) | 0.93 (0.62-1.40) |
| Nonliver | 1422 | 0.81 (0.64-1.03) | 0.83 (0.71-0.97) |
| Allograft | 266 | 1.11 (0.68-1.79) | 1.09 (0.78-1.54) |
| Nonallograft | 1364 | 0.80 (0.63-1.01) | 0.80 (0.68-0.94) |
| By PTLD pathology† | |||
| Polymorphic | 584 | 0.99 (0.69-1.41) | 0.98 (0.78-1.24) |
| Monomorphic | 825 | 0.86 (0.64-1.15) | 0.83 (0.67-1.02) |
| Other/unknown | 221 | 0.49 (0.24-1.00) | 0.56 (0.35-0.90) |
| By PTLD cell type | |||
| B cell | 1100 | 0.90 (0.70-1.16) | 0.85 (0.71-1.02) |
| T cell | 127 | 0.80 (0.36-1.74) | 1.09 (0.67-1.75) |
| Unknown | 403 | 0.70 (0.44-1.12) | 0.76 (0.56-1.03) |
| By PTLD EBV status | |||
| Negative | 238 | 1.04 (0.63-1.70) | 1.07 (0.75-1.53) |
| Positive | 785 | 0.75 (0.53-1.08) | 0.85 (0.69-1.05) |
| Unknown | 607 | 0.87 (0.63-1.20) | 0.75 (0.58-0.97) |
| By PTLD latency | |||
| Less than 1 y after transplantation | 627 | 0.71 (0.50-1.01) | 0.88 (0.69-1.11) |
| 1 to 4 y after transplantation | 535 | 1.02 (0.75-1.39) | 0.85 (0.67-1.07) |
| Over 5 y after transplantation | 468 | 0.77 (0.45-1.32) | 0.78 (0.58-1.06) |
| By organ type | |||
| Heart and/or lung | 513 | 1.53 (0.79-2.96) | 0.84 (0.63-1.11) |
| Kidney and/or pancreas | 698 | 0.55 (0.30-1.01) | 0.85 (0.67-1.08) |
| Liver | 382 | 0.83 (0.61-1.13) | 0.73 (0.56-0.96) |
| Other | 37 | 1.84 (0.52-6.53) | 0.65 (0.30-1.37) |
| . | Patients with PTLD, no. . | HCV+, HR (95% CI)* . | HCV status unknown, HR (95% CI)* . |
|---|---|---|---|
| Total | 1630 | 0.84 (0.68-1.05) | 0.84 (0.73-0.98) |
| By PTLD site | |||
| Liver | 208 | 1.06 (0.62-1.82) | 0.93 (0.62-1.40) |
| Nonliver | 1422 | 0.81 (0.64-1.03) | 0.83 (0.71-0.97) |
| Allograft | 266 | 1.11 (0.68-1.79) | 1.09 (0.78-1.54) |
| Nonallograft | 1364 | 0.80 (0.63-1.01) | 0.80 (0.68-0.94) |
| By PTLD pathology† | |||
| Polymorphic | 584 | 0.99 (0.69-1.41) | 0.98 (0.78-1.24) |
| Monomorphic | 825 | 0.86 (0.64-1.15) | 0.83 (0.67-1.02) |
| Other/unknown | 221 | 0.49 (0.24-1.00) | 0.56 (0.35-0.90) |
| By PTLD cell type | |||
| B cell | 1100 | 0.90 (0.70-1.16) | 0.85 (0.71-1.02) |
| T cell | 127 | 0.80 (0.36-1.74) | 1.09 (0.67-1.75) |
| Unknown | 403 | 0.70 (0.44-1.12) | 0.76 (0.56-1.03) |
| By PTLD EBV status | |||
| Negative | 238 | 1.04 (0.63-1.70) | 1.07 (0.75-1.53) |
| Positive | 785 | 0.75 (0.53-1.08) | 0.85 (0.69-1.05) |
| Unknown | 607 | 0.87 (0.63-1.20) | 0.75 (0.58-0.97) |
| By PTLD latency | |||
| Less than 1 y after transplantation | 627 | 0.71 (0.50-1.01) | 0.88 (0.69-1.11) |
| 1 to 4 y after transplantation | 535 | 1.02 (0.75-1.39) | 0.85 (0.67-1.07) |
| Over 5 y after transplantation | 468 | 0.77 (0.45-1.32) | 0.78 (0.58-1.06) |
| By organ type | |||
| Heart and/or lung | 513 | 1.53 (0.79-2.96) | 0.84 (0.63-1.11) |
| Kidney and/or pancreas | 698 | 0.55 (0.30-1.01) | 0.85 (0.67-1.08) |
| Liver | 382 | 0.83 (0.61-1.13) | 0.73 (0.56-0.96) |
| Other | 37 | 1.84 (0.52-6.53) | 0.65 (0.30-1.37) |
Adjusted for age, sex, race/ethnicity, and education. HCV− recipients were used as the referent group in all analyses.
PTLD pathology refers to morphologic classification of tumor cell size and shape as heterogeneous (″polymorphic PTLD″) or homogeneous (″monomorphic PTLD″). Both disease entities are thought to be monoclonal.
Among the small number of individuals who were not reported to have received any maintenance immunosuppression medications prior to hospital discharge (N = 5959 total, N = 38 patients with PTLD), HCV was positively associated with PTLD risk (HR = 3.09; 95% CI, 1.14-8.42). In contrast, HCV was not associated with PTLD among individuals who were reported to have received maintenance immunosuppression medications prior to hospital discharge (HR = 0.81; 95% CI, 0.65-1.01; P interaction = .007). Compared with individuals who were reported to have received maintenance immunosuppression medications prior to hospital discharge, individuals who were not reported to have received maintenance immunosuppression medications prior to hospital discharge were substantially more likely to have received an organ transplant early in the study period (1994-1996; 55% vs 22%), and were more likely to have received a liver transplant than another organ (27% vs 20%). The groups did not differ dramatically in terms of age, donor type, or HLA mismatch level. Among those who were not reported to have received maintenance immunosuppression medications prior to hospital discharge, 42% (2527 of 5959) of patients were reported to have received induction immunosuppression medications and/or treatment for early episodes of acute graft rejection, whereas 58% were not reported to have received immunosuppression medications for any reason prior to hospital discharge. The association between HCV and PTLD was similar in these 2 subgroups (HR = 2.93; 95% CI, 0.87-9.95; and HR = 2.62; 95% CI, 0.46-14.84, respectively). We did not have a sufficient number of patients to analyze the effect of HCV on PTLD risk by PTLD latency or subtype among individuals who were not reported to have received immunosuppression medications prior to hospital discharge.
The null effect of HCV on PTLD risk was similar across other subgroups defined by age (P interaction = .23), sex (P interaction = .95), race/ethnicity (P interaction = .67), HLA mismatch level (P interaction = .95), use of medication to induce immunosuppression (P interaction = .23), or use of medication to treat early acute rejection (P interaction = .08) (data not shown).
Discussion
Our findings from this large, population-based study including more than 200 000 organ transplant recipients in the United States suggest that HCV is not a major risk factor for PTLD. In an unadjusted analysis, HCV actually appeared somewhat protective against PTLD (HR = 0.63; 95% CI, 0.51-0.78), but after adjustment for demographic factors, this association was attenuated and no longer significant (HR = 0.84; 95% CI, 0.68-1.05). With additional adjustments for various clinical factors, the HCV relative risk estimate generally remained below 1.00, suggesting that additional unmeasured factors might be acting as confounders. We found no strong evidence to support that the relationship between HCV and PTLD risk varied in magnitude across type of organ transplanted, demographic subgroups, or characteristics of PTLD. Interestingly, we did see evidence that HCV may increase risk of PTLD among the small number of individuals who were not reported to have received immunosuppression maintenance medications prior to hospital discharge.
The relationship between HCV and PTLD has been investigated systematically in 5 previous reports. In the largest of these reports, Bustami et al17 used the SRTR database to examine risk factors for PTLD among 41 000 cadaveric first kidney transplant recipients and found no association between HCV and PTLD (multivariate adjusted HR = 0.70; 95% CI, 0.30-1.61). The remaining 4 studies were conducted in individual transplantation centers, and were thus substantially smaller (each with fewer than 700 transplant recipients and fewer than 15 patients with PTLD). One study of heart transplant recipients18 and 2 studies of liver transplant recipients15,19 reported increased risk of PTLD associated with HCV infection, whereas another study of liver transplant recipients reported no association between HCV and PTLD.16 Our study represents the first comprehensive examination of the association between HCV and PTLD among all solid organ transplant recipients, with detailed analysis by organ type and PTLD characteristics, and consideration of potential confounding and effect modification by demographic and clinical factors.
HCV infection is a well-established risk factor for the lymphoproliferative syndrome type II mixed cryoglobulinemia, in which approximately 90% of patients are HCV+.6 Although less well-established, the link between HCV and NHL in the general population has been demonstrated consistently in different populations of varying HCV prevalence, with estimated relative risks of 1.3 to 5.0.7,,,,,–13 It is unclear whether the association between HCV and NHL is specific to particular NHL subtypes, but associations have been noted with marginal zone lymphoma, lymphoplasmacytic lymphoma, and other low-grade NHLs. Successful antiviral therapy to treat HCV has been shown to lead to regression of marginal zone lymphoma29,30 and to eliminate t(14;18) translocation–positive B cells (the defining event of follicular lymphoma).31
Although our finding that HCV is not an important risk factor for PTLD may seem surprising, it is consistent with the lack of association between HCV and lymphoma observed in persons with HIV/AIDS,32,,,,–37 another immunosuppressed population. In the setting of solid organ transplantation, immunosuppression medications prevent graft rejection by suppressing T-cell function,38 which can also result in uncontrolled proliferation of B-lymphocytes and development of PTLD. Similarly, T-cell function is suppressed in persons with HIV/AIDS as the virus impairs and destroys CD4+ T cells, again allowing for uncontrolled proliferation of B-lymphocytes.39 Among both transplant recipients and people with HIV, EBV infection of lymphocytes also plays an important role in driving lymphoproliferation.
It is possible that HCV infection does not increase risk of lymphoproliferative disorders in immunosuppressed populations because HCV may have an indirect oncogenic effect on lymphocytes, and thus requires an intact immune system. Specifically, HCV infection may increase risk of lymphoma through chronic antigenic stimulation and the development of clonal B-lymphocyte populations.6,14,40 The impairment of T-cell function via immunosuppression may prevent the immune system from mounting a sustained response to persistent HCV infection. Thus, although immunosuppressed individuals are at increased risk for lymphoproliferative disorders, these disorders are not related to clonal expansion of HCV-specific B-lymphocytes. This model of antigen-driven lymphomagenesis directly contrasts with EBV-driven lymphomagenesis. EBV has been shown consistently to infect and transform B-lymphocytes.41 In the setting of an incompetent immune system, the lack of T-cell control of infected B cells permits EBV-driven transformation.2
Of interest, the only group in which we found an association between HCV and PTLD was the small number of individuals (2.8% of all transplant recipients) who were not reported to have received immunosuppression maintenance medications prior to hospital discharge. This observation is consistent with the model described in the previous paragraph, in which an intact immune system is necessary for development of HCV-related lymphoproliferation. We recognize that it is implausible that a solid organ transplant recipient would never receive any immunosuppression medications to prevent graft rejection. However, transplantation practices have varied over time, and it is possible that some patients might not have received immunosuppression medication before hospital discharge due to specific protocols or medical complications that delayed initiation of therapy. Unfortunately, our analyses of available data did not allow us to make conclusions regarding the reasons why some transplant recipients were not reported to have received baseline immunosuppression. Medication use could be incompletely reported by transplantation centers. However, in more than 98% of patients for whom no specific medications were reported, the transplantation center explicitly noted that no immunosuppression medications were administered (Greg Levine, SRTR/Arbor Research, personal written communication, June 6, 2007). These results should be interpreted cautiously due to the relatively small number of patients in this subgroup and the lack of information on why they did not receive immunosuppression medications.
Several strengths of this analysis should be considered in the interpretation of our findings. The SRTR database contains detailed baseline and follow-up data for all solid organ transplant recipients in the United States as reported by transplantation centers and organ procurement organizations. The large cohort, which is substantially larger than any previous study, enabled us to thoroughly evaluate the risk of PTLD associated with HCV infection by type of organ transplanted and PTLD subtype. The incidence of PTLD by organ type and time since transplantation in our study were similar to that reported in the literature,1,2,5 reflecting the population-based nature of the cohort.
The main limitation of this study was the lack of detailed dosage information for the medications used at baseline to induce and maintain immunosuppression and to treat early episodes of acute graft rejection. Immunosuppression is the major risk factor for PTLD, and it is possible that the intensity of immunosuppression differed by HCV status, resulting in unmeasured confounding. The inclusion of various demographic factors in the model resulted in a substantial change in the relative risk estimate for HCV. It is possible that residual confounding by other unmeasured factors remains, although it is unlikely that any residual confounding would be strong enough to have obscured a major effect of HCV on PTLD. Our study was also limited by potential underascertainment of PTLD, although it is likely that any underascertainment was unrelated to HCV status, and the incomplete ascertainment of HCV status for all recipients. Our main measure of HCV status was a baseline enzyme immunoassay screening test, which cannot distinguish between persistent and resolved HCV infection. However, among the small number of people with an informative HCV RNA confirmatory test, 86.1% of individuals had detectable HCV RNA, indicating persistent HCV infection. This estimate is slightly higher than the rate of persistence (60%-85%) observed in the general population,42 likely due to a high proportion of liver recipients with HCV-related cirrhosis and other medical comorbidities. Notably, our results were similar regardless of whether we considered all enzyme immunoassay–positive subjects or only those with detectable HCV RNA as being infected with HCV. In addition, we had no data on treatments for HCV given during or shortly after the time of transplantation. HCV treatment is used among liver transplant recipients to try to prevent reinfection of the transplanted organ, but HCV treatment is not used widely among other solid organ transplant recipients because it does not generally provide a sustained virologic response and increases risk of graft rejection.43 Therefore, it is unlikely that HCV treatment would have strongly affected our results. During follow-up, HCV status changed for 2.6% of patients. We observed a low incidence of PTLD in the small subgroup of individuals who may have had acute HCV infection, although the implications of this finding are unclear.
In conclusion, our results suggest that HCV is not an important risk factor for PTLD, which is consistent with the lack of association between HCV and lymphoma observed in persons with HIV/AIDS, another immunosuppressed population. Our findings support a model in which HCV infection indirectly promotes lymphomagenesis via chronic antigenic stimulation, which does not appear to occur in persons with impaired T-cell function.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The Scientific Registry of Transplant Recipients (SRTR) is supported by contract 231–00-0116 from the US Department of Health Resources and Services Administration (HRSA), US Department of Health and Human Services.
The authors gratefully acknowledge David M. Dickinson, Greg Levine, and James Welch (SRTR/Arbor Research) for their assistance with the SRTR database, particularly relating to the reporting of immunosuppression medication use. We also thank Alan B. Leichtman for his thoughtful review of this manuscript.
The data reported here have been supplied by the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
National Institutes of Health
Authorship
Contribution: L.M.M., O.L., and E.A.E. conceived of the study. E.A.E. obtained the data. D.C. and R.P. provided data management and, together with N.C., provided statistical support. L.M.M. conducted the data analysis and drafted the report. All authors interpreted the data and contributed to the final version of this report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lindsay M. Morton, Hormonal and Reproductive Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, DHHS, 6120 Executive Blvd, EPS 5100, Rockville, MD 20852; e-mail:mortonli@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal