Abstract
Sirolimus-based immunosuppressive regimens in organ transplantation have been associated with a lower than expected incidence of cytomegalovirus (CMV) disease. Whether sirolimus has a similar effect on CMV reactivation after allogeneic hematopoietic stem cell transplantation (HSCT) is not known. We evaluated 606 patients who underwent HSCT between April 2000 and June 2004 to identify risk factors for CMV reactivation 100 days after transplantation. The cohort included 252 patients who received sirolimus-tacrolimus for graft-versus-host disease (GVHD) prophylaxis; the rest received non–sirolimus-based regimens. An initial positive CMV DNA hybrid capture assay was observed in 225 patients (37.1%) at a median 39 days after HSCT for an incidence rate of 0.50 cases/100 patient-days (95% confidence interval [CI], 0.44-0.57). Multivariable Cox modeling adjusting for CMV donor-recipient serostatus pairs, incident acute GVHD, as well as other important covariates, confirmed a significant reduction in CMV reactivation associated with sirolimus-tacrolimus–based GVHD prophylaxis, with an adjusted HR of 0.46 (95% CI, 0.27-0.78; P = .004). The adjusted HR was 0.22 (95% CI, 0.09-0.55; P = .001) when persistent CMV viremia was modeled. Tacrolimus use without sirolimus was not significantly protective in either model (adjusted HR, 0.66; P = .14 and P = .35, respectively). The protective effect of sirolimus-containing GVHD prophylaxis regimens on CMV reactivation should be confirmed in randomized trials.
Introduction
Cytomegalovirus (CMV) infection remains a challenging problem after allogeneic hematopoietic stem cell transplantation (HSCT).1,2 CMV reactivation and disease was the leading cause of infectious morbidity and mortality in the 1970s and 1980s in hematopoietic stem cell (HSC) transplant recipients.3-6 The discovery and clinical use of the deoxyguanosine analogues acyclovir7,8 and ganciclovir9,10 for the prevention of CMV reactivation, and the implementation of preemptive ganciclovir treatment based on systematic CMV antigen11 or nucleic acid monitoring12 in peripheral blood, significantly decreased the impact of CMV infection after HSCT1,2,13 by the late 1990s. Valganciclovir has allowed the oral administration of effective doses of ganciclovir,14-16 thus minimizing the additional risks associated with prolonged administration of intravenous medications and central catheter use. Although preemption has reduced unnecessary ganciclovir exposure and consequent toxicity, it is still limited by its associated myelosuppression and risk of secondary infections that affect those who require treatment at the time of CMV reactivation.1,2,13
The effect of CMV seropositivity of donor-recipient pairs, as well as that of acute graft-versus-host disease (GVHD) and its treatment, on the risk of CMV reactivation and disease after HSCT is well established.3,4,17-20 While the relative importance of different HSCT regimens on CMV infection, including nonmyeloablative or reduced-intensity conditioning,21,22 T-cell depletion,23,24 and donor relatedness20,25,26 is established, the effect of GVHD prophylaxis strategies other than T-cell depletion has not been well studied. A small cohort study27 suggested that a mycophenolate-containing regimen may increase the cumulative incidence of CMV reactivation, but most studies have not had enough patients or variability to study the contribution of GVHD prophylaxis regimens to the risk of CMV reactivation.1,2
Randomized trials of sirolimus-based immunosuppressive regimens in solid organ transplantation have observed decreased cumulative incidence of CMV disease in patients receiving sirolimus.28,29 We reported a reduced incidence of CMV reactivation in an initial experience with a combination of sirolimus and tacrolimus for GVHD prophylaxis,30 although that trial was limited to matched-related HSC transplant recipients and the incidence of acute GVHD was low.31 Thus, in the present study we sought to evaluate whether sirolimus exposure during early transplantation had an effect on CMV infection particularly compared with calcineurin inhibitors cyclosporine and tacrolimus, as well as other risk factors for early CMV reactivation, in a recent cohort of adult HSC transplant recipients at our institution.
Patients, materials, and methods
Patients
All patients who underwent an allogeneic HSCT between April 2000 and June 2004 were identified through the clinical database at Dana-Farber Cancer Institute/Brigham and Women's Hospital (DFCI/BWH) Hematopoietic Stem Cell Transplantation Service. The Office for the Protection of Research Subjects of Dana-Farber/Harvard Cancer Center approved the study.
Six hundred sixty procedures were performed during the study period. Six umbilical cord blood HSCTs were excluded. There were 18 patients who underwent 2 or more transplantations: only their first transplantation was included in this analysis, such that 21 HSCTs were excluded, and their survival was censored at the time of the second transplantation. Three patients with missing data on donor CMV serologic status were excluded. Twenty-four patients were excluded because they did not undergo blood CMV DNA testing during the study period, the primary outcome of interest, mostly because of early death after HSCT. Thus a final cohort of 606 patients undergoing an initial HSCT during the study period was used for the present study.
Covariates and definitions
Data on covariates of interest were identified through the DFCI/BWH HSCT database, the Partners Healthcare System Research Patient Data Repository, and review of the electronic and paper medical records. Covariates included age at the time of HSCT, sex, race, primary disease that necessitated HSCT, date of transplantation, donor and recipient CMV IgG antibody status, bone marrow transplantation (BMT) disease risk group, HLA-matching between donor and recipient, relatedness of donor (sibling or not), mode in which stem cells were obtained (bone marrow versus peripheral blood stem cells [PBSCs]), whether acute GVHD occurred and date of diagnosis, organ-specific and overall acute GVHD grade (according to the consensus scale32 ), and date of death or last clinic visit on or before November 30, 2005.
Conditioning regimens were stable during the study period and were coded as myeloablative versus reduced-intensity as individual conditioning drugs were collinear with the conditioning regimens. Myeloablative conditioning consisted of cyclophosphamide followed by total body irradiation,33 or cyclophosphamide and oral busulfan.33 Reduced-intensity conditioning consisted of fludarabine and intravenous busulfan.34 The choice of conditioning regimen was decided by the treating transplant physician.
Patients received one of several GVHD prophylaxis regimens, including combinations of cyclosporine-methotrexate,35,36 cyclosporine-prednisone, cyclosporine–mycophenolate mofetil, tacrolimus-methotrexate,35,36 tacrolimus-sirolimus-methotrexate,33 or tacrolimus-sirolimus.30,37 All modes of T-cell depletion (CD8−38 or CD6− depletion,39 CD34+ selection) used during the study period were analyzed as a single group given the few patients who underwent a specific T-cell manipulation procedure. Methotrexate, cyclosporine, tacrolimus, mycophenolate mofetil, and prednisone were included individually in the analysis, but as patients who received sirolimus always received concomitant tacrolimus for initial GVHD prophylaxis during the study period, one variable was created to consider both of these medications together, and one variable was created that considered the effects of tacrolimus in the absence of sirolimus. With the exception of methotrexate, individual GVHD prophylaxis drugs were started during conditioning, tapered starting between day +60 and day +100, and discontinued by day +180 unless GVHD occurred. Given this exposure pattern, individual drugs were modeled as baseline risks in this analysis.
Overall acute GVHD severity was coded as grades 0-I versus grades II-IV because the latter received systemic treatment with corticosteroids and other immunosuppressants, which are known to increase CMV reactivation,1,2,13 and to allow comparability with previous studies, which have consistently used this dichotomization. Time to acute GVHD was defined as the time between transplantation (day 0) and diagnosis of GVHD. Donor and recipient CMV serology was coded as a 4-level covariate (D−/R−, D−/R+, D+/R−, D+/R+).3,18 Given the relatively few HSC transplant recipients belonging to each of several nonwhite races, these were analyzed as a group. As the cumulative incidence of CMV reactivation was noted to have decreased steadily over the study period, a 4-level transplantation period covariate was created to account for potential unmeasured covariates and secular trends of CMV reactivation.
CMV testing and preemptive treatment
Whole blood CMV DNA testing using a hybrid capture assay (Digene, Gaithersburg, MD)40,41 for monitoring of CMV reactivation at DFCI/BWH started in April 2000 and was used consistently throughout the study period. Patients were tested once or twice a week after engraftment, while hospitalized for any reason, and on every clinic visit after discharge and beyond day +100 after HSCT. Clinic visits usually took place every week to every 2 weeks, depending on the time after transplantation, intercurrent problems, and immune reconstitution. Patients with a positive CMV hybrid capture result were treated at the discretion of their transplant oncologist, who decided whether to start preemptive treatment immediately or wait for a repeat positive result for confirmation. Patients usually received preemptive intravenous ganciclovir 5 mg/kg twice daily, oral ganciclovir 1000 mg thrice daily, or valganciclovir 900 mg twice daily (adjusted for renal function as needed) for 10 to 14 days, or until CMV testing became negative, followed by a 2- to 4-week suppression period.
All patients received acyclovir prophylaxis (400 mg orally or 200 mg intravenously 3 times daily) beginning with conditioning until a year after HSCT, or longer if they remained on immunosuppression for GVHD; no patient received prophylactic ganciclovir or valganciclovir. Red cell and platelet support was provided with irradiated, leukocyte-reduced products obtained locally or through the American Red Cross during the study period. All platelets transfused were single-donor apheresis products. Leukocyte-reduced products were considered CMV-free by the hospital blood bank and administered to all patients who underwent HSCT (including CMV D−/R− recipients) independent of the blood donor's CMV status.
A patient's first positive CMV hybrid capture result, independent of the CMV DNA concentration, was considered an event for the purpose of this analysis40 ; indeterminate tests results were considered negative. Time to first positive CMV test result was calculated from the date of transplantation until the date of the first positive test. Those with persistently negative or indeterminate CMV assays were censored at day +100 if they were still alive on that day; patients who died within 100 days after HSCT were censored on the day of death if CMV reactivation had not occurred. A censoring variable for CMV positivity was created to this effect. Given the possibility of false-positive CMV hybrid capture results,40,42 a sensitivity analysis defining persistent CMV viremia as 2 consecutive positive CMV hybrid capture assays was performed. Similarly, time to persistent CMV viremia was calculated from the date of transplantation until the date of the second positive CMV test and its corresponding censoring variable was created; patients with single or nonconsecutive positive or negative test results were censored at day +100 if they were still alive on that day, independent of whether they received specific CMV treatment.
CMV pneumonia was diagnosed on the basis of positive CMV immunohistochemistry of a lung biopsy specimen or by positive CMV detection in culture of bronchoalveolar lavage specimens in patients with compatible clinical and radiographic findings. Gastrointestinal CMV infection was diagnosed by positive biopsy CMV immunohistochemistry.
Statistical analysis
Initial univariate exploration of the baseline covariates was performed using 2-sided Fisher exact test or Wilcoxon tests. We generated univariate Kaplan-Meier survival curves of time to CMV reactivation for all baseline covariates; log-rank was used to test for equality over strata. In addition, CMV reactivation cumulative probability plots were generated for covariates of particular interest. Incidence rates, incidence rate ratios, and their 95% confidence intervals were calculated with the methods of Rothman and Greenland43 and Fisher, respectively, using OpenEpi version 1.1 (http://www.openepi.com; Atlanta, GA). Possible predictors of CMV reactivation were evaluated in univariate Cox proportional hazard models. The hazard ratio and 95% confidence intervals were determined for all candidate covariates. GVHD was modeled as a time-varying covariate. Candidate covariates were included in the multivariate Cox regression model if they were associated with CMV reactivation in the univariate analysis (P < .20), or had been previously identified in the literature as significant risk factors. Final models were created including possible variations of a covariate with inclusion of dummy variables if any particular level of the covariate was associated with CMV reactivation. Using the multivariate model, we calculated the adjusted hazards ratios for CMV reactivation. The proportional hazards assumption was tested by including time*covariate products for each covariate in individual multivariate regression models at a significance level of .05. Tied failure times were dealt with exact partial likelihood ratios. Statistical analysis was done with SAS version 9.1 (Cary, NC).
Results
CMV reactivation in the cohort during the first 100 days after HSCT
A total of 5016 CMV DNA hybrid capture assays (CMV-HCAs) were performed during the first 100 days after HSCT in the cohort with a median of 8 assays per patient (interquartile range [IQR], 6-10; range, 1-21). An initial positive CMV-HCA was detected in 225 patients (37.1%) a median of 39 days after HSCT (IQR, 27-52; range, 2-100 days). These events occurred over 45 064 patient-days at-risk of CMV reactivation through day + 100, for an overall incidence rate of 0.50 cases/100 patient-days (95% CI, 0.44-0.57). The cumulative incidence and crude incidence rates of CMV reactivation during this period were similar among patients of different age, sex, underlying disease, and BMT disease risk group (Table 1). When stratified by the 4 possible categories of CMV donor/recipient serostatus pairs, there was a discrete and significant gradation in the incidence of CMV detection after HSCT. Patients who were CMV D−/R+ had the highest cumulative incidence of CMV-HCA positivity (65.9%), followed by CMV D+/R+ (50.0%), CMV D+/R− (31.3%) and CMV D−/R− (18.3%, P < .001).
Characteristics of allogeneic HSCT cohort, cumulative CMV reactivation, and crude incidence rates to day +100, DFCI/BWH April 2000-June 2004
| Characteristic . | No. . | CMV reactivation* . | % . | P . | Days after HSCT† . | IR (95% CI)‡ . | IRR (95% CI)§ . |
|---|---|---|---|---|---|---|---|
| Cohort | 606 | 225 | 37.1 | — | 45064 | 0.499 (0.438-0.569) | — |
| CMV serostatus‖ | < .001 | ||||||
| CMV D−/R− | 246 | 45 | 18.3 | 20982 | 0.215 (0.160-0.287) | 1 | |
| CMV D+/R− | 115 | 36 | 31.3 | 8881 | 0.405 (0.292-0.562) | 1.89 (1.19-3.00) | |
| CMV D+/R+ | 110 | 55 | 50.0 | 7289 | 0.755 (0.579-0.983) | 3.52 (2.33-5.34) | |
| CMV D−/R+ | 135 | 89 | 65.9 | 7912 | 1.130 (0.914-1.390) | 5.25 (3.63-7.68) | |
| Recipient age, quartiles, y | .50 | ||||||
| 18 to 35 | 152 | 66 | 43.4 | 11089 | 0.595 (0.468-0.758) | 1 | |
| 36 to 45 | 154 | 48 | 31.2 | 11873 | 0.404 (0.305-0.537) | 0.679 (0.458-1.00) | |
| 46 to 53 | 152 | 55 | 36.2 | 10938 | 0.503 (0.386-0.655) | 0.845 (0.580-1.23) | |
| 54 to 70 | 148 | 56 | 37.8 | 11164 | 0.502 (0.386-0.652) | 0.843 (0.580-1.22) | |
| Recipient sex | .55 | ||||||
| Female | 249 | 96 | 38.6 | 18586 | 0.517 (0.423-0.631) | 1.06 (0.813-1.38) | |
| Male | 357 | 129 | 36.1 | 26478 | 0.487 (0.410-0.579) | — | |
| Recipient race | < .001 | ||||||
| Nonwhite | 42 | 30 | 71.4 | 2384 | 1.260 (0.880-1.800) | 2.75 (1.85-4.00) | |
| White | 564 | 195 | 34.6 | 42680 | 0.457 (0.397-0.526) | — | |
| Primary disease | .25 | ||||||
| AML | 177 | 68 | 38.4 | 12713 | 0.535 (0.422-0.678) | 1 | |
| CML | 90 | 35 | 38.9 | 6605 | 0.530 (0.381-0.738) | 0.991 (0.639-1.51) | |
| MDS | 86 | 36 | 41.9 | 5859 | 0.614 (0.443-0.852) | 1.150 (0.745-1.75) | |
| NHL | 84 | 27 | 32.1 | 6935 | 0.389 (0.267-0.568) | 0.728 (0.448-1.15) | |
| Other | 61 | 16 | 26.2 | 4881 | 0.328 (0.201-0.535) | 0.613 (0.332-1.07) | |
| ALL | 58 | 27 | 46.6 | 4163 | 0.649 (0.445-0.946) | 1.210 (0.746-1.92) | |
| CLL | 50 | 16 | 32.0 | 3908 | 0.409 (0.251-0.668) | 0.765 (0.414-1.33) | |
| Conditioning regimen | .17 | ||||||
| Reduced-intensity | 238 | 80 | 33.6 | 18573 | 0.431 (0.346-0.536) | 0.787 (0.597-1.03) | |
| Myeloablative | 368 | 145 | 39.4 | 26491 | 0.547 (0.465-0.644) | — | |
| HLA match¶ | .08 | ||||||
| Mismatched donor | 65 | 31 | 47.7 | 4097 | 0.757 (0.532-1.760) | 1.60 (1.06-2.34) | |
| Matched donor | 541 | 194 | 35.9 | 40967 | 0.474 (0.411-0.545) | — | |
| Donor relatedness | .02 | ||||||
| Unrelated donor | 315 | 131 | 41.6 | 22395 | 0.585 (0.493-0.694) | 1.41 (1.07-1.86) | |
| Related donor | 291 | 94 | 32.3 | 22669 | 0.415 (0.339-0.508) | — | |
| Stem cell source | .002 | ||||||
| Peripheral blood | 415 | 136 | 32.8 | 31764 | 0.428 (0.362-0.507) | 0.640 (0.490-0.838) | |
| Bone marrow | 191 | 89 | 46.6 | 13300 | 0.669 (0.544-0.824) | — | |
| BMT disease risk group** | .61 | ||||||
| Low | 177 | 71 | 40.1 | 12727 | 0.558 (0.442-0.704) | 1 | |
| Intermediate | 324 | 116 | 35.8 | 24431 | 0.475 (0.396-0.570) | 0.851 (0.628-1.16) | |
| High | 105 | 38 | 36.2 | 7906 | 0.481 (0.350-0.661) | 0.862 (0.565-1.30) | |
| Acute GVHD | .002 | ||||||
| Grades II-IV | 196 | 90 | 45.9 | 13572 | 0.663 (0.539-0.815) | 1.55 (1.17-2.04) | |
| None–grade I | 410 | 135 | 32.9 | 31492 | 0.429 (0.362-0.507) | — | |
| GVHD prophylaxis regimen†† | |||||||
| Cyclosporine | 127 | 55 | 43.3 | .12 | 9172 | 0.600 (0.460-0.781) | 1.27 (0.919-1.73) |
| No cyclosporine | 479 | 170 | 35.5 | 35892 | 0.473 (0.407-0.549) | — | |
| Tacrolimus‡‡ | 199 | 80 | 40.2 | .28 | 14087 | 0.568 (0.456-0.707) | 1.21 (0.912-1.61) |
| No tacrolimus | 407 | 145 | 35.6 | 30977 | 0.468 (0.398-0.551) | — | |
| Sirolimus + tacrolimus | 252 | 78 | 31 | .008 | 19789 | 0.394 (0.316-0.492) | 0.678 (0.508-0.898) |
| No sirolimus + tacrolimus | 354 | 147 | 41.5 | 25275 | 0.582 (0.495-0.684) | — | |
| Prednisone | 110 | 45 | 40.9 | .38 | 8155 | 0.552 (0.412-0.739) | 1.13 (0.797-1.58) |
| No prednisone | 496 | 180 | 36.3 | 36909 | 0.488 (0.421-0.564) | — | |
| Methotrexate | 382 | 150 | 39.3 | .16 | 27559 | 0.544 (0.464-0.639) | 1.27 (0.957-1.70) |
| No methotrexate | 224 | 75 | 33.5 | 17505 | 0.428 (0.342-0.537) | — | |
| Mycophenolate mofetil | 48 | 20 | 41.7 | .53 | 3455 | 0.579 (0.374-0.897) | 1.18 (0.703-1.86) |
| No mycophenolate mofetil | 558 | 205 | 36.7 | 41609 | 0.493 (0.430-0.565) | — | |
| T-cell depletion§§ | 75 | 29 | 38.7 | .80 | 5513 | 0.526 (0.366-0.757) | 1.06 (0.693-1.57) |
| No T-cell depletion | 531 | 196 | 36.9 | 39551 | 0.496 (0.431-0.570) | — | |
| Transplantation period | .03 | ||||||
| 4/2000-6/2001 | 157 | 73 | 46.5 | 10653 | 0.685 (0.545-0.862) | 1 | |
| 7/2001-6/2002 | 144 | 51 | 35.4 | 10717 | 0.476 (0.362-0.626) | 0.695 (0.476-1.010) | |
| 7/2002-6/2003 | 150 | 46 | 30.7 | 11919 | 0.386 (0.289-0.515) | 0.563 (0.381-0.826) | |
| 7/2003-6/2004 | 155 | 55 | 35.5 | 11775 | 0.467 (0.359-0.608) | 0.682 (0.471-0.981) |
| Characteristic . | No. . | CMV reactivation* . | % . | P . | Days after HSCT† . | IR (95% CI)‡ . | IRR (95% CI)§ . |
|---|---|---|---|---|---|---|---|
| Cohort | 606 | 225 | 37.1 | — | 45064 | 0.499 (0.438-0.569) | — |
| CMV serostatus‖ | < .001 | ||||||
| CMV D−/R− | 246 | 45 | 18.3 | 20982 | 0.215 (0.160-0.287) | 1 | |
| CMV D+/R− | 115 | 36 | 31.3 | 8881 | 0.405 (0.292-0.562) | 1.89 (1.19-3.00) | |
| CMV D+/R+ | 110 | 55 | 50.0 | 7289 | 0.755 (0.579-0.983) | 3.52 (2.33-5.34) | |
| CMV D−/R+ | 135 | 89 | 65.9 | 7912 | 1.130 (0.914-1.390) | 5.25 (3.63-7.68) | |
| Recipient age, quartiles, y | .50 | ||||||
| 18 to 35 | 152 | 66 | 43.4 | 11089 | 0.595 (0.468-0.758) | 1 | |
| 36 to 45 | 154 | 48 | 31.2 | 11873 | 0.404 (0.305-0.537) | 0.679 (0.458-1.00) | |
| 46 to 53 | 152 | 55 | 36.2 | 10938 | 0.503 (0.386-0.655) | 0.845 (0.580-1.23) | |
| 54 to 70 | 148 | 56 | 37.8 | 11164 | 0.502 (0.386-0.652) | 0.843 (0.580-1.22) | |
| Recipient sex | .55 | ||||||
| Female | 249 | 96 | 38.6 | 18586 | 0.517 (0.423-0.631) | 1.06 (0.813-1.38) | |
| Male | 357 | 129 | 36.1 | 26478 | 0.487 (0.410-0.579) | — | |
| Recipient race | < .001 | ||||||
| Nonwhite | 42 | 30 | 71.4 | 2384 | 1.260 (0.880-1.800) | 2.75 (1.85-4.00) | |
| White | 564 | 195 | 34.6 | 42680 | 0.457 (0.397-0.526) | — | |
| Primary disease | .25 | ||||||
| AML | 177 | 68 | 38.4 | 12713 | 0.535 (0.422-0.678) | 1 | |
| CML | 90 | 35 | 38.9 | 6605 | 0.530 (0.381-0.738) | 0.991 (0.639-1.51) | |
| MDS | 86 | 36 | 41.9 | 5859 | 0.614 (0.443-0.852) | 1.150 (0.745-1.75) | |
| NHL | 84 | 27 | 32.1 | 6935 | 0.389 (0.267-0.568) | 0.728 (0.448-1.15) | |
| Other | 61 | 16 | 26.2 | 4881 | 0.328 (0.201-0.535) | 0.613 (0.332-1.07) | |
| ALL | 58 | 27 | 46.6 | 4163 | 0.649 (0.445-0.946) | 1.210 (0.746-1.92) | |
| CLL | 50 | 16 | 32.0 | 3908 | 0.409 (0.251-0.668) | 0.765 (0.414-1.33) | |
| Conditioning regimen | .17 | ||||||
| Reduced-intensity | 238 | 80 | 33.6 | 18573 | 0.431 (0.346-0.536) | 0.787 (0.597-1.03) | |
| Myeloablative | 368 | 145 | 39.4 | 26491 | 0.547 (0.465-0.644) | — | |
| HLA match¶ | .08 | ||||||
| Mismatched donor | 65 | 31 | 47.7 | 4097 | 0.757 (0.532-1.760) | 1.60 (1.06-2.34) | |
| Matched donor | 541 | 194 | 35.9 | 40967 | 0.474 (0.411-0.545) | — | |
| Donor relatedness | .02 | ||||||
| Unrelated donor | 315 | 131 | 41.6 | 22395 | 0.585 (0.493-0.694) | 1.41 (1.07-1.86) | |
| Related donor | 291 | 94 | 32.3 | 22669 | 0.415 (0.339-0.508) | — | |
| Stem cell source | .002 | ||||||
| Peripheral blood | 415 | 136 | 32.8 | 31764 | 0.428 (0.362-0.507) | 0.640 (0.490-0.838) | |
| Bone marrow | 191 | 89 | 46.6 | 13300 | 0.669 (0.544-0.824) | — | |
| BMT disease risk group** | .61 | ||||||
| Low | 177 | 71 | 40.1 | 12727 | 0.558 (0.442-0.704) | 1 | |
| Intermediate | 324 | 116 | 35.8 | 24431 | 0.475 (0.396-0.570) | 0.851 (0.628-1.16) | |
| High | 105 | 38 | 36.2 | 7906 | 0.481 (0.350-0.661) | 0.862 (0.565-1.30) | |
| Acute GVHD | .002 | ||||||
| Grades II-IV | 196 | 90 | 45.9 | 13572 | 0.663 (0.539-0.815) | 1.55 (1.17-2.04) | |
| None–grade I | 410 | 135 | 32.9 | 31492 | 0.429 (0.362-0.507) | — | |
| GVHD prophylaxis regimen†† | |||||||
| Cyclosporine | 127 | 55 | 43.3 | .12 | 9172 | 0.600 (0.460-0.781) | 1.27 (0.919-1.73) |
| No cyclosporine | 479 | 170 | 35.5 | 35892 | 0.473 (0.407-0.549) | — | |
| Tacrolimus‡‡ | 199 | 80 | 40.2 | .28 | 14087 | 0.568 (0.456-0.707) | 1.21 (0.912-1.61) |
| No tacrolimus | 407 | 145 | 35.6 | 30977 | 0.468 (0.398-0.551) | — | |
| Sirolimus + tacrolimus | 252 | 78 | 31 | .008 | 19789 | 0.394 (0.316-0.492) | 0.678 (0.508-0.898) |
| No sirolimus + tacrolimus | 354 | 147 | 41.5 | 25275 | 0.582 (0.495-0.684) | — | |
| Prednisone | 110 | 45 | 40.9 | .38 | 8155 | 0.552 (0.412-0.739) | 1.13 (0.797-1.58) |
| No prednisone | 496 | 180 | 36.3 | 36909 | 0.488 (0.421-0.564) | — | |
| Methotrexate | 382 | 150 | 39.3 | .16 | 27559 | 0.544 (0.464-0.639) | 1.27 (0.957-1.70) |
| No methotrexate | 224 | 75 | 33.5 | 17505 | 0.428 (0.342-0.537) | — | |
| Mycophenolate mofetil | 48 | 20 | 41.7 | .53 | 3455 | 0.579 (0.374-0.897) | 1.18 (0.703-1.86) |
| No mycophenolate mofetil | 558 | 205 | 36.7 | 41609 | 0.493 (0.430-0.565) | — | |
| T-cell depletion§§ | 75 | 29 | 38.7 | .80 | 5513 | 0.526 (0.366-0.757) | 1.06 (0.693-1.57) |
| No T-cell depletion | 531 | 196 | 36.9 | 39551 | 0.496 (0.431-0.570) | — | |
| Transplantation period | .03 | ||||||
| 4/2000-6/2001 | 157 | 73 | 46.5 | 10653 | 0.685 (0.545-0.862) | 1 | |
| 7/2001-6/2002 | 144 | 51 | 35.4 | 10717 | 0.476 (0.362-0.626) | 0.695 (0.476-1.010) | |
| 7/2002-6/2003 | 150 | 46 | 30.7 | 11919 | 0.386 (0.289-0.515) | 0.563 (0.381-0.826) | |
| 7/2003-6/2004 | 155 | 55 | 35.5 | 11775 | 0.467 (0.359-0.608) | 0.682 (0.471-0.981) |
AML indicates acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; and Other, other hematologic malignancies (myeloproliferative syndromes, Hodgkin disease, multiple myeloma, etc); ALL, acute lymphocytic leukemia; and CLL, chronic lymphocytic leukemia.
Positive CMV-HCA, whole blood CMV DNA hybrid capture assay, within the first 100 days after HSCT.
Censored at day of positive CMV reactivation, death, or day +100.
IR indicates crude incidence rate. Cases/100 patient-days after HSCT. Confidence intervals were calculated using the Rothman-Greenland method.
IRR indicates crude incidence rate ratio, when compared with other levels of the covariate. Confidence intervals were calculated using the Fisher Exact method.
D indicates donor; R, recipient; +, CMV IgG positive; and −, CMV IgG negative.
Patients were considered HLA-matched if all 6/6 HLA A, B, and DR were identical.
Diseases considered low risk were AML or ALL in first complete remission, CML in first chronic phase, aplastic anemia, MDS in patients with refractory anemia or refractory anemia with ringed sideroblasts. Patients with relapsed or refractory disease were considered high risk. Patients not meeting either criterion were considered intermediate risk.
Most regimens included more than one agent or procedure.
Patients who received tacrolimus prophylaxis without concomitant sirolimus.
Includes CD6– or CD8–depletion or CD34+ selection.
Other covariates associated with an increased incidence of CMV-HCA positivity after HSCT were recipients with race other than white (incidence rate ratio [IRR], 2.75; 95% CI, 1.85-4.00), those who received HSC transplant from an unrelated (IRR, 1.41; 95%, CI 1.07-1.86) or mismatched (IRR, 1.60; 95% CI, 1.06-2.34) donor, and those who experienced grades II-IV acute GVHD (IRR, 1.55; 95% CI, 1.17-2.04).
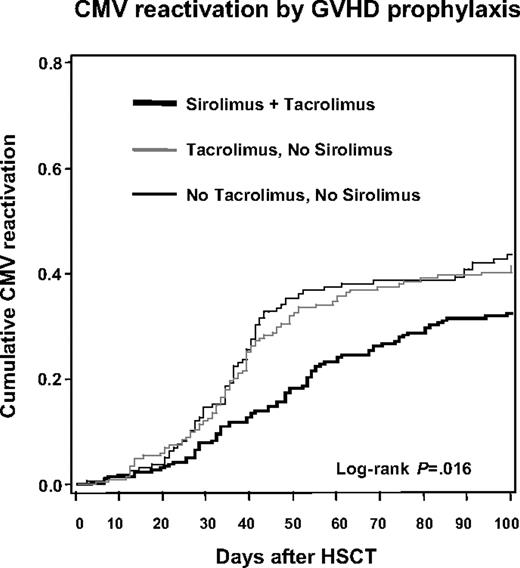
Patients who received PBSCs (IRR, 0.64; 95% CI, 0.49-0.84) and those who received sirolimus-tacrolimus prophylaxis for GVHD (IRR, 0.68; 95% CI, 0.51-0.90) had lower incidence rates of CMV-HCA positivity. There was a trend toward a decreased CMV reactivation rate among patients who received reduced-intensity conditioning (IRR, 0.79; 95% CI, 0.60-1.03). Patients who underwent transplantation in the first period of the cohort (April 2000–June 2001) had a higher incidence of CMV reactivation when compared with subsequent periods (P = .03). Cumulative probability curves demonstrated a significantly lower probability of CMV reactivation among patients who received sirolimus-tacrolimus GVHD prophylaxis when compared with patients who received tacrolimus without sirolimus, and to those who received GVHD prophylaxis with other regimens (Figure 1; log-rank, P = .016).
Kaplan-Meier plot of time to first positive CMV hybrid capture assay by acute GVHD prophylaxis regimen group.
Kaplan-Meier plot of time to first positive CMV hybrid capture assay by acute GVHD prophylaxis regimen group.
Sirolimus exposure in the cohort and its distribution among known risk factors for CMV reactivation
The distribution of sirolimus-tacrolimus use for GVHD prophylaxis was explored in order to identify possible confounding among covariates that were associated with CMV reactivation (Table 2).
Baseline characteristics of allogeneic HSCT cohort according to sirolimus use for GVHD prophylaxis (DFCI/BWH April 2000-June 2004)
| Characteristic . | Sirolimus GVHD prophylaxis (%)* . | Nonsirolimus GVHD prophylaxis (%) . | P . |
|---|---|---|---|
| No. of patients | 252 | 354 | — |
| CMV serostatus | .087 | ||
| CMV D-/R- | 90 (35.7) | 156 (44.1) | |
| CMV D+/R- | 48 (19.1) | 67 (18.9) | |
| CMV D+/R+ | 56 (22.2) | 54 (15.2) | |
| CMV D-/R+ | 58 (23.0) | 77 (21.8) | |
| Median age, years (IQR, range) | 45 (34-53, 18-69) | 45 (36-53, 18-70) | .68 |
| Male sex | 156 (61.9) | 201 (56.8) | .21 |
| Race | .05 | ||
| White | 241 (95.6) | 323 (91.2) | |
| Nonwhite | 11 (4.4) | 31 (8.8) | |
| Primary disease | .03 | ||
| ALL | 19 (7.5) | 39 (11.0) | |
| AML | 61 (24.2) | 116 (32.8) | |
| CLL | 20 (7.9) | 30 (8.5) | |
| CML | 45 (17.9) | 45 (12.7) | |
| MDS | 34 (13.5) | 52 (14.7) | |
| NHL | 45 (17.9) | 39 (11.0) | |
| Other | 28 (11.1) | 33 (9.3) | |
| Conditioning | .67 | ||
| Myeloablative | 156 (61.7) | 212 (59.9) | |
| Reduced-intensity | 96 (38.1) | 142 (40.1) | |
| Stem cell source | .013 | ||
| Peripheral blood | 187 (74.2) | 228 (64.4) | |
| Bone marrow | 65 (25.8) | 126 (35.6) | |
| HLA match | .29 | ||
| Matched | 221 (87.7) | 320 (90.4) | |
| Mismatched | 31 (12.3) | 34 (9.6) | |
| Donor relatedness | .001 | ||
| Related | 100 (39.7) | 191 (54.0) | |
| Unrelated | 152 (60.3) | 163 (46.0) | |
| BMT disease risk group | .72 | ||
| Low | 74 (29.4) | 103 (29.1) | |
| Intermediate | 138 (54.7) | 186 (52.5) | |
| High | 40 (15.9) | 65 (18.4) | |
| Acute GVHD | .01 | ||
| None or grade I | 185 (73.4) | 225 (63.6) | |
| Grades II-IV | 67 (26.6) | 129 (36.4) | |
| GVHD prophylaxis regimen | < .001 | ||
| Prednisone | 0 | 110 (31.1) | |
| Cyclosporine | 0 | 127 (35.9) | |
| Tacrolimus | 252 (100) | 199 (56.2) | |
| Methotrexate | 188 (74.6) | 194 (54.8) | |
| Mycophenolate mofetil | 5 (2.0) | 43 (12.1) | |
| T-cell depletion | 0 | 75 (21.2) | |
| Transplantation period | < .001 | ||
| 4/2000-6/2001 | 38 (15.1) | 119 (33.6) | |
| 7/2001-6/2002 | 18 (7.1) | 126 (35.6) | |
| 7/2002-6/2003 | 84 (33.3) | 66 (18.6) | |
| 7/2003-6/2004 | 112 (44.4) | 43 (12.2) |
| Characteristic . | Sirolimus GVHD prophylaxis (%)* . | Nonsirolimus GVHD prophylaxis (%) . | P . |
|---|---|---|---|
| No. of patients | 252 | 354 | — |
| CMV serostatus | .087 | ||
| CMV D-/R- | 90 (35.7) | 156 (44.1) | |
| CMV D+/R- | 48 (19.1) | 67 (18.9) | |
| CMV D+/R+ | 56 (22.2) | 54 (15.2) | |
| CMV D-/R+ | 58 (23.0) | 77 (21.8) | |
| Median age, years (IQR, range) | 45 (34-53, 18-69) | 45 (36-53, 18-70) | .68 |
| Male sex | 156 (61.9) | 201 (56.8) | .21 |
| Race | .05 | ||
| White | 241 (95.6) | 323 (91.2) | |
| Nonwhite | 11 (4.4) | 31 (8.8) | |
| Primary disease | .03 | ||
| ALL | 19 (7.5) | 39 (11.0) | |
| AML | 61 (24.2) | 116 (32.8) | |
| CLL | 20 (7.9) | 30 (8.5) | |
| CML | 45 (17.9) | 45 (12.7) | |
| MDS | 34 (13.5) | 52 (14.7) | |
| NHL | 45 (17.9) | 39 (11.0) | |
| Other | 28 (11.1) | 33 (9.3) | |
| Conditioning | .67 | ||
| Myeloablative | 156 (61.7) | 212 (59.9) | |
| Reduced-intensity | 96 (38.1) | 142 (40.1) | |
| Stem cell source | .013 | ||
| Peripheral blood | 187 (74.2) | 228 (64.4) | |
| Bone marrow | 65 (25.8) | 126 (35.6) | |
| HLA match | .29 | ||
| Matched | 221 (87.7) | 320 (90.4) | |
| Mismatched | 31 (12.3) | 34 (9.6) | |
| Donor relatedness | .001 | ||
| Related | 100 (39.7) | 191 (54.0) | |
| Unrelated | 152 (60.3) | 163 (46.0) | |
| BMT disease risk group | .72 | ||
| Low | 74 (29.4) | 103 (29.1) | |
| Intermediate | 138 (54.7) | 186 (52.5) | |
| High | 40 (15.9) | 65 (18.4) | |
| Acute GVHD | .01 | ||
| None or grade I | 185 (73.4) | 225 (63.6) | |
| Grades II-IV | 67 (26.6) | 129 (36.4) | |
| GVHD prophylaxis regimen | < .001 | ||
| Prednisone | 0 | 110 (31.1) | |
| Cyclosporine | 0 | 127 (35.9) | |
| Tacrolimus | 252 (100) | 199 (56.2) | |
| Methotrexate | 188 (74.6) | 194 (54.8) | |
| Mycophenolate mofetil | 5 (2.0) | 43 (12.1) | |
| T-cell depletion | 0 | 75 (21.2) | |
| Transplantation period | < .001 | ||
| 4/2000-6/2001 | 38 (15.1) | 119 (33.6) | |
| 7/2001-6/2002 | 18 (7.1) | 126 (35.6) | |
| 7/2002-6/2003 | 84 (33.3) | 66 (18.6) | |
| 7/2003-6/2004 | 112 (44.4) | 43 (12.2) |
— indicates not applicable.
Percentages in parenthesis refer to the total of patients in each column. Total numbers are the same as in Table 1.
Age, sex, BMT disease risk groups, and conditioning regimens were similar among patients prescribed sirolimus-based GVHD prophylaxis and those who received other regimens. The distribution of disease groups that necessitated HSCT was different among patients who received sirolimus-tacrolimus, with higher proportions of patients with chronic myeloid leukemia (CML) and nonHodgkin lymphoma (NHL), but lower proportion of patients undergoing HSCT for acute myeloid leukemia (AML). However, none of these baseline covariates was associated with an increased incidence proportion of CMV reactivation.
Interestingly, the distribution of sirolimus-tacrolimus use in the cohort was higher among some covariates associated with decreased risk of CMV reactivation, but also with others associated with increased risk of CMV reactivation. The sirolimus-tacrolimus group had lower incident acute GVHD grades II-IV when compared with the rest of the cohort (26.6% vs 36.4%, P = .01). The proportion of nonwhite recipients was lower (4.4% vs 8.8%, P = .05) as well as that of recipients of stem cells collected from bone marrow aspiration (25.8% vs 35.6%, P = .01). The proportion of sirolimus-tacrolimus use in the 2 most recent cohort periods was significantly higher than in the 2 earlier periods: 72.3% of the patients undergoing HSCT between July 2003 to June 2004 received sirolimus-tacrolimus for GVHD prophylaxis when only 24.2% did so between April 2000 to June 2001 (P < .001). All these covariates were associated with decreased cumulative incidence of CMV reactivation in the cohort.
On the other hand, more sirolimus-tacrolimus recipients were CMV D+ and/or R+ (64.3% vs 55.9%, P = .04) and more of them received hematopoietic stem cells from unrelated donors (60.3% vs 46.0%, P = .001). Both covariates were associated with increased cumulative incidence of CMV reactivation in the cohort. Physicians were unaware of any relationship between sirolimus use and CMV infection during the study period.
Multivariate model of CMV reactivation
Univariate Cox models for time to a first positive CMV-HCA were computed for covariates found to be associated with CMV reactivation in the cohort (Table 3). The initial multivariate proportional hazards model included donor-recipient CMV serology as a 4-level covariate with D−/R− as reference group, race, conditioning regimen, donor-recipient HLA-mismatch and unrelatedness, stem cell source, incident acute grades II-IV GVHD as a time-varying covariate, transplantation period (April 2000–June 2001 as referent), and GVHD prophylaxis with sirolimus-tacrolimus, tacrolimus, prednisone, methotrexate, mycophenolate mofetil (MMF), and T-cell depletion, with cyclosporine as the reference group. The proportional hazards assumption was confirmed for all time*covariate products with the exception of PBSC use. An overall hazard plot of the risk of CMV reactivation stratified by stem cell source confirmed a small but relatively higher hazard of CMV reactivation early after HSCT in patients receiving PBSCs, with crossing of the hazard curves at day +20, with a subsequent persistently lower hazard of CMV reactivation for the rest of the initial 100 days after HSCT. As the primary objective of the study was to analyze the contribution of GHVD prophylaxis regimens to the risk of CMV reactivation after HSCT, the final multivariate model was stratified by stem cell source and is presented in Table 3.
Proportional hazards modeling of risk of CMV reactivation after allogeneic HSCT, stratified by stem cell source (PBSC vs BM)
| Characteristic . | Univariate HR (95% CI) . | P . | Multivariate HR (95% CI) . | P . |
|---|---|---|---|---|
| Donor/recipient CMV serostatus | < .001 | |||
| CMV D-/R- | 1 | — | 1 | — |
| CMV D+/R- | 1.867 (1.204-2.894) | — | 2.016 (1.292-3.146) | .002 |
| CMV D+/R+ | 3.476 (2.343-5.159) | — | 4.176 (2.762-6.315) | < .001 |
| CMV D-/R+ | 5.210 (3.634-7.468) | — | 5.655 (3.873-8.257) | < .001 |
| Recipient race: non white | 2.659 (1.808-3.910) | < .001 | 1.434 (0.924-2.223) | .11 |
| Conditioning: reduced-intensity | 0.802 (0.610-1.054) | .11 | 0.738 (0.487-1.121) | .15 |
| HLA matching: mismatched donor | 1.545 (1.057-2.258) | .025 | 1.243 (0.813-1.899) | .32 |
| Donor relatedness: unrelated donor | 1.391 (1.067-1.813) | .015 | 1.138 (0.834-1.553) | .42 |
| Stem cell source: peripheral blood | 0.655 (0.501-0.855) | .002 | —† | — |
| Transplantation period | ||||
| 4/2000-6/2001 | 1 | — | 1 | — |
| 7/2001-6/2002 | 0.708 (0.495-1.013) | .06 | 1.108 (0.709-1.729) | .65 |
| 7/2002-6/2003 | 0.570 (0.394-0.825) | .003 | 0.746 (0.444-1.254) | .27 |
| 7/2003-6/2004 | 0.688 (0.485-0.977) | .036 | 1.005 (0.598-1.690) | .98 |
| Acute GVHD*: grades II-IV | 1.532 (1.159-2.025) | .003 | 1.518 (1.088-2.118) | .01 |
| GVHD prophylaxis regimen | ||||
| Cyclosporine | 1.275 (0.941-1.729) | .12 | 1 | — |
| Tacrolimus alone | 1.212 (0.922-1.592) | .17 | 0.664 (0.391-1.127) | .14 |
| Sirolimus + tacrolimus | 0.672 (0.511-0.885) | .005 | 0.455 (0.266-0.779) | .004 |
| Prednisone | 1.140 (0.822-1.580) | .43 | 1.270 (0.706-2.286) | .42 |
| Methotrexate | 1.258 (0.953-1.660) | .10 | 1.741 (1.072-2.827) | .02 |
| Mycophenolate mofetil | 1.190 (0.752-1.884) | .46 | 1.056 (0.584-1.907) | .86 |
| T-cell depletion | 1.081 (0.732-1.596) | .67 | 1.136 (0.662-1.952) | .64 |
| Characteristic . | Univariate HR (95% CI) . | P . | Multivariate HR (95% CI) . | P . |
|---|---|---|---|---|
| Donor/recipient CMV serostatus | < .001 | |||
| CMV D-/R- | 1 | — | 1 | — |
| CMV D+/R- | 1.867 (1.204-2.894) | — | 2.016 (1.292-3.146) | .002 |
| CMV D+/R+ | 3.476 (2.343-5.159) | — | 4.176 (2.762-6.315) | < .001 |
| CMV D-/R+ | 5.210 (3.634-7.468) | — | 5.655 (3.873-8.257) | < .001 |
| Recipient race: non white | 2.659 (1.808-3.910) | < .001 | 1.434 (0.924-2.223) | .11 |
| Conditioning: reduced-intensity | 0.802 (0.610-1.054) | .11 | 0.738 (0.487-1.121) | .15 |
| HLA matching: mismatched donor | 1.545 (1.057-2.258) | .025 | 1.243 (0.813-1.899) | .32 |
| Donor relatedness: unrelated donor | 1.391 (1.067-1.813) | .015 | 1.138 (0.834-1.553) | .42 |
| Stem cell source: peripheral blood | 0.655 (0.501-0.855) | .002 | —† | — |
| Transplantation period | ||||
| 4/2000-6/2001 | 1 | — | 1 | — |
| 7/2001-6/2002 | 0.708 (0.495-1.013) | .06 | 1.108 (0.709-1.729) | .65 |
| 7/2002-6/2003 | 0.570 (0.394-0.825) | .003 | 0.746 (0.444-1.254) | .27 |
| 7/2003-6/2004 | 0.688 (0.485-0.977) | .036 | 1.005 (0.598-1.690) | .98 |
| Acute GVHD*: grades II-IV | 1.532 (1.159-2.025) | .003 | 1.518 (1.088-2.118) | .01 |
| GVHD prophylaxis regimen | ||||
| Cyclosporine | 1.275 (0.941-1.729) | .12 | 1 | — |
| Tacrolimus alone | 1.212 (0.922-1.592) | .17 | 0.664 (0.391-1.127) | .14 |
| Sirolimus + tacrolimus | 0.672 (0.511-0.885) | .005 | 0.455 (0.266-0.779) | .004 |
| Prednisone | 1.140 (0.822-1.580) | .43 | 1.270 (0.706-2.286) | .42 |
| Methotrexate | 1.258 (0.953-1.660) | .10 | 1.741 (1.072-2.827) | .02 |
| Mycophenolate mofetil | 1.190 (0.752-1.884) | .46 | 1.056 (0.584-1.907) | .86 |
| T-cell depletion | 1.081 (0.732-1.596) | .67 | 1.136 (0.662-1.952) | .64 |
— indicates not applicable.
GVHD was modeled as a time-varying covariate.
Adjusted main effect of PBSC cannot be calculated, as analysis was stratified by PBSC given crossing of hazards.
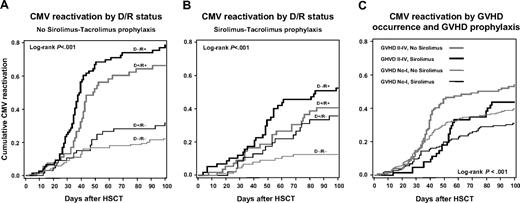
In the multivariable adjusted model, only donor-recipient CMV serostatus pairs, incident acute grades II-IV GVHD (adjusted HR, 1.52; 95% CI, 1.09-2.12; P = .01), and sirolimus-tacrolimus use for GVHD prophylaxis (adjusted HR, 0.46; 95% CI, 0.27-0.78; P = .004) remained significant predictors of CMV reactivation at a P < .05 significance level. In addition, methotrexate use for GVHD prophylaxis became a significant risk of CMV reactivation in the adjusted model (adjusted HR, 1.74; 95% CI, 1.07-2.83; P = .02). The adjusted risk of PBSCs on CMV reactivation cannot be directly calculated as this covariate was stratified in this analysis. The gradation of the risk of CMV-HCA positivity according to all possible CMV donor-recipient pairs persisted in the adjusted model: the adjusted HR for CMV D−/R+ patients was 5.66 (95% CI, 3.87-8.26; P < .001), 4.18 (95% CI, 2.76-6.32; P < .001) for CMV D+/R+ patients, and 2.02 (95% CI, 1.29-3.15; P = .002) for D+/R− patients when compared with D−/R− recipients. To illustrate the findings of the multivariable model, the effect of CMV serostatus pairs and sirolimus-tacrolimus prophylaxis on the cumulative probability of CMV reactivation is seen in Figure 2A-B. The effect of sirolimus-tacrolimus prophylaxis and incident acute GVHD grades II-IV on the cumulative probability of CMV reactivation is presented in Figure 2C. There was no interaction (effect modification) between acute GVHD and sirolimus-tacrolimus use in the model on the adjusted HR of CMV reactivation (P = .67).
Time to first positive CMV hybrid capture assay. (A) Kaplan-Meier plot of time to first positive CMV DNA hybrid capture assay by CMV donor/recipient serostatus among HSC transplant recipients that did not receive sirolimus-tacrolimus prophylaxis. (B) Kaplan-Meier plot of time to first positive CMV DNA hybrid capture assay by CMV donor/recipient serostatus among HSC transplant recipients that received sirolimus-tacrolimus prophylaxis. (C) Kaplan-Meier plot of time to first positive CMV DNA hybrid capture assay stratified by sirolimus-tacrolimus GVHD prophylaxis and incident grades II-IV GVHD.
Time to first positive CMV hybrid capture assay. (A) Kaplan-Meier plot of time to first positive CMV DNA hybrid capture assay by CMV donor/recipient serostatus among HSC transplant recipients that did not receive sirolimus-tacrolimus prophylaxis. (B) Kaplan-Meier plot of time to first positive CMV DNA hybrid capture assay by CMV donor/recipient serostatus among HSC transplant recipients that received sirolimus-tacrolimus prophylaxis. (C) Kaplan-Meier plot of time to first positive CMV DNA hybrid capture assay stratified by sirolimus-tacrolimus GVHD prophylaxis and incident grades II-IV GVHD.
Sensitivity analysis
One interesting feature of the data is that 45 (18.3%) CMV D−/R− patients had at least one positive CMV-HCA result. Although the cumulative probability of CMV reactivation in D−/R− recipients is similar to previous reports,3,20 it is higher than recent cohorts in which the prevention of transfusion-associated CMV was minimized with use of leukoreduction by filtration.44 This may reflect the possibility of false-positive results40,42 by considering only a single CMV-HCA test positive. As laboratory personnel who perform CMV-HCAs are unaware of a patient's CMV serostatus, the probability of false-positive testing should be similar among CMV D/R strata. Nondifferential misclassification43 should attenuate, but not bias, any association between the covariates and the risk of CMV reactivation. Nevertheless, as a sensitivity analysis and to test this hypothesis, we modeled the time to persistent CMV-HCA positivity defined as time to 2 consecutive positive CMV-HCAs as the event of interest with appropriate censoring.
There were 87 patients with persistent CMV viremia during the first 100 days after HSCT for a cumulative incidence of 14.4% and an overall incidence rate of 0.166 cases/100 patient-days after HSCT (95% CI, 0.134-0.204). The multivariate analysis of risk of persistent CMV-HCA positivity is presented in Table 4using the same model covariates as in the main analysis. Given that only 3 patients had persistent CMV-HCA in the CMV D−/R− group, the crude and adjusted HRs for the other CMV D/R strata are quite larger than in the main analysis. The adjusted HR for incident acute GVHD grades II-IV was 1.87 (95% CI, 1.23-3.11; P = .016) and that for use of sirolimus-tacrolimus for GVHD prophylaxis was 0.22 (95% CI, 0.09-0.55; P = .001) using cyclosporine as reference group. In this stringent model, reduced-intensity conditioning became significantly protective with an adjusted HR of 0.40 (95% CI, 0.17-0.94; P = .036), whereas methotrexate use did not maintain its significance as a risk factor. Removing patients with CMV D−/R− serostatus and using patients with CMV D+/R− serostatus as the reference group provided similar adjusted HR estimates (data not shown).
Proportional hazards modeling of risk of persistent* CMV-HCA positivity after allogeneic HSCT, stratified by stem cell source (PBSC vs BM)
| Characteristic . | Univariate HR (95% CI) . | P . | Multivariate HR (95% CI) . | P . |
|---|---|---|---|---|
| Donor/recipient CMV serostatus | < .001 | |||
| CMV D-/R- | 1 | — | 1 | — |
| CMV D+/R- | 4.403 (1.101-17.60) | — | 5.259 (1.308-21.15) | .02 |
| CMV D+/R+ | 24.01 (7.284-79.15) | — | 37.60 (11.24-125.7) | < .001 |
| CMV D-/R+ | 38.44 (12.00-123.2) | — | 51.13 (15.70-166.5) | < .001 |
| Recipient race: nonwhite | 3.169 (1.842-5.452) | < .001 | 0.867 (0.463-1.625) | .66 |
| Conditioning | ||||
| Reduced-intensity HLA matching | 0.488 (0.301-0.792) | .004 | 0.404 (0.173-0.944) | .036 |
| Mismatched donor | 2.147 (1.248-3.693) | .006 | 1.915 (0.993-3.691) | .06 |
| Donor relatedness: unrelated donor | 1.504 (0.978-2.314) | .06 | 1.464 (0.863-2.484) | .16 |
| Stem cell source: peripheral blood | 0.515 (0.338-0.785) | .002 | —‡ | — |
| Transplantation period | ||||
| 4/2000-6/2001 | 1 | — | 1 | — |
| 7/2001-6/2002 | 0.619 (0.361-1.064) | .08 | 1.141 (0.536-2.427) | .73 |
| 7/2002-6/2003 | 0.378 (0.203-0.703) | .002 | 0.456 (0.180-1.160) | .10 |
| 7/2003-6/2004 | 0.453 (0.254-0.809) | .007 | 0.867 (0.358-2.099) | .75 |
| Acute GVHD†: grades II-IV | 2.549 (1.654-3.929) | < .001 | 1.871 (1.123-3.117) | .016 |
| GVHD prophylaxis regimen | ||||
| Cyclosporine | 1.553 (0.976-2.471) | .06 | 1 | — |
| Tacrolimus alone | 1.443 (0.940-2.215) | .09 | 0.661 (0.277-1.573) | .35 |
| Sirolimus + tacrolimus | 0.613 (0.474-0.795) | .001 | 0.224 (0.091-0.549) | .001 |
| Prednisone | 1.087 (0.640-1.846) | .76 | 1.160 (0.423-3.180) | .77 |
| Methotrexate | 0.899 (0.586-1.380) | .63 | 1.192 (0.531-2.678) | .67 |
| Mycophenolate mofetil | 1.213 (0.586-2.510) | .60 | 0.943 (0.353-2.521) | .91 |
| T-cell depletion | 2.463 (1.507-4.026) | .001 | 1.440 (0.633-3.275) | .38 |
| Characteristic . | Univariate HR (95% CI) . | P . | Multivariate HR (95% CI) . | P . |
|---|---|---|---|---|
| Donor/recipient CMV serostatus | < .001 | |||
| CMV D-/R- | 1 | — | 1 | — |
| CMV D+/R- | 4.403 (1.101-17.60) | — | 5.259 (1.308-21.15) | .02 |
| CMV D+/R+ | 24.01 (7.284-79.15) | — | 37.60 (11.24-125.7) | < .001 |
| CMV D-/R+ | 38.44 (12.00-123.2) | — | 51.13 (15.70-166.5) | < .001 |
| Recipient race: nonwhite | 3.169 (1.842-5.452) | < .001 | 0.867 (0.463-1.625) | .66 |
| Conditioning | ||||
| Reduced-intensity HLA matching | 0.488 (0.301-0.792) | .004 | 0.404 (0.173-0.944) | .036 |
| Mismatched donor | 2.147 (1.248-3.693) | .006 | 1.915 (0.993-3.691) | .06 |
| Donor relatedness: unrelated donor | 1.504 (0.978-2.314) | .06 | 1.464 (0.863-2.484) | .16 |
| Stem cell source: peripheral blood | 0.515 (0.338-0.785) | .002 | —‡ | — |
| Transplantation period | ||||
| 4/2000-6/2001 | 1 | — | 1 | — |
| 7/2001-6/2002 | 0.619 (0.361-1.064) | .08 | 1.141 (0.536-2.427) | .73 |
| 7/2002-6/2003 | 0.378 (0.203-0.703) | .002 | 0.456 (0.180-1.160) | .10 |
| 7/2003-6/2004 | 0.453 (0.254-0.809) | .007 | 0.867 (0.358-2.099) | .75 |
| Acute GVHD†: grades II-IV | 2.549 (1.654-3.929) | < .001 | 1.871 (1.123-3.117) | .016 |
| GVHD prophylaxis regimen | ||||
| Cyclosporine | 1.553 (0.976-2.471) | .06 | 1 | — |
| Tacrolimus alone | 1.443 (0.940-2.215) | .09 | 0.661 (0.277-1.573) | .35 |
| Sirolimus + tacrolimus | 0.613 (0.474-0.795) | .001 | 0.224 (0.091-0.549) | .001 |
| Prednisone | 1.087 (0.640-1.846) | .76 | 1.160 (0.423-3.180) | .77 |
| Methotrexate | 0.899 (0.586-1.380) | .63 | 1.192 (0.531-2.678) | .67 |
| Mycophenolate mofetil | 1.213 (0.586-2.510) | .60 | 0.943 (0.353-2.521) | .91 |
| T-cell depletion | 2.463 (1.507-4.026) | .001 | 1.440 (0.633-3.275) | .38 |
— indicates not applicable.
Persistent is defined as 2 consecutive positive CMV-HCAs.
GVHD was modeled as a time-varying covariate.
Adjusted main effect of PBSC cannot be calculated, as analysis was stratified by PBSC given crossing of hazards.
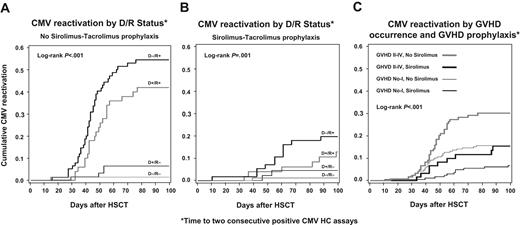
The effect of CMV serostatus pairs and sirolimus-tacrolimus prophylaxis on the cumulative probability of persistent CMVreactivation is presented graphically in Figure 3A-B. The effect of sirolimus-tacrolimus prophylaxis and incident acute GVHD grades II-IV on the cumulative probability of persistent CMV reactivation is presented in Figure 3C.
Time to 2 consecutive positive CMV hybrid capture assays. (A) Kaplan-Meier plot of time to 2 consecutive positive CMV DNA hybrid capture assays by CMV donor/recipient serostatus among HSC transplant recipients that did not receive sirolimus-tacrolimus prophylaxis. (B) Kaplan-Meier plot of time to 2 consecutive positive CMV DNA hybrid capture assays by CMV donor/recipient serostatus among HSC transplant recipients that received sirolimus-tacrolimus prophylaxis. (C) Kaplan-Meier plot of time to 2 consecutive CMV DNA hybrid capture assays stratified by sirolimus-tacrolimus GVHD prophylaxis and incident grades II-IV GVHD.
Time to 2 consecutive positive CMV hybrid capture assays. (A) Kaplan-Meier plot of time to 2 consecutive positive CMV DNA hybrid capture assays by CMV donor/recipient serostatus among HSC transplant recipients that did not receive sirolimus-tacrolimus prophylaxis. (B) Kaplan-Meier plot of time to 2 consecutive positive CMV DNA hybrid capture assays by CMV donor/recipient serostatus among HSC transplant recipients that received sirolimus-tacrolimus prophylaxis. (C) Kaplan-Meier plot of time to 2 consecutive CMV DNA hybrid capture assays stratified by sirolimus-tacrolimus GVHD prophylaxis and incident grades II-IV GVHD.
CMV disease during the first year after HSCT
There were 8 cases of CMV disease during the first year of follow-up after HSCT for a cumulative incidence of 1.32%. Four cases were diagnosed before day + 100; 3 of them hadCMV pneumonia. Five cases were confirmed by biopsy of the affected organ, while 3 had CMV cultures that eventually grew CMV in the setting of pneumonic syndromes without another etiologic explanation. Six cases were CMV D−/R+ and 2 cases CMV D+/R+, while no cases were diagnosed among CMV seronegative recipients. All patients but one received myeloablative conditioning, 6 of them from unrelated donors. All patients but one were diagnosed with acute GVHD, 5 with grade III. Two patients received GVHD prophylaxis with sirolimus-tacrolimus, while others received several other regimens. All patients developed persistently positive CMV-HCA viremia around the time CMV disease was diagnosed.
Univariate analysis of risk of CMV disease in the cohort demonstrated that CMV serostatus was significantly associated with CMV disease. The cumulative incidence of CMV disease was 4.4% among CMV D−/R+ HSC transplant recipients, 1.8% for CMV D+/R+ patients, while no CMV-seronegative recipients developed CMV disease a year after HSCT (P = .001). CMV disease developed in 6 patients who had been diagnosed with grade II or higher acute GVHD for a cumulative incidence of 3.1%, while 2 cases were observed among patients with no or grade I GVHD (0.49%; P = .016). No other covariate was significantly associated with CMV disease. Two patients who received sirolimus-tacrolimus GVHD prophylaxis developed CMV disease for a cumulative incidence of 0.79% while the remainder of patients were not on this regimen (cumulative incidence, 1.69%; P = .47).
Cohort survival
The one-year survival for the cohort was 58.6% (95% CI, 56.6-60.7). To explore the impact of CMV serostatus and sirolimus-tacrolimus use for GVHD prophylaxis on survival at one year, the cohort was divided into 4 possible strata according to CMV serostatus (D−/R− versus any seropositivity)18 and use of sirolimus-tacrolimus prophylaxis for GVHD. Among patients who did not receive sirolimus-tacrolimus prophylaxis, the crude one-year survival was 57.6% (95% CI, 53.6-61.5) among patients who were CMV D−/R− and 50.0% (95% CI, 46.4-53.5) among HSC transplant recipients with any CMV seropositivity. Among patients who received sirolimus-tacrolimus prophylaxis, the crude one-year survival was 72.2% (95% CI, 67.5-76.9) among CMV D−/R− HSC transplant recipients and 63.1% (95% CI, 59.3-67.0) for those with any CMV seropositivity (log-rank P = .006).
Discussion
In this study of a large recent cohort of HSC transplant recipients, CMV serostatus of donor-recipient pairs and incident grades II-IV acute GVHD remain the most significant and stable predictors of CMV reactivation,1-5,17,20,45 verifying the main observations by Meyers et al 20 years after their landmark study.3 In contrast to the availabel management in the 1980s, the overall cumulative incidence of CMV disease was 0.66% at day + 100 and 1.32% at one year after HSCT.
During the period studied, HSCT patients received a variety of treatments that included the evaluation and incorporation into practice of different conditioning strategies, stem cell sources, and GVHD prophylaxis regimens. This variability allowed us to study and model the adjusted contribution of several potential risk factors of CMV reactivation after HSCT.
In multivariable models that adjusted for CMV donor-recipient serostatus pairs, incident acute GVHD, race, conditioning regimens, HLA-matching, donor relatedness, transplantation period, other GVHD prophylaxis drugs, and stratified by stem cell source, we demonstrated a significant reduction in the incidence of CMV reactivation associated with a GVHD prophylaxis regimen containing sirolimus and tacrolimus with an adjusted HR of 0.46 (95% CI, 0.27-0.78; P = .004) in the absence of specific CMV antiviral prophylaxis. This adjusted HR was even lower when persistent CMV viremia was modeled (adjusted HR of 0.22; 95% CI, 0.09-0.55; P = .001). This extends the reported low incidence of CMV infection following solid organ transplantation in patients receiving sirolimus prophylaxis28,29,46 and is the first demonstration of such an effect in the HSCT population. The crude risk of CMV end-organ disease was not reduced among sirolimus-tacrolimus recipients (0.79% versus 1.69%), but the overall cumulative incidence of CMV disease was quite low in the cohort. Although baseline CMV seropositivity in donor or recipient remained associated with a reduced survival a year after transplantation,18 sirolimus-tacrolimus–based prophylaxis did not increase overall mortality (with the possibility of reducing time-at-risk for CMV reactivation), but in fact seemed to confer a survival advantage such that CMV-seropositive HSC transplant recipients who received sirolimus-tacrolimus had a similar one-year survival as CMV-seronegative patients who received other GVHD prophylaxis regimens.
As sirolimus was consistently given with tacrolimus during the study period, we cannot address whether the protective effect observed on the risk of CMV reactivation is due to sirolimus alone or to the combination of sirolimus and tacrolimus. Tacrolimus was frequently used in the cohort during the study period, and although its adjusted HR for risk of CMV reactivation was 0.66 when compared with cyclosporine as reference group for initial or persistent CMV viremia, it did not reach statistical significance in either model (P = .14 and P = .35, respectively).
The measured protective effect of sirolimus-based GVHD prophylaxis observed is probably a conservative estimate as false-positive CMV-HCAs likely occurred,42 thus attenuating the observed association. All GVHD prophylaxis regimens were modeled as baseline exposures in the present study, which may have further attenuated the observed sirolimus-tacrolimus protective association for patients in whom sirolimus was subsequently discontinued due to adverse effects,47 concern for drug interactions,48 or withdrawal of immunosuppression to increase graft-versus-leukemia effect in cases of relapsed disease. Furthermore, whether the observed association is maintained beyond day +100 was not studied, given the further investment needed for capturing sirolimus exposure later in the transplantation process when GVHD prophylaxis is systematically tapered. Of note, the observed decreased risk of CMV reactivation was additional and adjusted to the lower incidence of acute grades II-IV GVHD observed in the cohort among sirolimus-tacrolimus recipients (Tables 3–4; Figures 2–3). We did not study the effect of sirolimus on CMV reactivation and disease when it was administered as a second drug in patients who developed acute or chronic GVHD. Whether sirolimus-containing regimens provide protection against CMV infection when initiated for the treatment of acute or chronic GVHD remains to be determined.
Sirolimus inhibits the kinetics of CMV replication in experimentally infected human foreskin fibroblasts through binding to rictor complex (mammalian target of rapamycin kinase complex 2), which is modified in CMV-infected cells.49 Yet the effect of sirolimus in this in vitro system only delays and decreases the peak levels of CMV titers during productive infection49,50 and it is not likely to explain the clinical effect on CMV infection observed in the cohort. In HSCT, reactivation of CMV latent in recipient tissues early after transplantation is the predominant phenomenon that leads to detectable viremia and clinical disease.2,51 The potential effect of sirolimus on the mechanisms of CMV desilencing and reactivation at the cellular level has not been studied,51 but could perhaps be due to enhanced inhibition of rictor-mediated blockade as suggested in the productive infection models,49 but more likely due to effects on other mechanisms such as histone modifications52 or chromatin remodeling53 of epigenetic CMV genomes.51 Sirolimus-based regimens perhaps facilitate CMV-specific lymphocyte immune reconstitution,54 but this remains to be studied.
The data presented confirm several reported risk factors1,2 of CMV reactivation including the risks associated with unrelated26,55 donors, and the protective effect of reduced-intensity21 conditioning in the absence of T-cell depletion.22,23 However, reports that found age to be a significant risk factor for CMV infection included children,3-7,25 where as our cohort comprised patients 18 years or older at the time of transplantation and age did not have an impact on CMV reactivation.21 Methotrexate use had an increased adjusted HR of CMV reactivation in the main model, but it did not remain significant in the stringent model of persistent CMV positivity, whereas reduced-intensity conditioning became significantly protective for CMV reactivation in the stringent model.
The role of PBSCs on the risk of CMV reactivation deserves mention. Although the overall risk of CMV reactivation was lower among patients who received PBSCs, its adjusted HR could not be addressed in the multivariate models due to crossing hazards, but was controlled for by stem cell source stratification in the multivariable analysis. The initial increased risk when compared with bone marrow transplantation is likely due to earlier engraftment observed with PBSCs that allows detection of CMV reactivation earlier when using assays that depend on leukocyte counts such as the CMV-HCA. The overall decreased risk and decreased hazard after day +20 is likely due to earlier immune reconstitution that has been observed when using PBSCs for HSCT.54,56
The observed association on the decreased risk of CMV reactivation with a sirolimus-tacrolimus GVHD prophylaxis regimen when patients are exposed early after HSCT is robust. Confirmation of this finding from data on CMV reactivation of randomized GVHD prophylaxis trials currently under way57,58 that include sirolimus arms is welcomed given the observational nature of the present study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by NIH grant HL070149.
We are thankful for the data support from Qiheng Yang and the stem cell transplant data management team of the DFCI/BWH HSCT Service.
National Institutes of Health
Authorship
Contribution: F.M.M. conceived of and designed the study; J.B., S.K.B., T.S., F.M.M., and V.T.H. collected and assembled data; F.M.M., J.B., S.K.B., T.S., V.T.H., I.V.B., J.K., E.P.A., R.J.S., C.S.C., J.H.A., and L.R.B. analyzed and interpreted the data; F.M.M., I.V.B., and J.B. performed statistical analysis; F.M.M. drafted the article; J.B., S.K.B., T.S., V.T.H., I.V.B., J.K., E.P.A., R.J.S., C.S.C., J.H.A., and L.R.B. critically revised the article for important intellectual content.
Conflict-of-interest disclosure: F.M.M. has received research grant support from Astellas Pharma US and Wyeth Research for unrelated work. All other authors declare no competing financial interest.
Correspondence: Francisco Marty, Brigham & Women's Hospital, Division of Infectious Diseases, 75 Francis St, PBB-A4, Boston, MA 02115; e-mail: fmarty@partners.org.