Abstract
Macrophages (Mφ) in most solid tumors exhibit a distinct immunosuppressive phenotype, but the mechanisms that allow tumor microenvironments to “educate” Mφ are incompletely understood. Here, we report that culture supernatants (TSNs) from several types of tumor cell lines can drive monocytes to become immunosuppressive Mφ. Kinetic experiments revealed that soon after exposure to these TSNs, monocytes began to provoke transient proinflammatory responses and then became refractory to subsequent stimulation. Other TSNs that failed to cause such temporary preactivation did not alter Mφ polarization. Consistent with these results, we observed that the monocytes/Mφ in different areas of human tumor samples exhibited distinct activation patterns. Moreover, we found that hyaluronan fragments constitute a common factor produced by various tumors to induce the formation of immunosuppressive Mφ, and also that upregulation of hyaluronan synthase-2 in tumor cells is correlated with the ability of the cells to cause Mφ dysfunction. These results indicate that soluble factors derived from tumor cells, including hyaluronan fragments, co-opt the normal development of Mφ to dynamically educate the recruited blood monocytes in different niches of a tumor. The malignant cells can thereby avoid initiation of potentially dangerous Mφ functions and create favorable conditions for tumor progression.
Introduction
Macrophages (Mφ) are essential components of host defense and act as both antigen presenting cells (APCs) and effector cells. Under the influence of local conditions, they acquire specialized phenotypic characteristics with diverse functional programs.1-4 The M1 or classical Mφ are activated by microbial products and interferon (IFN)-γ, and they are capable of efficiently killing microorganisms and tumor cells and eliciting adaptive Th1 immunity. In contrast, M2 Mφ are distinctly activated by anti-inflammatory molecules, such as interleukin (IL)-4 and IL-10, and they express different receptors, have a poor antigen-presenting capacity, and also suppress T-cell responses.4,5 The M2 cells share an IL-12low/IL-10high phenotype and are generally better adapted to remodeling tissues.5-7
Mφ constitute a major component of the leukocyte infiltrate of tumors, and the tumor-associated Mφ (TAM) are derived almost entirely from circulating blood monocytes.3,8,9 Mφ in normal or inflamed tissues exhibit spontaneous antitumor activity, whereas TAM are polarized M2 cells that suppress antitumor immunity and promote tumor progression.5,8 Those findings agree with clinical studies showing that a high density of TAM is associated with poor prognosis in most solid tumors.8-11 Although the precise underlying mechanisms are not yet clear, it is generally assumed that the tumor microenvironment is a critical determinant of the phenotype of local Mφ. Tumor-derived factors, including IL-10 and transforming growth factor (TGF)-β1, “educate” the newly recruited monocytes to take on a M2 phenotype and perform a protumoral role.4,12 In contrast, overexpression or local delivery of IL-12 can re-establish the antitumor activity of Mφ, and in that case a high density of TAM is correlated with a marked reduction in tumor growth.13,14 Such opposing effects of Mφ on tumor progression indicate that selective modulation of Mφ polarization might serve as a novel strategy for cancer therapy. However, such an approach is hampered by the fact that the mechanisms by which tumor microenvironments educate Mφ to perform specific tasks have not been fully elucidated.
Tumor microenvironments comprise both cellular and noncellular (matrix) components.8,15 Hyaluronan (HA) is a major matrix constituent that normally exists as a high-molecular-weight (HMW) (> 1000 kDa) polysaccharide composed of repeating units of glucuronic acid and N-acetylglucosamine.16,17 Clinical and experimental studies have indicated that HA is not merely a space-filling material, but rather a key regulator for tumor progression.17-20 HA is overproduced in many types of tumors, and in some cases HA levels are predictive of malignancy and poor prognosis.19,20 Manipulation of HA production has been shown to markedly alter the course of tumor progression.21 In addition, although not directly related to TAM, HA was recently found to induce potent proinflammatory responses in dendritic cells and Mφ.22,23 Moreover, the effects of HA on tumor or immune cells are size-dependent: small or intermediate-sized HA (INT-HA) fragments activate APC via CD44 or TLR,22-24 whereas HMW-HA or fragments smaller than a hexasaccharide have no effect.17,22 Such behavior indicates that tissue context can determine the action of HA by regulating both the level and the turnover of HA. These mentioned findings, together with results showing that tumor cells deactivate monocytes via CD44 and TLR,25,26 suggest that HA fragments play an important role in the induction of TAM.
The present study shows that soluble factors derived from tumor cells induced transient activation of monocytes and subsequently drove them to develop into immunosuppressive Mφ. This observation, along with the distinct activation patterns of Mφ in tumor samples, indicates that tumor cells may co-opt the normal development of Mφ to dynamically educate the migrating monocytes in different niches of a tumor. Our results also suggest that the HA fragments are the common components released from tumor cells to induce early activation and subsequent formation of immunosuppressive Mφ. Moreover, we provide evidence that upregulation of HAS2 in tumor cells is associated with the ability to cause dysfunction of monocytes.
Materials and methods
The human tumor samples used in the present study were obtained from the Cancer Center of Sun Yat-Sen University and coded anonymously in accordance with local ethical guidelines. We obtained approval from the Review Board of Cancer Center of Sun Yat-Sen University and informed consent in accordance with the Declaration of Helsinki.
Reagents
The antibodies (Abs) and chemicals used and their sources were as follows: anti-IL-10, HLA-DR, and control Ab from R&D Systems (Abingdon, United Kingdom); anti-CD68 mAb and Envision System for immunohistochemistry from DakoCytomation (Glostrup, Denmark); anti-CD44 Ab from LabVision Corporation (Fremont, CA); Lipofectamine 2000, G418, Trizol, cell isolation and tissue culture reagents from Invitrogen (Grand Island, NY); and MMLV reverse-transcriptase from Promega (Madison, WI). The HA-specific blocking peptide Pep-1 (GAHWQFNALTVR) and a control peptide (WRHGFALTAVNQ) were synthesized by GL Biochem (Shanghai, China), as described previously,23 All other reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated in the text.
Tumor cell lines and preparation of tumor culture supernatants
Human cervical (HeLa), hepatoma (SK-Hep-1 and HepG2), and leukemia (THP1 and U937) cell lines were obtained from the American Type Culture Collection; glioma (U251), nasopharyngeal carcinoma (CNE1 and CNE2), and lung carcinoma (95D) cells were from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. All cells were tested for mycoplasma contamination using single-step polymerase chain reaction (PCR) method,27 and maintained in complete medium composed of RPMI 1640 (or Dulbecco modified Eagle medium [DMEM]) supplemented with 10% fetal bovine serum. Tumor culture supernatants (TSNs) were prepared by plating 5 × 106 tumor cells in 10 mL complete medium in 100-mm dishes for 24 hours, and thereafter changing the medium to complete DMEM medium supplemented with 10% human AB serum instead of fetal bovine serum. After 2-3 days, the supernatants were harvested, centrifuged, and stored in aliquots at −80°C.
Isolation and culture of monocytes
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats derived from the blood of healthy donors by Ficoll density gradient as described previously.28 The cells in DMEM alone were plated at 4 × 106/well in 24-well plates for 1.5 hours, washed, and then cultured in DMEM containing 10% human AB serum for 16 hours to remove residual lymphocytes. Thereafter, the monocytes in DMEM containing AB serum were cultured in the presence of HA, 15% TSN, or medium alone for 6 to 8 days to obtain Mφ.28,29 Mφ were activated by 10 ng/mL lipopolysaccharide (LPS) or 50 U/mL IFN-γ for 18 hours. In some experiments, the cells were pretreated with polymyxin B, CD44-blocking Ab, control IgG, Pep-1, or control peptide at the indicated concentrations before exposure to HA or TSN.
Flow cytometry
Monocytes/Mφ were detached with 5 mM EDTA, washed, and then resuspended in phosphate buffered saline (PBS) supplemented with 1% heat-inactivated fetal bovine serum. Thereafter, the cells were stained with fluorochrome-conjugated mAb for CD14, CD64, CD80, CD86, and HLA-DR or control Ab (BD PharMingen, San Diego, CA), and analyzed by flow cytometry (FACS VantageSE, BD Immunocytometry Systems) using CellQuest software version 7.5.3 (FACS Vantage-SE, BD Immunocytometry Systems, San Diego, CA).
Enzyme-linked immunosorbent assay
Concentrations of TNF-α, IL-12p70, IL-1β, and IL-10 were detected by enzyme-linked immunosorbent assay kits (eBioscience, San Diego, CA).
Preparation of HA fragments
INT-HA fragments were prepared by partial digestion of HMW-HA with limited amounts of testicular hyaluronidase exactly as previously described.30 The sizes of HA fragments were determined by 0.5% agarose gel electrophoresis and visualized with cationic dye Stain-All.
Immunohistochemisty
Samples of hepatocellular carcinoma, lung cancers, and nasopharyngeal carcinoma (NPC) (6 of each) were obtained from the Cancer Center of Sun Yat-Sen University and coded anonymously in accordance with local ethical guidelines. Paraffin-embedded and formalin-fixed samples were cut into 5-μm sections, which were then processed for immunohistochemistry as previously described.31 After incubation with the indicated mAb, the adjacent sections were stained using the Envision System with diaminobenzidine or amino-ethylcarbazide.
Total RNA extraction and reverse-transcription PCR
Total RNA was isolated using Trizol reagent. Aliquots containing 2 μg of total RNA were transcribed reversely using MMLV reverse transcriptase. The specific primers used to amplify the genes are listed in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). PCR products were resolved in 1.5% agarose gel and visualized by ethidium bromide staining.
Construction of siRNA expression plasmids and stable transfection
Based on plasmid pcDNA3.0 (Invitrogen), we constructed the plasmid pSi vector (Cheng J. et al. Human macrophages promote the motility and invasiveness of osteopontin–knockdown tumor cells. Cancer Res. 2007. In Press.), which contains the human U6 RNA promoter and an enhanced green fluorescent protein expression cassette under the control of the CMV promoter. The candidate sequence (5′-GGAGTCGTCACATTCTATA-3′) in human HAS2 gene was selected for RNAi. A scrambled siRNA sequence (5′-CACTAGACTACTCATGGTGTGAGAT-3′) that is not homologous to any human DNA sequence was used as a negative control. Two complementary oligonucleotides that contain sense and antisense siRNA sequences were synthesized chemically; these were the loop sequence (5′-TTCAAGACA-3′) and the flanking EcoRI and XbaI sites, which were subsequently annealed and ligated into the linearized pSi vector. Each construct was sequenced to confirm that the sequence of the insert was correct. The construct containing siRNA against the target sequence of HAS2 was designated pSi-HAS2, and the plasmid with scrambled siRNA was named Mock.
The plasmids were amplified, purified with a QIAGEN Plasmid Midi Kit (Qiagen, Valencia, CA), and then transfected into SK-Hep-1 and U251 cells using Lipofectamine 2000 according to the manufacturer's instructions. Transfected cells were selected with 800 μg/mL G418 for 1 month, and stable clones were maintained with 400 μg/mL G418.
Immunoblotting
The proteins from exponentially growing cells were extracted as previously described.28 Equal amounts of cellular proteins were separated by 10% SDS-PAGE, immunoblotted with anti-HAS2 Ab (kindly donated by Dr P. Heldin, Ludwig Institute for Cancer Research, Uppsala, Sweden), and visualized with a ECL kit.
Results
Induction of immunosuppressive macrophages by TSN from cells of solid tumors
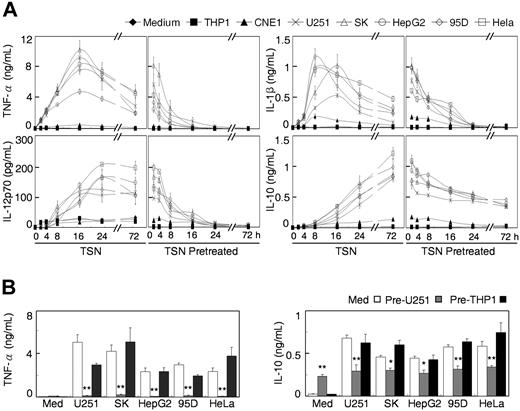
To study the mechanisms involved in the induction of immunosuppressive Mφ by a tumor environment, we first set out to establish conditions under which this process can be reliably reproduced in vitro. Human monocytes were cultured for 6 days in the presence or absence of TSN from 6 different solid tumors and 2 leukemia cell lines. On day 6, the control cells had differentiated into Mφ with reduced expression of CD14 and increased expression of HLA-DR and CD86 on their surface. Exposure of monocytes to 15% TSN obtained from most solid tumor types used in our study, including SK-Hep-1, HepG2, HeLa, U251, and 95D cells, resulted in Mφ with a markedly altered phenotype showing retained CD14 molecules and reduced expression of HLA-DR and CD86 (Figure 1A). These TSN-exposed Mφ also released significant amounts of IL-10, but not TNF-α and IL-12p70 (Figure 1B).
Exposure to TSN redirected monocytes to develop into immunosuppressive Mφ. Human monocytes were cultured for 6 days in medium alone (solid lines) or with 15% TSN from various tumor cells (dashed lines). (A and B) Expression of surface markers and production of cytokines were determined by flow cytometry and enzyme-linked immunosorbent assay, respectively. (C and D) Thereafter, the Mφ were left untreated (Med) or were stimulated with LPS or IFN-γ for 18 hours and were subsequently analyzed. (C) Results for Mφ that were exposed to TSN from U251 cells. The histograms in (A) and (C) are representative of 10 separate experiments, and the mean fluorescence intensity (MFI) values for control and TSN-treated cells (mean ± standard deviation [SD]) are indicated in Table S2. The data on cytokine production represent the mean (± standard error [SE]) of 10 experiments. Statistical differences between groups was calculated by Student t test. *P < .05 and **P < .01, compared with Mφ that were cultured in medium alone.
Exposure to TSN redirected monocytes to develop into immunosuppressive Mφ. Human monocytes were cultured for 6 days in medium alone (solid lines) or with 15% TSN from various tumor cells (dashed lines). (A and B) Expression of surface markers and production of cytokines were determined by flow cytometry and enzyme-linked immunosorbent assay, respectively. (C and D) Thereafter, the Mφ were left untreated (Med) or were stimulated with LPS or IFN-γ for 18 hours and were subsequently analyzed. (C) Results for Mφ that were exposed to TSN from U251 cells. The histograms in (A) and (C) are representative of 10 separate experiments, and the mean fluorescence intensity (MFI) values for control and TSN-treated cells (mean ± standard deviation [SD]) are indicated in Table S2. The data on cytokine production represent the mean (± standard error [SE]) of 10 experiments. Statistical differences between groups was calculated by Student t test. *P < .05 and **P < .01, compared with Mφ that were cultured in medium alone.
To further elucidate the functional states of TSN-exposed Mφ, we next examined how those cells responded to LPS and IFN-γ. In normal Mφ, those 2 stimuli induce the classical activation pattern, such as the upregulation of HLA-DR, CD86 and CD64, and production of TNF-α. However, with the exception of an augmented production of IL-10, that pattern was completely absent in Mφ that had been exposed to TSN from most solid tumors (Figure 1C,D and Table S2). In contrast, the TSN from NPC (CNE1 and CNE2) and leukemia (THP1 and U937) cells did not significantly affect the expression of surface markers or the profile of cytokine production in either resting or stimulated Mφ (Figure 1; Table S2), even when used at a high concentration (40% TSN). These results clearly indicate that one or more soluble mediators released from solid tumors compel monocytes to develop into immunosuppressive Mφ.
TSN caused transient early activation and subsequent deactivation of monocytes
TAM are almost entirely derived from circulating blood monocytes, which suggests that monocytes are educated by a tumor environment to adopt a specific phenotype during their early migration/differentiation stage.8,9 Therefore, we investigated the initial effects of TSN on freshly isolated monocytes, and the results showed that the TSN originating from most solid tumors induced transient activation of monocytes. The expression of HLA-DR, CD80, and CD86 was significantly upregulated on monocytes after exposure to TSN for 18 hours, but returned to a normal level within 48 hours (Table S3). Measuring cytokines produced by TSN-stimulated monocytes over time revealed rapid accumulation of TNF-α, IL-12, and IL-1β in the culture supernatants, reaching a maximum or a plateau within 24 hours. In particular, concentrations of TNF-α and IL-1β peaked sharply between 8 and 16 hours, and then gradually declined. In contrast, IL-10 exhibited a delayed onset but a sustained elevation that lasted until at least 72 hours after stimulation (Figure 2A). Polymyxin B did not affect the TSN-stimulated cytokine production, whereas it effectively blocked the activity of LPS in parallel experiments (Figure S1).
TSN caused transient activation and subsequent deactivation of monocytes. (A) Monocytes were cultured with the indicated TSN for 0 to 72 hours, after which the cytokine concentrations were determined by enzyme-linked immunosorbent assay (left panels). For the TSN pretreatment (right panels), the cells were incubated with TSN for the indicated time periods, washed, and re-incubated with the same TSN for 18 hours. TSN from CNE1 and THP1 cells did not induce the transient activation of monocytes. (B) Monocytes were pretreated with TSN from U251 (shaded bars), THP1 (solid bars), or medium (open bars) or cells for 10 hours. Thereafter, the cells were washed twice and recultured in medium alone or with the indicated TSN for 18 hours. Values given represent the mean (± SE) of 4 separate experiments; *P < .05 and **P < .01, compared with untreated monocytes.
TSN caused transient activation and subsequent deactivation of monocytes. (A) Monocytes were cultured with the indicated TSN for 0 to 72 hours, after which the cytokine concentrations were determined by enzyme-linked immunosorbent assay (left panels). For the TSN pretreatment (right panels), the cells were incubated with TSN for the indicated time periods, washed, and re-incubated with the same TSN for 18 hours. TSN from CNE1 and THP1 cells did not induce the transient activation of monocytes. (B) Monocytes were pretreated with TSN from U251 (shaded bars), THP1 (solid bars), or medium (open bars) or cells for 10 hours. Thereafter, the cells were washed twice and recultured in medium alone or with the indicated TSN for 18 hours. Values given represent the mean (± SE) of 4 separate experiments; *P < .05 and **P < .01, compared with untreated monocytes.
The distinct kinetics of different cytokines in the TSN-treated monocytes indicate that these cells might become “exhausted” and subsequently acquire a polarized M2 phenotype. To test this possibility, we washed monocytes at various time points after exposure to TSN and then recultured them for 18 hours with the same TSN. As shown in Figure 2A, the ability of monocytes to produce TNF-α, IL-12, and IL-1β decreased with increasing pre-exposure time and was completely lost after 24 hours, although the cells still produced IL-10. Monocytes that had been exposed to TSN for 72 hours became refractory to further stimulation and exhibited an immunosuppressive phenotype (data not shown). In contrast, culture supernatants from CNE1 and THP1 cells, which did not affect the polarization of Mφ (Figure 1), failed to stimulate such preactivated and subsequently exhausted monocytes (Figure 2A). The TSN used in our study did not contain any measurable levels of TNF-α, IL-10, IL-1β, and IL-12.
To ascertain whether TSN have a common mechanism of action, monocytes were pretreated with TSN from U251 or THP1 cells for 10 hours, washed, and then either recultured for 18 hours in medium alone (to measure residual cytokine production) or re-exposed to different TSN. Monocytes that were pretreated with TSN from U251 cells were no longer capable of producing TNF-α and were unresponsive to a second stimulation with TSN, although they still produced IL-10. In contrast, THP1 TSN, which was unable to induce the transient activation of monocytes (Figure 2A), did not affect the capacity of monocytes to respond to other TSN (Figure 2B).
Distinct activation patterns of monocytes/Mφ in tumor samples
These results suggested that TSN may act sequentially on migrating monocytes in different niches of a solid tumor, which results in the formation of immunosuppressive Mφ in a cancer nest. To test this hypothesis, we examined the phenotype of Mφ in serial sections of human tumor samples stained for CD68 (marker for monocytes/Mφ), HLA-DR, and IL-10. In hepatocellular carcinomas, CD68-positive cells were present in all areas of the tissue, but often predominantly in the stroma surrounding the cancer nest. Most Mφ in the peritumoral stroma had a smaller volume and showed marked expression of HLA-DR, which implies that they were newly recruited and activated Mφ (Figure 3A). In contrast, most Mφ in the cancer nests were negative for HLA-DR but positive for IL-10, whereas the Mφ in adjacent normal tissue exhibited moderate expression of HLA-DR (Figure 3A). The IL-10 expression in Mφ was further confirmed with confocal microscopic analysis showing that most Mφ in the cancer nest were positively stained with IL-10 (Figure S2). Similar results were obtained in lung cancer tissues, including the accumulation of Mφ with high HLA-DR expression in the immediate vicinity of cancer nests (data not shown).
Distinct activation patterns of Mφ in tumor samples. Adjacent sections of paraffin-embedded hepatocellular (A) or nasopharyngeal (B) carcinoma were stained with an anti-CD68, anti-HLA-DR, or anti-IL-10 Ab (magnification ×160 in panel A and ×100 in panel B). The micrographs at higher magnification show the stained cancer nest,1 peritumoral stroma,2 and adjacent normal tissue.3 IL-10–positive Mπ are seen in the cancer nest, and Mφ with high HLA-DR expression have accumulated in the immediate vicinities of the cancer nest in hepatocellular carcinoma in panel A. Slides were viewed with a Leica DM IRB inverted research microscope (Leica Microsystems, Wetzlar, Germany) using a HI PLAN CY lens at 10 ×/0.25 PH and 40 ×/0.65 PH2 0.17/, 0.36 and Klear Mount medium (GBI Inc, Mukilteo, WA). Images were acquired using a QImaging camera (Surrey, BC, Canada), model Retiga 4000RV Fast 1394 Mono Cooled, and were processed with Image-Pro Plus 5.0 (Media Cybernetics, Silver Spring, MD) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software.
Distinct activation patterns of Mφ in tumor samples. Adjacent sections of paraffin-embedded hepatocellular (A) or nasopharyngeal (B) carcinoma were stained with an anti-CD68, anti-HLA-DR, or anti-IL-10 Ab (magnification ×160 in panel A and ×100 in panel B). The micrographs at higher magnification show the stained cancer nest,1 peritumoral stroma,2 and adjacent normal tissue.3 IL-10–positive Mπ are seen in the cancer nest, and Mφ with high HLA-DR expression have accumulated in the immediate vicinities of the cancer nest in hepatocellular carcinoma in panel A. Slides were viewed with a Leica DM IRB inverted research microscope (Leica Microsystems, Wetzlar, Germany) using a HI PLAN CY lens at 10 ×/0.25 PH and 40 ×/0.65 PH2 0.17/, 0.36 and Klear Mount medium (GBI Inc, Mukilteo, WA). Images were acquired using a QImaging camera (Surrey, BC, Canada), model Retiga 4000RV Fast 1394 Mono Cooled, and were processed with Image-Pro Plus 5.0 (Media Cybernetics, Silver Spring, MD) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software.
Because the TSN from CNE1 did not induce either early activation or subsequent deactivation of Mφ (Figures 1–2), we also examined the phenotype of Mφ in the biopsy samples of NPC tissue. In Figure 3B, significant infiltration of Mφ can be seen in NPC nests. However, most of the Mφ were negative for IL-10 but expressed HLA-DR, which indicates that the Mϕ in NPC were of a normal phenotype rather than being like the immunosuppressive cells seen in most solid tumors (Figure 3A).3,8,11
HA induced transient activation and subsequent deactivation of macrophages
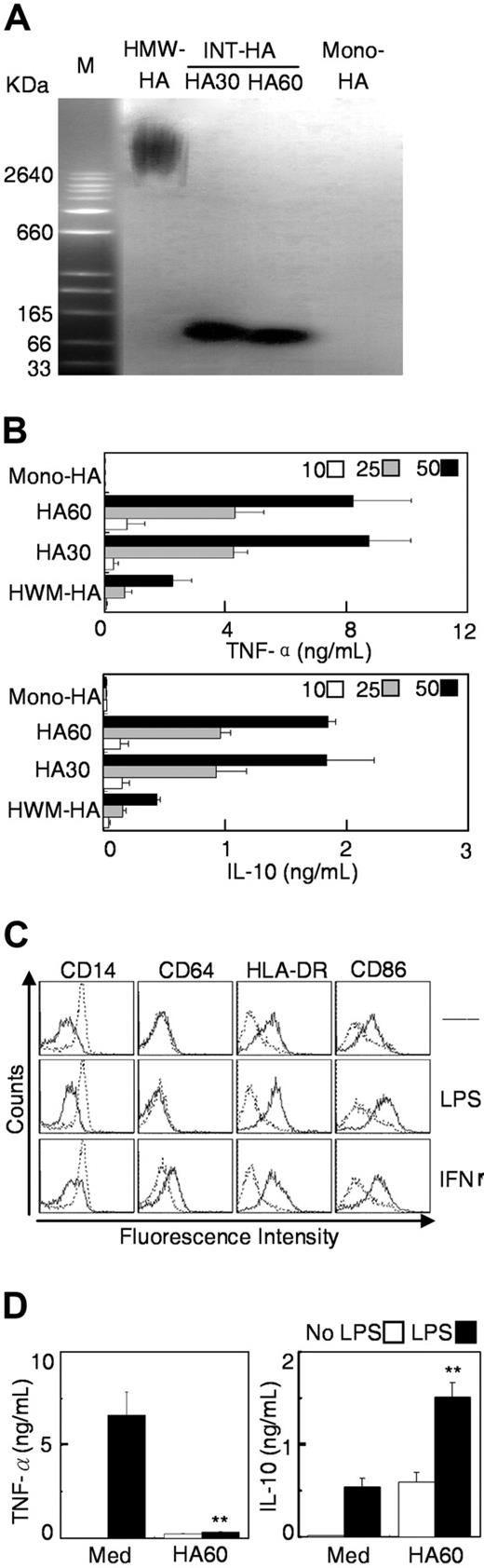
In some cancer patients, high levels of HA are associated with poor prognosis.17-20 However, studies have shown that HA fragments can induce potent proinflammatory responses in Mφ and dendritic cells.22,23 Therefore, we studied the kinetic effect of HA on polarization of Mφ. INT-HA (50-200 kDa) was prepared by partial digestion of HMW-HA with limited amounts of hyaluronidase for 30 and 60 minutes (Figure 4A). Stimulation for 18 hours with INT-HA elicited a dose-dependent monocyte activation that included a marked increase in cytokine production (Figure 4B) and upregulation of CD86 and HLA-DR expression (Table S3). HA fragments obtained after 30 or 60 minutes of digestion were similar with regard to their stimulatory effects on monocytes, whereas HMW-HA or the HA monomer had little or no impact (Figure 4B).
INT-HA induced early activation of monocytes and subsequent formation of immunosuppressive Mφ. (A) HMW-HA (lane 1) was digested by exposure to hyaluronidase for 30 (lane 2) or 60 (lane 3) minutes, and the resulting INT-HA fragments were separated by agarose gel electrophoresis and visualized with Stain-All. HA monomer was loaded in lane 4. (B) Monocytes were cultured for 18 hours in the presence of 10 (open bars), 25 (shaded bars), or 50 (solid bars) μg/mL of the different HA preparations. Levels of TNF-α and IL-10 in the medium were determined by enzyme-linked immunosorbent assay. (C-D) Cells were cultured for 6 days in medium alone (solid lines) or with 50 μg/mL INT-HA from 60-minute digestion (dashed lines). Thereafter, Mφ were left untreated (Med) or were stimulated with LPS or IFN-γ for 18 hours. Expression of cell surface markers and release of cytokines were determined as described in Figure 1. Data given are means (± SE; n = 4); **P < .01, compared with Mφ cultured in medium alone.
INT-HA induced early activation of monocytes and subsequent formation of immunosuppressive Mφ. (A) HMW-HA (lane 1) was digested by exposure to hyaluronidase for 30 (lane 2) or 60 (lane 3) minutes, and the resulting INT-HA fragments were separated by agarose gel electrophoresis and visualized with Stain-All. HA monomer was loaded in lane 4. (B) Monocytes were cultured for 18 hours in the presence of 10 (open bars), 25 (shaded bars), or 50 (solid bars) μg/mL of the different HA preparations. Levels of TNF-α and IL-10 in the medium were determined by enzyme-linked immunosorbent assay. (C-D) Cells were cultured for 6 days in medium alone (solid lines) or with 50 μg/mL INT-HA from 60-minute digestion (dashed lines). Thereafter, Mφ were left untreated (Med) or were stimulated with LPS or IFN-γ for 18 hours. Expression of cell surface markers and release of cytokines were determined as described in Figure 1. Data given are means (± SE; n = 4); **P < .01, compared with Mφ cultured in medium alone.
Moreover, exposure to INT-HA for 6 days induced monocytes to develop into suppressive Mφ that displayed preserved expression of CD14, had decreased levels of HLA-DR and CD86, and showed significant release of IL-10. The INT-HA–treated cells were refractory to stimulation with LPS or IFN-γ, although they did show augmented expression of IL-10 (Figure 4C,D), whereas HMW-HA or the HA monomer did not affect Mφ polarization (data not shown). These results indicate that, similar to TSN, INT-HA can induce transient activation of monocytes and sequential formation of immunosuppressive Mφ.
The role of HA fragments in TSN-induced monocyte dysfunction
In light of these results, we performed 3 sets of experiments to investigate the role of HA in TSN-induced polarization of Mφ. In these experiments, release of TNF-α and IL-10 was used as a measure of monocyte dysfunction.
First, monocytes were pretreated with INT-HA for 10 hours, washed, and then re-incubated for 18 hours in the presence of INT-HA or TSN. The results showed that the 10-hour pre-exposure to INT-HA blocked the ability of the cells to produce TNF-α on restimulation with TSN or INT-HA, although these monocytes still produced IL-10 (Figure 5A).
The role of HA in TSN-induced monocyte dysfunction. (A) Human monocytes were left untreated (open bars) or were pretreated with INT-HA (shaded bars) or TSN from U251 (solid bars) cells for 10 hours, and the cells were then washed and recultured in medium alone or with INT-HA or the indicated TSN for 18 hours. (B) Monocytes were preincubated for 4 hours in medium alone (open bars) or with 10 μg/mL CD44-blocking Ab (solid bars) or control mAb (IgG1, shaded bars), after which they were left untreated (Med) or stimulated with 15% TSN or 50μg/mL INT-HA for 18 hours. (C) Cells were pre-incubated for 4 hours in medium alone (open bars) or with 100 μg/mL Pep-1 (solid bars) or control peptide (shaded bars) and then stimulated with TSN or INT-HA for 18 h. Levels of TNF-α and IL-10 in the culture supernatants were determined by enzyme-linked immunosorbent assay, and the illustrated values represent the mean (± SE) of 4 separate experiments; *P < .05 and **P < .01, compared with monocytes pre-incubated in medium alone.
The role of HA in TSN-induced monocyte dysfunction. (A) Human monocytes were left untreated (open bars) or were pretreated with INT-HA (shaded bars) or TSN from U251 (solid bars) cells for 10 hours, and the cells were then washed and recultured in medium alone or with INT-HA or the indicated TSN for 18 hours. (B) Monocytes were preincubated for 4 hours in medium alone (open bars) or with 10 μg/mL CD44-blocking Ab (solid bars) or control mAb (IgG1, shaded bars), after which they were left untreated (Med) or stimulated with 15% TSN or 50μg/mL INT-HA for 18 hours. (C) Cells were pre-incubated for 4 hours in medium alone (open bars) or with 100 μg/mL Pep-1 (solid bars) or control peptide (shaded bars) and then stimulated with TSN or INT-HA for 18 h. Levels of TNF-α and IL-10 in the culture supernatants were determined by enzyme-linked immunosorbent assay, and the illustrated values represent the mean (± SE) of 4 separate experiments; *P < .05 and **P < .01, compared with monocytes pre-incubated in medium alone.
CD44 is the major cell surface receptor for HA. Therefore, we next determined the effect of mAb 5F12, which specifically blocks the binding of HA to CD44,26,32,33 on the TSN-induced monocyte dysfunction. As shown in Figure 5B, pretreatment of monocytes with this anti-CD44 mAb effectively inhibited the production of TNF-α in these cells stimulated by exposure to TSN or INT-HA; the rates of inhibition ranged from approximately 50% for U251 TSN to more than 90% for HepG2 TSN (Figure 5B). IL-10 production induced by various TSN or INT-HA was also significantly decreased in monocytes that were pre-incubated with the blocking Ab, whereas the isotype-matched control Ab had no effect (Figure 5B).
In the third set of experiments conducted to gain further evidence that HA fragments in TSN are responsible for inducing monocyte activation, we tested the ability of an HA-specific blocking peptide (Pep-1)23,34 to hinder cytokine induction. As shown in Figure 5C, Pep-1 markedly inhibited the production of TNF-α and IL-10 in monocytes that had been activated by TSN or INT-HA, whereas the random control peptide had no effect. Pep-1 did not interfere with LPS induction of TNF-α or IL-10 in monocytes (Figure 5C). In addition, compared with the HA levels in the TSN from NPC or leukemia cells, an average of 6-fold higher amounts of HA was found in the TSN from tumors that induce immunosuppressive Mφ (Figure S3). These results clearly show that HA is a common component of different types of TSN that induce early activation and subsequent formation of immunosuppressive Mφ.
HAS2 expression in tumor cells was correlated with their ability to cause dysfunction of monocytes/Mφ
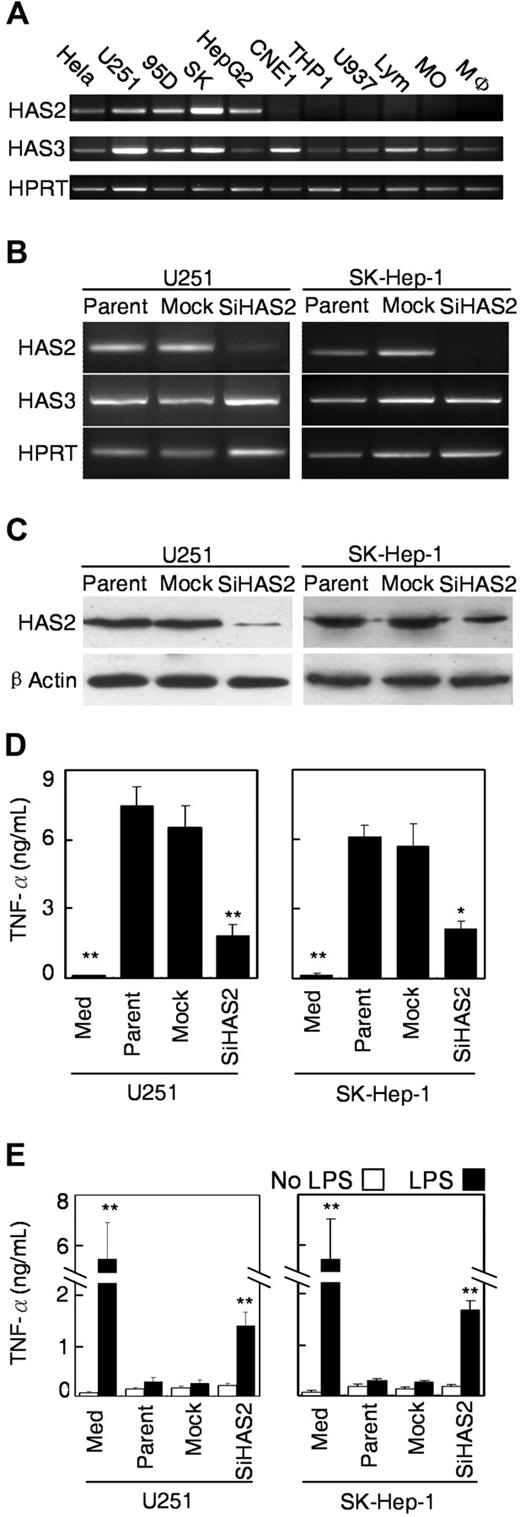
Three HA synthase (HAS) isoforms have been found in mammals.17,35 Therefore, we performed reverse-transcription PCR using the specific primers to examine their expression in both tumor cells and monocytes/Mφ. For semiquantitative comparisons, we used 30 cycles for HAS2 and 26 cycles for HAS3 and hypoxanthine guanine phosphoribosyl transferase. The results revealed that PCR products for HAS3 were detected in all types of cells we examined. Interestingly, the expression of HAS2 mRNA in tumor cells coincided with the ability of the cells to induce Mφ dysfunction; more specifically, the mRNA was detected in HeLa, U251, 95D, SK-Hep-1, and HepG2 cells, but not in other cells (Figure 6A). In contrast, using 2 separate sets of primers, HAS1 could not be detected in any of the cells examined even after 35 cycles of PCR (data not shown). However, other investigators have been successful in using one primer set to amplify HAS1 in myeloma cells.36
Silencing of HAS2 in tumor cells attenuated their ability to cause dysfunction of monocytes/Mφ. (A) Expression of HAS family in cells from different cells. The levels of mRNA for HAS2 and HAS3 were determined by reverse-transcription PCR using specific primers. The hypoxanthine guanine phosphoribosyl transferase gene (Hprt) was served as an internal control. (B) Reverse-transcription PCR analysis showing that the level of HAS2 was dramatically reduced in stably transfected pSi-HAS2-U251 and pSi-HAS2-SK-Hep-1 clones, compared with the parental and mock-transfected cells. The hypoxanthine guanine phosphoribosyl transferase gene served as an internal control. (C) Western blot analysis demonstrating that the expression of HAS2 protein was markedly reduced in both of the stably pSi-HAS2–transfected clones. The blots were stripped and reprobed with antiactin to confirm equal protein loading. (D) Silencing of HAS2 attenuated the ability of the tumor cells to activate monocytes. Monocytes were cultured with TSN from parental, mock-transfected, or pSi-HAS2-transfected cells for 18 hours, and the release of TNF-α from the monocytes was determined by enzyme-linked immunosorbent assay. (E) Monocytes were cultured with TSN from the indicated tumor cells for 7 days, and the Mφ were subsequently stimulated with 10 ng/mL LPS for 18 hours. Values represent the mean (± SE) of 4 separate experiments; P < .05 and ** P < .01 indicate significantly different from parental and mock cells.
Silencing of HAS2 in tumor cells attenuated their ability to cause dysfunction of monocytes/Mφ. (A) Expression of HAS family in cells from different cells. The levels of mRNA for HAS2 and HAS3 were determined by reverse-transcription PCR using specific primers. The hypoxanthine guanine phosphoribosyl transferase gene (Hprt) was served as an internal control. (B) Reverse-transcription PCR analysis showing that the level of HAS2 was dramatically reduced in stably transfected pSi-HAS2-U251 and pSi-HAS2-SK-Hep-1 clones, compared with the parental and mock-transfected cells. The hypoxanthine guanine phosphoribosyl transferase gene served as an internal control. (C) Western blot analysis demonstrating that the expression of HAS2 protein was markedly reduced in both of the stably pSi-HAS2–transfected clones. The blots were stripped and reprobed with antiactin to confirm equal protein loading. (D) Silencing of HAS2 attenuated the ability of the tumor cells to activate monocytes. Monocytes were cultured with TSN from parental, mock-transfected, or pSi-HAS2-transfected cells for 18 hours, and the release of TNF-α from the monocytes was determined by enzyme-linked immunosorbent assay. (E) Monocytes were cultured with TSN from the indicated tumor cells for 7 days, and the Mφ were subsequently stimulated with 10 ng/mL LPS for 18 hours. Values represent the mean (± SE) of 4 separate experiments; P < .05 and ** P < .01 indicate significantly different from parental and mock cells.
Silencing of HAS2 in tumor cells inhibited their ability to cause monocyte dysfunction
To investigate whether HAS2 in tumor cells plays a role in inducing monocyte dysfunction, we established a HAS2-knockdown model in U251 and SK-Hep-1 cells. The vector-based RNAi system was applied to stably suppress the expression of HAS2 in these tumor cells. When we compared the endogenous level of HAS2 mRNA and protein expression in parental cells and mock transfectants, we found no apparent differences between the 2 cell lines. In contrast, expression of both mRNA and the protein was significantly decreased in cells stably transfected with pSi-HAS2, compared with parental cells and mock transfectants. Inhibition of HAS2 expression did not alter the production of HAS3 (Figure 6B,C).
We next examined the effects of TSN from pSi-HAS2 transfectants on early monocyte activation and on the Mφ response to LPS stimulation. Compared with parental and mock-transfected cells, U251 or SK-Hep-1 cells with silenced HAS2 showed an attenuated ability to stimulate production of TNF-α in monocytes (Figure 6D). Furthermore, in Mφ that were exposed to TSN from HAS2-knockdown cells for 7 days, the capacity to release TNF-α on stimulation with LPS was partially restored (Figure 6E).
Discussion
Macrophages in most solid tumors exhibit a distinct phenotype with key properties similar to the immunosuppressive M2 cells.5-7 The present study showed that soluble tumor-derived factors promote the development of immunosuppressive Mφ by triggering a transient early activation of monocytes. This dynamic regulation of monocyte activity may represent a novel escape mechanism by which tumors co-opt the normal development of Mφ to educate the recruited monocytes to adopt specific phenotypes in different niches in a lesion. Moreover, we found that HA fragments constitute a common factor produced by a variety of human tumors to induce the suppressive Mφ, and also that upregulation of HAS2 in tumor cells is correlated with their ability to cause Mφ dysfunction.
Although TAMs originate from prototypical inflammatory cells, they are strongly impaired with regard to various functions related to inflammation.5,37 In the current study, we observed that TSN from several different kinds of tumor cells effectively induced the formation of TAM. Interestingly, kinetic analysis revealed 2 opposing functional stages in the TAM life cycle: monocytes are rapidly activated during a narrow time window, 4 to 16 hours after their first exposure to TSN, and afterward the same cells become exhausted and their production of cytokines is extinguished, with the exception of IL-10. Because TAMs are derived from blood monocytes, such sequential preactivation and exhaustion of cells may reflect a novel immune-escape mechanism by which tumors dynamically regulate the functions of migrating monocytes at distinct intratumoral sites. More precisely, this means that during their first exposure to the tumor microenvironment, the newly recruited monocytes may be transiently activated while they are approaching the stroma surrounding the tumor, with the aim of minimizing their potential to damage tumor cells. Thereafter, when these Mφ are in close proximity to the tumor cells, they become exhausted and thus fail to mount an effective antitumor immune response. This notion is supported by our observations in human hepatocellular carcinoma and lung cancer tissues, indicating that most CD68-positive cells are smaller and show high expression of HLA-DR in the peritumoral stromal region, whereas they exhibit a HLA-DRlowIL10high phenotype in the cancer nest. Moreover, NPC cells that failed to trigger preactivation of monocytes did not alter the polarization of Mφ and, accordingly, Mφ in both peritumoral and cancer nest regions of NPC tissues exhibited a normal phenotype.
The exhaustion of inflammatory cytokine production in TSN-exposed Mφ is reminiscent of a phenomenon that has been described as LPS tolerance in APC.38-41 Soon after initial exposure to LPS, Mφ and dendritic cells extinguish their synthesis of proinflammatory cytokines and switch from a Th1-inducing to a Th2-inducing mode.40,41 Apparently, the TSN we used triggered Mφ preactivation and exhaustion via a mechanism different from that associated with LPS, which is not affected by polymyxin B. After pre-exposure to TSN, the Mφ become unresponsive not only to activation by the homologous TSN but also to other stimuli, such as LPS and IFNγ. Thus, exhaustion is a general phenomenon that may also apply to other stimuli or cell lineages. For instance, Rissoan et al42 observed that plasmacytoid dendritic cells stimulated with CD40L for 6 days were exhausted and induced Th2 responses, whereas in another study43 in which exhaustion was avoided, it was found that such CD40L-activated cells preferentially primed Th1 responses. These findings further support the idea that the kinetics of monocyte activation, rather than the lineage of the monocytes, may be a critical factor in the M1-M2 polarization of Mφ.
In cancer patients, HA concentrations are usually higher in malignant tumors than in corresponding benign or normal tissues, and in some cases the levels are associated with poor prognosis.17-20 The present study provides evidence that HA is a common factor that is derived from solid tumors to alter Mφ polarization. The results of 3 sets of experiments support this conclusion. First, purified HA fragments were able to mimic the kinetic effect of TSN in inducing the formation of immunosuppressive Mφ. Second, pretreatment with anti-CD44 mAb or Pep-1, to antagonize the interactions between HA and its receptors, markedly inhibited the TSN-mediated monocyte dysfunction. Third, upregulation of HAS2 in tumor cells coincided with their ability to alter Mφ polarization, and silencing of HAS2 in tumor cells partially blocked the induction of monocyte/Mφ dysfunction. Therefore, it is tempting to suggest that HA fragments generated in tumor microenvironments constitute a common mediator of the sequential activation and exhaustion of newly recruited monocytes, resulting in the formation of immunosuppressive Mφ. This concept is supported by previous studies showing that HA fragments can rapidly stimulate the proinflammatory responses of Mφ and dendritic cells both in vitro and in vivo, whereas prolonged exposure to HA results in monocyte deactivation.25,26
In the present study, we found that INT-HA fragments had the greatest capacity to induce monocyte dysfunction, whereas HMW-HA or the HA monomer had little or no effect. This observation agrees with the general view that the HA exerts size-dependent effects and that INT-HA fragments are potent activators of immune cells.22,23,44 Thus, the exact balance between the HA polymer and its degradation products within tumors is of major importance in the regulation of immune responses. It has been suggested that both the concentration and the size of HA are determined by the tissue context, which represents the net outcome of the intricate balance between the synthesis and degradation of HA within tumors.17,23 Three isoforms of HAS, which differ with regard to their enzymatic properties and expression patterns, have been identified in humans.17,35,45 HAS1 is the least active and drives the synthesis of HMW-HA (2000 kDa). HAS2 is also associated with the synthesis of HMW-HA and its activity is essential for initiation and progression of breast cancer.16,35 HAS3 synthesizes short HA chains, where its expression seems to be activated to produce smaller HA fragments for the pericellular matrix. We found that expression of HAS2 mRNA in tumor cells coincided with their ability to induce Mφ dysfunction, and that effect could be partially reversed by silencing HAS2 in tumor cells (Figure 6). These results indicate that HAS2 is an important common factor for tumor cells to alter Mφ polarization, although the precise underlying mechanism is not yet known. Apparently, the ultimate size of HA fragments is determined by the concerted action of hyaluronidase or nonenzymatic degradation.17,35 Therefore, it is possible that, in the presence of hyaluronidases, the HMW-HA synthesized by HAS2 is degraded into smaller HA fragments that apply their regulatory effect on immune cells. This idea is supported by a recent study in which invasive breast cancer cells expressing primarily HAS2 were found to liberate large quantities of HMW-HA, which, in the presence of active hyaluronidases, were rapidly degraded into the HA fragments of 10-100 kDa.46 In addition, we have found that both Mφ and tumor cells express the hyaluronidases Hyal-2 and Hyal-3 (unpublished results).
In all our experiments, anti-CD44 mAb or Pep-1 could only partially block the TSN-mediated monocyte/Mφ dysfunction, which indicates that the effect of the TSN involved additional soluble factors from tumor cells. Besides producing numerous immunosuppressive cytokines and chemokines,4,8,11 tumor cells also secrete molecules (eg, gangliosides), which induced early activation of monocyte-derived APC and ultimately impaired their differentiation and activation.47,48 The suppressive mechanisms of tumor associated macrophages are less well understood in human. Arginase and inducible nitric oxide synthase (iNOS) have been reported to be implicated in macrophage-mediated immunosuppression in mouse. However, arginase I is barely detectable and iNOS is generally thought to be inactive in human Mφ.49,50 Interestingly, it has been recently reported that expression of inhibitory molecule B7-H4 on human Mφ is essential for their suppressive activity. Moreover, Treg cells can trigger the IL-10 production by APC, which in turn stimulate APC B7-H4 expression in an autocrine manner and render APC immunosuppressive via B7-H4.50,51 Therefore, characterization of the suppressive mechanisms may provide new avenues for development of novel immune-based therapies to enhance antitumor immunity in human cancer.
Our results give important new insights into the formation of immunosuppressive Mφ in tumors. HA fragments and other soluble factors derived from some cancer cells can alter the normal developmental process of Mφ that is intended to dynamically regulate monocyte activation at distinct sites, and in that way avoid the potentially dangerous actions of the Mφ and create conditions that are conducive to tumor progression. Apparently, the phenotype of Mφ is determined by the local environment and varies with the type of tumor. Consistent with our results, other investigators have observed that large numbers of Mφ in hepatocellular carcinoma and lung cancer samples were positively correlated with metastasis and reduced survival in the patients they studied.10,52 In contrast, we noted that Mφ exposed to NPC cells or found in NPC samples exhibited a normal phenotype (Figure 3B), and their presence was associated with good prognosis in NPC patients (unpublished results). Therefore, studying the mechanisms that can selectively modulate or reverse the phenotype of Mφ might provide a novel strategy for anticancer therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Paraskevi Heldin for providing the anti-HAS2 Ab and Ms Patricia Ödman for linguistic revision of the manuscript.
This work was supported by the Outstanding Young Scientist Fund and project grants from the NSFC, China (30425025 and 30672388), the “973” program (2004CB518801 and 2005CB724600), Asia-Swedish Research Partnership Programme (2004-4893), and the Natural Science Foundation of Guangdong (05200303).
Authorship
Contribution: D.M.K. designed and performed most of the research. Y.W. and N.C. performed Western blot and reverse-transcription PCR analysis. J.C. constructed the siRNA plasmid. S.M.Z. and L.Z. designed and supervised research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Limin Zheng, College of Life Sciences, Sun Yat-Sen (Zhongshan) University, Guangzhou 510275, PR China; e-mail: zhenglm@mail.sysu.edu.cn; or Shi-Mei Zhuang, College of Life Sciences, Sun Yat-Sen (Zhongshan) University, Guangzhou 510275, PR China; email: lsszsm@mail.sysu.edu.cn.

![Figure 1. Exposure to TSN redirected monocytes to develop into immunosuppressive Mφ. Human monocytes were cultured for 6 days in medium alone (solid lines) or with 15% TSN from various tumor cells (dashed lines). (A and B) Expression of surface markers and production of cytokines were determined by flow cytometry and enzyme-linked immunosorbent assay, respectively. (C and D) Thereafter, the Mφ were left untreated (Med) or were stimulated with LPS or IFN-γ for 18 hours and were subsequently analyzed. (C) Results for Mφ that were exposed to TSN from U251 cells. The histograms in (A) and (C) are representative of 10 separate experiments, and the mean fluorescence intensity (MFI) values for control and TSN-treated cells (mean ± standard deviation [SD]) are indicated in Table S2. The data on cytokine production represent the mean (± standard error [SE]) of 10 experiments. Statistical differences between groups was calculated by Student t test. *P < .05 and **P < .01, compared with Mφ that were cultured in medium alone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2007-01-068031/4/m_zh80130704290001.jpeg?Expires=1764961593&Signature=HGCegDvt4B5rhPhDGh5Xan9w1QfxWS9rnm-nsZBYyWw~CN9bBDnQqke16O4n9XQBrraeHYnlwgKFISykwQjP4MR1AkJ~e9isEMTxF-eb9PuJWyqo4yaxr4PNRwC1g92O1NPqNW03BW9G1SZ4f4Lr0QXN8FVhGXw5MZ7u-cTpqmUbv60fTl4HJ6GD0KKbxwyOQgh0tNVhzzLwCrMBnKUgFp~bnmpQfU7uWp4v3Kdr41~bDCfO2PsEimM7J-Ana2nxkdycxzb5A~zH~uL1SHIhigD-u5J-OwHWEEOqoOOqfl7wLIQGWkqMsCzk9OyUwVggMo6fxFkxC~JB25dZJ8a7fg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal