Abstract
Vascular endothelial growth factor (VEGF), a major factor in tumor-host interactions, plays a critical role in the aberrant hematopoiesis observed in cancer-bearing hosts. To dissect the roles of VEGF receptor (VEGFR)-1 and VEGFR-2 in cancer-associated hematopoiesis in vivo, we selectively stimulated VEGFR-1 and VEGFR-2 by continuous infusion of receptor-specific ligands or selective blockade with VEGF receptor-specific antibodies in mice infused with recombinant VEGF at levels observed in tumor-bearing animals. We found that the effect of VEGF on the accumulation of Gr1+CD11b+ cells is mediated by VEGFR-2, but that the 2 receptors have opposite effects on lymphocyte development. Pathophysiologic levels of VEGF strongly inhibit T-cell development via VEGFR-2, whereas VEGFR-1 signaling decreases this inhibition. VEGFR-1, and not VEGFR-2, signaling is responsible for the observed increase of splenic B cells. Both receptors are capable of inhibiting dendritic cell function. These data suggest that most of observed aberrant hematopoiesis caused by excess tumor-derived VEGF is mediated by VEGFR-2, and VEGFR-1 alone has very limited independent effects but clearly both positively and negatively modulates the effects of VEGFR-2. Our findings suggest that selective blockade of VEGFR-2 rather than of both receptors may optimally overcome the adverse hematologic consequences of elevated VEGF levels found in malignancy.
Introduction
Vascular endothelial growth factor (VEGF) is a secreted growth factor that plays a critical role in the formation and maintenance of blood vessels (angiogenesis) and of blood cells (hematopoiesis). The essential role of VEGF and VEGF receptors in developmental hematopoiesis is underscored by the fact that knockouts of VEGF or either of its receptors results in embryonic lethality with defects in vascular and hematopoietic cell development.1-3 These biologic effects of VEGF are mediated by VEGFR-1 (also referred to as Flt1) and VEGFR-2 (also referred to as KDR or Flk1), which differ considerably in their signaling characteristics.4,5
VEGF and its receptors also have profound effects on hematopoiesis in adults. VEGF receptors are expressed in subsets of adult hematopoietic cells, and it is now also known that VEGF is secreted by hematopoietic stem cells after stimulation with cytokines.6-11 VEGF-deficient hematopoietic stem cells fail to repopulate lethally irradiated mice and stimulation of VEGFR-1 fully rescues this phenotype in vivo.12 Clonogenic assays reveal that VEGF inhibits total colony formation from less mature progenitor cells and at the same time promotes the formation of myeloid, mixed and erythroid colonies from lineage-committed progenitors.13,14 VEGFR-1 has also been suggested as regulating the cell cycle and differentiation of hematopoietic stem cells and promoting hematopoietic cell mobilization.7,15-17
Important roles of VEGF and its receptors in hematopoiesis in adults are also observed in pathologic conditions associated with elevated levels of VEGF. VEGF is produced by almost tumor cells and is found to be elevated in the serum of cancer patients,18,19 where its level is closely associated with a poor prognosis.20,21 In tumor-bearing patients and animals, tumor-derived factors, including VEGF, inhibit dendritic cell (DC) differentiation, induce Gr1+CD11b+ cell production, cause thymus atrophy, and inhibit T-cell development.6,21,22 DCs are the most potent antigen-presenting cells and play a central role in immune induction, and tumor-derived Gr1+CD11b+ cells have the ability to inhibit antigen-specific CD8+ T cell responses.23 Overexpression and activation of VEGFR-2 in transplanted bone marrow leads to the accumulation of these Gr1+ and CD11b+ cell populations.24 Consistent with these observations, chronic exposure of mice to recombinant VEGF165 at concentrations similar to those observed in advanced stage cancer patients not only inhibits DC differentiation and increases the production of immature Gr1+ myeloid cell and B cells but also induces profound thymic atrophy and decreases in T-cell subpopulations.13,21,25 These studies also show that selective blockade of VEGF in tumor-bearing animals and humans can reverse the majority of these effects, demonstrating that VEGF is a major component of the overall impact of the tumor on these host systems. In this study, we dissect the receptors responsible for these effects.
Although it is clear that VEGF receptor signaling plays important roles in hematopoiesis in adults, much remains to be learned about the specific roles of VEGFR-1 and VEGFR-2 in postnatal hematopoiesis in vivo. To clarify this question, in this study we used a murine model of chronic infusion of VEGF with an osmotic pump.13,21,26 This mouse model mimics the pathophysiologic VEGF concentrations observed in patients with advanced-stage cancer.13,21 In this study, we used 2 highly receptor-selective VEGF165 variants to compare the different functions of VEGFR-1 and VEGFR-2 in vivo. One, VEGF165 mutant (Flt-sel) with 4 amino acid substitutions, binds with native affinity to Flt1 and approximately 470-fold less well to KDR compared with wild-type VEGF165. The second variant (KDR-sel), with 3 amino acid substitutions, has wild-type affinity for KDR but approximately a 2000-fold reduced affinity for Flt1.27-29 We also used 2 specific anti-VEGF receptor blocking antibodies to confirm the roles of VEGF receptors in adult hematopoiesis in vivo. These antibodies, MF1 (anti-VEGFR-1) and DC101 (anti-VEGFR-2), specifically block the interaction between VEGF and each receptor in vivo.30-34
We find that the effect of VEGF on the accumulation of Gr1+CD11b+ cells in spleen is mediated by VEGFR-2. Unexpectedly, these 2 receptors have antagonistic effects on lymphoid cell development; VEGF promotes bone marrow B-cell development and increases total splenic B cells via VEGFR1, and VEGFR-2 has the opposite effect. VEGF inhibits thymic T-cell differentiation and decreases splenic T cells via VEGFR-2, and VEGFR-1 slightly decreases the inhibition of VEGFR-2. The inhibition of DC function by VEGF is mediated by both VEGFR-1 and VEGFR-2, but blockade of VEGFR-2 is sufficient to restore DC function. Our data suggest that VEGFR-2 plays major in vivo roles in aberrant hematopoiesis and immunodeficiency in cancer, and VEGFR-1 and VEGFR-2 have distinct and sometimes opposing effects.
Materials and methods
Animals
Female Balb/c mice were purchased from Harlan (Indianapolis, IN). Female mice (6 to 8 weeks old) used in the study were housed in pathogen-free units at Vanderbilt University School of Medicine, in compliance with Institutional Animal Care and Use Committee regulations.
Medium and reagents
VEGF165, KDR-sel (VEGFR2-sel), and Flt-sel (VEGFR1-sel) were generous gifts from Genentech (South San Francisco, CA). The KDR-sel mutant was VEGF D63S/G65M/L66R, and Flt-sel was VEGF I43A/I46A/Q79A/I83A.27,29 DC101 and MF1 are generous gifts from ImClone system (New York, NY). DC101 and MF1 are rat-blocking monoclonal antibodies specifically against mouse VEGFR-2 and VEGFR-1, respectively (ImClone Systems, New York, NY). The control antibody rat IgG was purchased from Sigma-Aldrich (St Louis, MO). Osmotic pumps were purchased from Alzet (Durect, Cupertino, CA).
VEGF administration
VEGF165 (50 ng/hr), VEGFR2-sel (also KDR-sel, 50 ng/hr), or VEGFR1-sel (also Flt-sel, 50 ng/hr) was delivered into mice via Alzet osmotic pumps (Durect) as previously described13,21,26 for 28 days. Control pumps were filled with phosphate-buffered saline (PBS). In another group experiment, PBS-pump mice were treated by intraperitoneal injection 1 day after pump implantation and every 3 days thereafter with 800 μg rat IgG. VEGF-pump mice were treated by intraperitoneal injection 1 day after pump implantation and every 3 days thereafter with 800 μg of rat IgG, anti-VEGFR-2 (DC101), or anti-VEGFR-1 (MF1).30-34
FACS analysis
Fresh single-cell suspensions of splenocytes, bone marrow cells, or thymocytes (106 each)were washed once with cold buffer and resuspended in 100 μL cold buffer (1% bovine serum albumin, 0.1% NaN3 in PBS). Cells were stained with monoclonal antibodies (mAbs) in the dark on ice for 30 minutes, washed with cold buffer (1% bovine serum albumin, 0.1% NaN3 in PBS), and resuspended in 0.5 mL cold buffer (1% bovine serum albumin, 0.1% NaN3 in PBS). Fluorescence-activated cell sorting (FACS) data were acquired using a FACSCalibur (Becton Dickinson, San Diego, CA) and analyzed by WinList 5.0 software. Appropriate fluorochrome-conjugated, isotype-matched control IgGs were used in all experiments. The following monoclonal antimouse antibodies were used: Gr1/Ly-6G-FITC, CD11b-PE, B220/CD45R-FITC, B220/CD45R-PerCP, IgMa-PE, CD43-PE, CD19-PE, CD3e-FITC, CD3e-PerCP, CD4-FITC, CD8a-PE, and Streptavidin-PerCP (Purchased from BD PharMingen). Biotin anti-mo/huB220 and biotin antimouse CD3e were purchased from eBioscience (San Diego, CA).
DC preparation
Spleens were harvested from pump mice infused with PBS or VEGF receptor agonists via osmotic pump as described in Figure 6. Single-cell suspensions were prepared by enzymatic disaggregation with collagenase D. Splenic DCs were then positively selected using anti-CD11c (N418) MicroBeads and LS columns (MACS; Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. All procedures except the collagenase digestion step were performed on ice. Purified cells were routinely more than 95% CD11c+.
Allogeneic mixed leukocyte reaction
Antigen-presenting cell function was assessed by mixed leukocyte reaction as described previously.25 Briefly, T cells were separated from allogeneic C57BL/6 mice using mouse T-cell enrichment columns (R&D Systems, Minneapolis, MN); 105 purified DCs were incubated with 4 × 105 T cells (C57BL/6) in triplicate for 3 days. The cultures were pulsed overnight with 1 μCi of [3H]thymidine (Amersham, Arlington Heights, IL). Cells were harvested using a cell harvester (Skatron Instruments, Sterling, VA). [3H]Thymidine uptake was counted using a liquid scintillation counter.
Results
The effect of VEGF on accumulation of immature myeloid cells (Gr1+CD11b+) is mediated by VEGFR-2 in vivo
The accumulation of Gr1+CD11b+ cells is observed in tumor-bearing hosts, and these cells clearly contribute to tumor immunosuppression.13,22,35 To clarify the role of VEGF as well as its individual receptors in the production of these Gr1+CD11b+ cells in tumor-bearing hosts, we infused non–tumor-bearing mice with recombinant human VEGF165 or highly receptor-selective VEGF mutants, VEGFR2-sel (KDR-sel) and VEGFR1-sel (Flt-sel), using an osmotic pump (referred to as “VEGF-pump mice”). In the spleen, the fraction of Gr1+CD11b+ cells was increased approximately 2-fold in VEGF-pump mice, compared with that of PBS-pump mice (chronically infused with PBS as a control; Figure 1A,B). Selective stimulation of VEGFR-2 by VEGFR2-sel resulted in comparable increases of Gr1+CD11b+ cells (2.2-fold), but selective activation of VEGFR-1 by VEGFR1-sel did not increase this population compared with that observed in PBS-pump mice (Figure 1A,B). To confirm these data, we treated VEGF-pump mice with specific VEGF receptor antibodies, anti-VEGFR-2 (DC101) or anti-VEGFR-1 (MF1), dosed at 800 μg every 3 days for 28 days. Blockade of VEGFR-2 by anti-VEGFR-2 completely normalized the accumulation of Gr1+CD11b+ cells in the spleen (Figure 1C). Treatment with anti-VEGFR-1 showed an increase in Gr1+CD11b+ cells equivalent to that of VEGF-IgG mice (VEGF-pump mice treated with rat IgG; Figure 1C). These data show that VEGFR-2 activation is necessary and sufficient for the accumulation of Gr1+CD11b+ cells by VEGF165 in the spleen. Consistent with the increase of Gr1+CD11b+ cells in the spleen, these cells are also increased approximately 2-fold in the peripheral blood in VEGF-pump mice and VEGFR2-sel-pump mice (chronic administration of VEGFR2-sel to nontumor mouse via an osmotic pump), but not in VEGFR1-sel-pump mice (chronic administration of VEGFR1-sel to nontumor mouse via an osmotic pump; Figure 1D,1E). Together, these data demonstrate that the effect of VEGF on the accumulation of Gr1+CD11b+ cells in the spleen and peripheral blood is mediated by VEGFR-2 but not VEGFR-1 in vivo.
Immature myeloid cells (Gr1+CD11b+) were increased in spleen and peripheral blood of VEGF-pump mice. The 8- to 10-week-old Balb/c mice were given a continuous infusion of PBS, rhVEGF-A (50 ng/hr), VEGFR2-sel (KDR-sel, 50 ng/hr), or VEGFR1-sel (Flt-sel, 50 ng/hr) over a period of 28 days. (A) Splenocytes were analyzed by FACS for the expression of Gr1 and CD11b. The representative FACS plots were shown. The first plot is the isotype control. (B) The percentage of Gr1+CD11b+ cells in spleens. (C) The 8- to 10-week-old Balb/c mice given a continuous infusion of PBS treated with rat IgG or infusions of rhVEGF-A (50 ng/hr) were treated with rat IgG, anti-R1 (MF1), or anti-R2 (DC101), 800 μg every 3 days over a period of 28 days. The percentage of Gr1+CD11b+ cells in spleens is shown. The data (mean ± standard error of the mean [SEM]; n ≥ 5) are repeated 2 times. (D) Red blood cells from murine peripheral blood were lysed and then Gr1+CD11b+ cell population was analyzed by FACS. The representative FACS plots are shown. The first plot is the isotype control. (E) The percentage of Gr1+CD11b+ cells in peripheral blood. All of the other data (mean ± SEM, n ≥ 3) repeat 3 times. *P < .05; **P < .01.
Immature myeloid cells (Gr1+CD11b+) were increased in spleen and peripheral blood of VEGF-pump mice. The 8- to 10-week-old Balb/c mice were given a continuous infusion of PBS, rhVEGF-A (50 ng/hr), VEGFR2-sel (KDR-sel, 50 ng/hr), or VEGFR1-sel (Flt-sel, 50 ng/hr) over a period of 28 days. (A) Splenocytes were analyzed by FACS for the expression of Gr1 and CD11b. The representative FACS plots were shown. The first plot is the isotype control. (B) The percentage of Gr1+CD11b+ cells in spleens. (C) The 8- to 10-week-old Balb/c mice given a continuous infusion of PBS treated with rat IgG or infusions of rhVEGF-A (50 ng/hr) were treated with rat IgG, anti-R1 (MF1), or anti-R2 (DC101), 800 μg every 3 days over a period of 28 days. The percentage of Gr1+CD11b+ cells in spleens is shown. The data (mean ± standard error of the mean [SEM]; n ≥ 5) are repeated 2 times. (D) Red blood cells from murine peripheral blood were lysed and then Gr1+CD11b+ cell population was analyzed by FACS. The representative FACS plots are shown. The first plot is the isotype control. (E) The percentage of Gr1+CD11b+ cells in peripheral blood. All of the other data (mean ± SEM, n ≥ 3) repeat 3 times. *P < .05; **P < .01.
VEGF increases splenic B cells via VEGFR-1, and VEGFR-2 has the opposite effect in vivo
After 28 days of VEGF infusion, splenomegaly was observed in most of the VEGF-pump mice and VEGFR2-sel-pump mice (Figure 2A). The total number of splenocytes was increased approximately 3-fold, compared with that of PBS-pump mice (Figure 3B). Although there is no apparent splenomegaly in VEGFR1-sel-pump mice, the total number of splenocytes was still increased (81.8 ± 3.2 × 106 in PBS-pump mice and 109.9 ± 7.5 × 106 in VEGFR1-sel-pump mice; P = .02; Figure 2B). The percentage of CD19+ B cells in spleens was decreased approximately 3-fold in VEGF-pump mice and VEGFR2-sel-pump mice compared with that of PBS-pump mice (Figure 2C). The total number of splenic CD19+ B cells was comparable among these 3 groups (Figure 2D). However, the total splenic B cells were increased in VEGFR1-sel-pump mice (Figure 2D). Accordingly, the total number of splenic B cells was significantly decreased in VEGF-pump mice treated with anti-VEGFR-1 and increased in VEGF-pump mice treated with anti-VEGFR-2 (Figure 2E). To further characterize this population, we also analyzed the effects on B220+CD19+ cell populations in spleens. The percentages of B220+, CD19+ and B220+CD19+ from each mouse were equivalent (data not shown). These data indicated that VEGF increases total splenic B cells via VEGFR-1 and VEGFR-2 has the opposite effect in vivo.
VEGF increases splenic B cells via VEGFR-1 and VEGFR-2 has opposite effect. The 8- to 10-week-old Balb/c mice were given PBS or VEGF receptor agonists for 28 days as described in Figure 1. (A) The representative spleens from PBS, VEGF-A, VEGFR2-sel, and VEGFR1-sel mice (from left to right). (B) The total splenocytes of spleens from the pump mice. (C) The splenocytes were analyzed for expression of CD19. The representative FACS histograms were shown. (D) Total number of B cells (CD19+) in spleens. (E) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1, or anti-R2 as described in Figure 1C. Total number of B cells (CD19+) in spleens is shown. The data (mean ± SEM, n ≥ 5) repeat 2 times. All of the other data (mean ± SEM, n ≥ 3) repeat 3 times. *P < .05; **P < .01.
VEGF increases splenic B cells via VEGFR-1 and VEGFR-2 has opposite effect. The 8- to 10-week-old Balb/c mice were given PBS or VEGF receptor agonists for 28 days as described in Figure 1. (A) The representative spleens from PBS, VEGF-A, VEGFR2-sel, and VEGFR1-sel mice (from left to right). (B) The total splenocytes of spleens from the pump mice. (C) The splenocytes were analyzed for expression of CD19. The representative FACS histograms were shown. (D) Total number of B cells (CD19+) in spleens. (E) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1, or anti-R2 as described in Figure 1C. Total number of B cells (CD19+) in spleens is shown. The data (mean ± SEM, n ≥ 5) repeat 2 times. All of the other data (mean ± SEM, n ≥ 3) repeat 3 times. *P < .05; **P < .01.
VEGF promotes bone marrow B-cell development via VEGFR-1 and VEGFR-2 has the opposite effect. (A) Flow cytometric analysis of bone marrow cells expressed CD43 and B220. The representative FACS plots were shown. The first plot is the isotype control. (B) The proportion of pre-B/pro-B (left) and the percentage of B-cell subsets (right) in total bone marrow cells: pro-B cells (CD43+B220+) and pre-B cells (CD43−B220+). The data (mean ± SEM, n ≥ 3) repeat 3 times. (C) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1 or anti-R2 as described in Figure 1C. The proportion of pre-B/pro-B (left) and the percentage of B-cell subtypes (right) in total bone marrow are shown. The data (mean ± SEM, n ≥ 5) repeat 2 times. *P < .05; **P < .01.
VEGF promotes bone marrow B-cell development via VEGFR-1 and VEGFR-2 has the opposite effect. (A) Flow cytometric analysis of bone marrow cells expressed CD43 and B220. The representative FACS plots were shown. The first plot is the isotype control. (B) The proportion of pre-B/pro-B (left) and the percentage of B-cell subsets (right) in total bone marrow cells: pro-B cells (CD43+B220+) and pre-B cells (CD43−B220+). The data (mean ± SEM, n ≥ 3) repeat 3 times. (C) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1 or anti-R2 as described in Figure 1C. The proportion of pre-B/pro-B (left) and the percentage of B-cell subtypes (right) in total bone marrow are shown. The data (mean ± SEM, n ≥ 5) repeat 2 times. *P < .05; **P < .01.
VEGF promotes bone marrow B-cell development via VEGFR-1, and VEGFR-2 has the opposite effect in vivo
The bone marrow is the primary organ for early B-cell development. There are 2 major B-cell subtypes: pro-B cells (B220+CD43+) and pre-B cells (B220+CD43−). The percentage of pro-B cells greatly decreased in both of VEGF-pump mice and VEGFR2-sel-pump mice compared with that of PBS-pump mice, but not in VEGFR1-sel-pump mice (Figure 3A,3B). These data suggest that VEGF inhibits the production of pro-B cells from early lymphoid progenitors via VEGFR-2. The proportion of pre-B to pro-B is comparable between PBS-pump mice and VEGF-pump mice, but decreases in VEGFR2-sel-pump mice and increases in VEGFR1-sel-pump mice (Figure 3B). These data suggest that VEGFR-1 and VEGFR-2 have opposite effects on pro-B to pre-B differentiation. Consistent with this, blockade of VEGFR-2 by anti-VEGFR-2 also increases the proportion of pre-B to pro-B cells (Figure 3C). When VEGFR-1 is blocked by anti-VEGFR-1, the proportion of pre-B to pro-B cells is still increased (Figure 3C), but the total number of CD19+ B cells in the spleen is dramatically decreased (Figure 2E). This may be attributable to inhibition of the exit of pre-B cells from bone marrow. In sum, these data indicate that VEGFR-1 and VGEFR-2 have opposite effect on pro-B to pre-B differentiation in the bone marrow.
VEGF decreases splenic T cells via VEGFR-2, and VEGFR-1 partially decreases the inhibition mediated by VEGFR-2 in vivo
T cells are the major immune effector cells. They are activated by antigen-presenting cells and then circulate to recognize and kill tumor cells. In VEGF-pump mice or VEGFR2-sel-pump mice, the total number of splenic T cells (CD3e+) and its subpopulations (CD4+ and CD8a+) were significantly decreased, compared with that of PBS-pump mice (Figure 4A,4B). It is interesting that total splenic T cells were slightly increased in VEGFR1-sel-pump mice (Figure 4A,4B), demonstrating an opposite effect of activation of the 2 receptors. To further investigate the role of VEGFR-1 and VEGFR-2 in T-cell development, we treated age-matched VEGF-pump mice with rat IgG, anti-VEGFR-1, or anti-VEGFR-2. When VEGFR-1 is blocked by anti-VEGFR-1, the total number of splenic T cells (CD3e+) and its subpopulations (CD4+ and CD8a+) were even lower than that of VEGF-IgG pump mice (Figure 4C). Anti-VEGFR-2 alone corrected all of the T-cell populations in the spleen (Figure 4C). These data suggest that VEGF decreases splenic T cells via VEGFR-2 and VEGFR-1 partially antagonizes the inhibition by VEGFR-2.
VEGF decreases splenic T cells via VEGFR-2 and VEGFR-1 slightly decreases the inhibition of VEGFR-2. Age-matched Balb/c mice were given PBS or VEGF receptor agonists for 28 days as described in Figure 1. (A) Flow cytometric analysis of the expression of CD4 and CD8a in splenocytes. The representative FACS plots were shown. The first plot is the isotype control. (B) The total numbers of T-cell subsets in spleens. The data (mean ± SEM, n ≥ 3) repeat 3 times. (C) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1, or anti-R2 as described in Figure 1C. The total numbers of T-cell subsets in spleen were shown. The data (mean ± SEM, n ≥ 5) repeat 2 times. *P < .05; **P < .01.
VEGF decreases splenic T cells via VEGFR-2 and VEGFR-1 slightly decreases the inhibition of VEGFR-2. Age-matched Balb/c mice were given PBS or VEGF receptor agonists for 28 days as described in Figure 1. (A) Flow cytometric analysis of the expression of CD4 and CD8a in splenocytes. The representative FACS plots were shown. The first plot is the isotype control. (B) The total numbers of T-cell subsets in spleens. The data (mean ± SEM, n ≥ 3) repeat 3 times. (C) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1, or anti-R2 as described in Figure 1C. The total numbers of T-cell subsets in spleen were shown. The data (mean ± SEM, n ≥ 5) repeat 2 times. *P < .05; **P < .01.
VEGF inhibits thymic T-cell development via VEGFR-2 in vivo
The thymus is the major organ for T-cell development. Thymocyte differentiation starts from triple-negative (CD3e−/low, CD4−, and CD8a−) cells, via intermediate single-positive (CD3e−/low and either CD4+ or CD8a+) cells, to double-positive (CD3e−/low, CD4+, and CD8a+) cells, and then ultimately mature single-positive cells.21,36-38 Previous studies have shown that the inhibition of VEGF on splenic T-cell populations appears to be caused by the effect of VEGF on thymocyte differentiation. Dramatic thymic atrophy is observed in mice chronically infused with VEGF. Using the selective ligands, we found that the thymuses show even greater atrophy in VEGFR2-sel-pump mice, whereas in VEGFR1-sel-pump mice they are a normal size (Figure 5A). Consistent with this, the T-cell populations in the thymus were greatly altered in VEGF-pump mice and VEGFR2-sel-pump mice, compared with those in PBS-pump mice (Figure 5C). T-cell subpopulations also showed a normal distribution in VEGFR1-sel-pump mice (Figure 5C). The proportions of intermediate single-positive CD3e−/lowCD4−CD8a+ and CD3e−/lowCD4+CD8a− cell fractions dramatically increased as well as triple-negative cells [(CD3e−/lowCD4−CD8a+)] PBS: 2.4 ± 0.2%; VEGF: 24.4 ± 7.0%; VEGFR2-sel: 22.6 ± 8.2%); and [(CD3e−/lowCD4+CD8a−] PBS: 4.0 ± 0.4%; VEGF: 11.1 ± 2.2%; VEGFR2-sel: 13.1 ± 3.3%)], but the proportion of double-positive CD3e−/lowCD4+CD8a+ (PBS: 86.8 ± 0.6%; VEGF: 36.3 ± 11.7%; VEGFR2-sel: 25.5 ± 11.5%) cell fractions dramatically decreased with VEGFR-2 stimulation (Figure 5C). These data suggest that VEGF suppress the progression from double-negative cells to double-positive cells via VEGFR-2. Because total thymocytes were significantly decreased in VEGF-pump and VEGFR2-sel-pump mice, the absolute numbers of all subsets were also dramatically decreased, compared with those of PBS-pump mice (Figure 5B and 5D). We obtained consistent data from VEGF-pump mice treated with anti-VEGFR-1 or anti-VEGFR-2 (data not shown), showing that VEGF inhibits thymic T-cell development apparently exclusively via VEGFR-2.
VEGF inhibits thymic T-cell development via VEGFR-2. (A) The representative thymuses from PBS, VEGF-A, VEGFR2-sel, and VEGFR1-sel mice (from left to right). (B) The total thymocytes. (C) Flow cytometric analysis of thymocytes expressed CD4 and CD8a in CD3elow/− cells. The representative FACS plots were shown. The first plot is the isotype control. (D) The total numbers of T-cell subtypes in thymus. All of the data (mean ± SEM, n ≥ 3) repeat 3 times. *P < .05; **P < .01.
VEGF inhibits thymic T-cell development via VEGFR-2. (A) The representative thymuses from PBS, VEGF-A, VEGFR2-sel, and VEGFR1-sel mice (from left to right). (B) The total thymocytes. (C) Flow cytometric analysis of thymocytes expressed CD4 and CD8a in CD3elow/− cells. The representative FACS plots were shown. The first plot is the isotype control. (D) The total numbers of T-cell subtypes in thymus. All of the data (mean ± SEM, n ≥ 3) repeat 3 times. *P < .05; **P < .01.
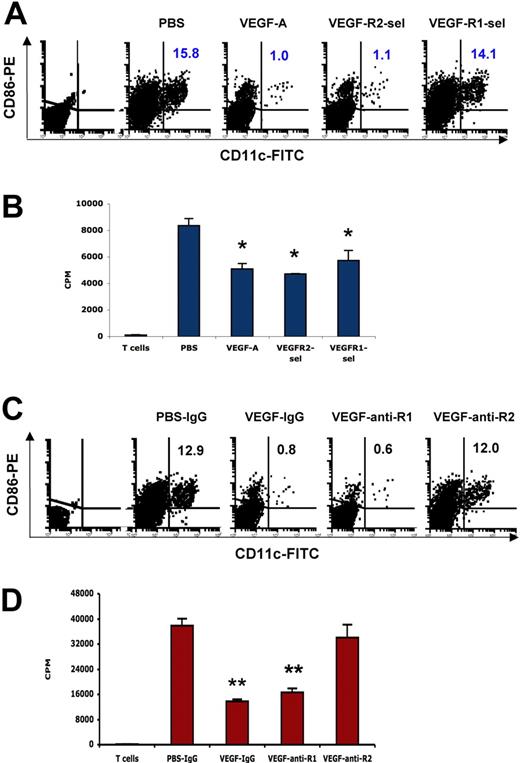
VEGF inhibits the ability of DCs to stimulate allogeneic T cell proliferation via both VEGFR-1 and VEGFR-2, but blockade of VEGFR-2 is sufficient to restore DC function in vivo
The inhibition of professional antigen-presenting cell function, particularly DCs, appears to be one of the major mechanisms of cancer-associated immunosuppression in tumor-bearing animals as well as cancer patients.39,40 Anti-VEGF antibody restores antigen-presenting cell function in tumor-bearing mice, and antigen-presenting cell dysfunction was observed in mice receiving continuous VEGF infusion.13,25 These results demonstrated that VEGF contributes to inhibition of antigen-presenting cell function, but the role of the individual VEGF receptors in this effect in vivo was unclear. We found that the proportions of splenic CD11c+CD86+ DCs significantly decreased in both VEGF-pump mice and VEGFR2-sel-pump mice, compared with that of PBS-pump mice (Figure 6A). Accordingly, the ability of DC to stimulate allogeneic T-cell proliferation was inhibited by VEGF165 and VEGFR2-sel (Figure 6B). Although the proportions of splenic CD11c+CD86+ DCs did not change in VEGFR1-sel-pump mice, the ability of DC to stimulate allogeneic T-cell proliferation was also inhibited (Figure 6A,B). Consistent with these observations, blockade of VEGFR-1 by anti-VEGFR-1 in mice infused with wild-type VEGF165 did not restore the proportion and function of splenic CD11c+CD86+ DCs (Figure 6C,D), but blockade of VEGFR-2 restored both the proportion and function of splenic CD11c+CD86+ DCs (Figure 6C,D). These data show that VEGF inhibits the ability of DC to stimulate allogeneic T-cell proliferation via both of VEGFR-1 and VEGFR-2, but blockade of VEGFR-2 is sufficient to restore DC function.
VEGF inhibits the ability of DCs to stimulate allogeneic T-cell proliferation via both of VEGFR-1 and VEGFR-2, and blockade of VEGFR-2 is sufficient to restore DC function. The 8- to 10-week-old Balb/c mice were given PBS or VEGF receptor agonists for 28 days as described in Figure 1. (A) Expression of CD11c and CD86 in CD3e−B220− splenocytes was analyzed by FACS. The representative FACS plots were shown. The numbers represent the means of the percentage of indicated cell fractions. The first plot is the isotype control. (B) The ability of DCs to stimulate allogeneic T-cell proliferation. DCs were isolated from splenocytes with CD11c Microbeads. DCs (105) were incubated with 4 × 105 T cells (C57BL/6) for 4 days and 3H-thymidine uptake was analyzed in triplicate. (C) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1, or anti-R2 as described in Figure 1C. Expression of CD11c and CD86 in CD3e−B220− splenocytes was analyzed by FACS. The representative FACS plots were shown. The numbers represent the means of the percentage of indicated cell fractions. The first plot is the isotype control. (D) The ability of DCs to stimulate allogeneic T-cell proliferation. DCs were isolated from splenocytes with CD11c Microbeads. DCs (105) were incubated with 4 × 105 T cells (C57BL/6) for 4 days and 3H-thymidine uptake was analyzed in triplicate. The data (mean ± SEM, n = 3) repeat 2 times.
VEGF inhibits the ability of DCs to stimulate allogeneic T-cell proliferation via both of VEGFR-1 and VEGFR-2, and blockade of VEGFR-2 is sufficient to restore DC function. The 8- to 10-week-old Balb/c mice were given PBS or VEGF receptor agonists for 28 days as described in Figure 1. (A) Expression of CD11c and CD86 in CD3e−B220− splenocytes was analyzed by FACS. The representative FACS plots were shown. The numbers represent the means of the percentage of indicated cell fractions. The first plot is the isotype control. (B) The ability of DCs to stimulate allogeneic T-cell proliferation. DCs were isolated from splenocytes with CD11c Microbeads. DCs (105) were incubated with 4 × 105 T cells (C57BL/6) for 4 days and 3H-thymidine uptake was analyzed in triplicate. (C) Balb/c mice were given PBS treated with rat IgG, or rhVEGF-treated rat IgG, anti-R1, or anti-R2 as described in Figure 1C. Expression of CD11c and CD86 in CD3e−B220− splenocytes was analyzed by FACS. The representative FACS plots were shown. The numbers represent the means of the percentage of indicated cell fractions. The first plot is the isotype control. (D) The ability of DCs to stimulate allogeneic T-cell proliferation. DCs were isolated from splenocytes with CD11c Microbeads. DCs (105) were incubated with 4 × 105 T cells (C57BL/6) for 4 days and 3H-thymidine uptake was analyzed in triplicate. The data (mean ± SEM, n = 3) repeat 2 times.
Discussion
It is clear from many studies that VEGF and its receptors are expressed in both normal and malignant hematopoietic cells, and that VEGF receptor signaling plays an important role in hematopoiesis in the adult as well as during development.6-8,10,11,41-44 The critical role of pathologic VEGF signaling in hematopoiesis and cancer-associated immunodeficiency has also been demonstrated in multiple tumor-bearing systems. Pathophysiologically elevated levels of VEGF causes aberrant hematopoiesis affecting DCs, B cells, and T cells, and these effects are manifested by measurable defects in immunity.6,13,21,22,25,45,46 A better understanding of the individual roles of the 2 major VEGF receptors on aberrant hematopoiesis might guide the development of improved immunotherapies for cancer patients, a task that is made more difficult by the fact that VEGFR-1 or VEGFR-2 knockout nice have an embryonic lethal phenotype as well as the fact that many hematopoietic cells express both VEGFR-1 and VEGFR-2.
In this study, we used a VEGF infusion osmotic pump mouse model that mimics the pathophysiologic concentrations of VEGF observed in advanced cancer patients, but additionally used highly receptor-selective ligands (VEGFR2-sel and VEGFR1-sel) as well as specific VEGF receptor antibodies: DC101 (anti-VEGFR-2) and MF1 (anti-VEGFR-1) to allow us to study the in vivo roles of each of these receptors in cancer-associated aberrant hematopoiesis and immunodeficiency.
Immature myeloid cells, which are defined as Gr1+CD11b+ cells in mice, have been demonstrated to accumulate in tumor-bearing hosts and contribute to tumor-associated immunosuppression by suppressing antigen-specific T-cell responses.22,23,35,46 Elimination of Gr1+CD11b+ cell populations from tumor-bearing mice can restore tumor-associated immunosuppression.47 Several recent reports also suggest that Gr1+CD11b+ cells directly contribute to tumor progression by promoting tumor angiogenesis.48,49 Our in vivo data showed that VEGFR-2 is responsible for the accumulation of this cell population in the spleens of VEGF-pump mice (Figure 1B,1C). We did not observe any effect on Gr1+CD11b+ cell production mediated by VEGFR-1 (Figure 1B,1C), even though the serum VEGF165 level in these animals was higher than IgG-treated controls (data not shown). This is consistent with the data from Flt1(TK−/−) mice. In wild-type or Flt1(TK−/−) tumor-bearing mice, the Gr1+CD11b+ cells in the spleen were increased to similar level, which indicates that the tyrosine kinase domain of Flt1 is not necessary for Gr1+CD11b+ cell production in the spleen (unpublished data). Consistent with our observations, Larrivee et al24 have reported that overexpression and specific activation of VEGFR-2 in the bone marrow microenvironment leads to the accumulation of Gr1+CD11b+ myeloid cells. Although these data support our conclusion about VEGFR-2 function, Gr1+CD11b+ cells did not increase in the bone marrow of our VEGF-pump mice, which more closely mimic the tumor-bearing host. One possible explanation for this discrepancy may lie in the nonphysiologic overexpression of VEGFR-2 in their mouse model. First, expression of VEGFR-2 is low in myeloid elements of normal marrow but high in myeloid progenitors of marrow cells from leukemia patients.50,51 Second, overexpression of VEGFR-2 also results in high serum levels of granulocyte-macrophage colony-stimulating factor,24,52 which induces myeloid cell expansion in the bone marrow.
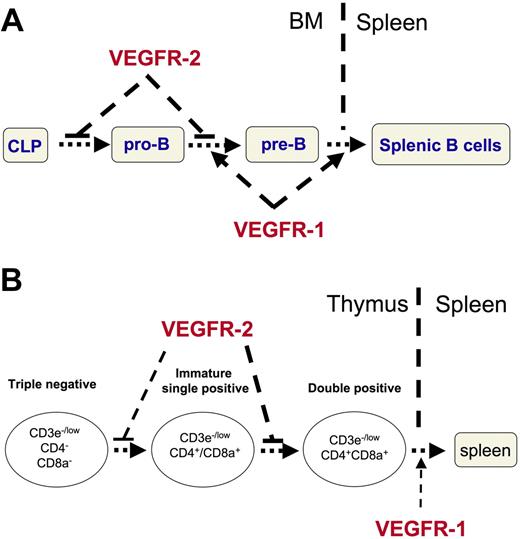
We found that VEGFR-1 and VEGFR-2 have opposite effects on lymphoid cell development. The total number of splenic CD19+ B cells increased (Figure 2) and the proportion of pre-B to pro-B increased in VEGFR1-sel-pump mice or VEGF-pump mice treated with anti-VEGFR-2 (Figure 3). These data suggest that VEGF promotes bone marrow B-cell development via VEGFR-1. However, the total splenic CD19+ B cells (Figure 2) and the proportion of pre-B to pro-B were comparable between PBS-pump mice and VEGF-pump mice (Figure 3). These data indicate that VEGFR-1 and VEGFR-2 have opposite effects on B-cell development, and that there is a balance between the effects of the 2 receptors on this system. The percentage of pro-B cells was decreased in VEGF-pump mice and VEGFR2-sel-pump mice compared with that of PBS-pump mice, but not in VEGFR1-sel-pump mice (Figure 3). These data suggest that VEGF suppresses the production of pro-B cells from early common lymphoid progenitors. It is interesting that the proportion of pre-B to pro-B cells in VEGF-pump mice treated with anti-VEGFR-1 was increased, although the absolute percentage of pro-B cells is similar to that of VEGF-pump mice (Figure 3C). It is well-known that VEGFR-1 functions in mobilization of bone marrow-derived cells.53,54 It is therefore possible that blockade of VEGFR-1 inhibits the exit of pre-B cells from the bone marrow and that this causes the observed accumulation of pre-B cells there. These data also explain why the total splenic CD19+ B cells were decreased in VEGF-pump animals treated with anti-VEGFR-1, but not in VEGFR2-sel-pump mice. A model for these effects in bone marrow is shown in Figure 7A. We hypothesize that VEGF suppresses the progression from common lymphoid progenitors to pro-B cells and from pro-B to pre-B via VEGFR-2; VEGF then promotes the progression from pro-B to pre-B, and the exit from bone marrow to spleen via VEGFR-1.
A model for the opposite effects of VEGFR-1 and VEGFR-2 on lymphoid cell development. (A) VEGF suppresses the progression from common lymphoid progenitors (CLP) to pro-B cells and from pro-B to pre-B cells via VEGFR-2; whereas VEGF promotes the progression from pro-B to pre-B cells, and the exit from bone marrow to spleen via VEGFR-1. (B) VEGF suppresses the progression from triple-negative to double-positive, especially from intermediate single-positive to double-positive thymocytes via VEGFR-2, and VEGF promotes the mobilization of related cells via VEGFR-1 (such as the exit of the mature single positive T cells from thymus to spleen).
A model for the opposite effects of VEGFR-1 and VEGFR-2 on lymphoid cell development. (A) VEGF suppresses the progression from common lymphoid progenitors (CLP) to pro-B cells and from pro-B to pre-B cells via VEGFR-2; whereas VEGF promotes the progression from pro-B to pre-B cells, and the exit from bone marrow to spleen via VEGFR-1. (B) VEGF suppresses the progression from triple-negative to double-positive, especially from intermediate single-positive to double-positive thymocytes via VEGFR-2, and VEGF promotes the mobilization of related cells via VEGFR-1 (such as the exit of the mature single positive T cells from thymus to spleen).
Interestingly, opposite effects of the 2 VEGF receptors were observed on T-cell development. VEGF not only decreased total splenic T-cell populations but also caused dramatic thymic atrophy (Figures 4,5). Surprisingly, total splenic T-cell populations were increased in VEGFR1-sel-pump mice and even more decreased in VEGF-pump mice treated with anti-VEGFR-1 (Figure 4). VEGFR2-sel infusion or blockade of VEGFR-1 also resulted in more profound thymic atrophy (Figure 5). These data suggest that VEGF inhibits T-cell development via VEGFR-2 and VEGFR-1 has an opposing effect. However, blockade of VEGFR-2 in wt VEGF165 infused animals completely restores the T-cell populations in both the spleen and the thymus (Figures 4,5), which indicates that signaling through VEGFR-1 itself does not affect T-cell development. One possible explanation for this effect is that VEGFR-1 promotes the mobilization of T-related cells (such as T precursors from the bone marrow and T cells from thymus to spleen). This may also explain why the number of T cells and their subpopulations were increased in the spleen (Figure 4B), whereas the T-cell differentiation is normal in the thymus (Figure 5) in VEGFR1-sel-pump mice. It is also possible that some of the functions of VEGFR-1 may be mediated by VEGFR-1 and VEGFR-2 heterodimers. We propose a model, shown in Figure 7B, for the effects of VEGF on T-cell development in which VEGF suppresses the progression from triple-negative to double-positive, especially from intermediate single-positive to double-positive via VEGFR-2, and VEGF promotes the mobilization of related cells via VEGFR-1.
Hematopoietic lineage differentiation and maturation occur in multiple discrete immune niches. For example, B-cell and T-cell differentiation occur in the bone marrow and spleen or bone marrow and thymus, respectively. During differentiation, these cells need to migrate to different compartments even within the same organ, and this migration is one potential mechanism for the regulation of T-cell and B-cell development. Our data show that elevated levels of VEGF inhibit T-cell and B-cell development via VEGFR-2, and VEGFR-1 has the opposite effect. We hypothesize that one mechanism for this observation may be that VEGFR-1 affects precursor cell mobility in transiting between immune niches required for full maturation, whereas VEGFR-2 is more involved in cell differentiation. This is consistent with the fact that hematopoiesis appears completely normal in VEGF-pump mice treated with anti-VEGFR-2, even in the presence of very high levels of VEGF.
These data show that VEGFR-2 plays a major in vivo role in aberrant hematopoiesis and immunodeficiency in cancer but that VEGFR-1 sometimes has opposite effects. Therefore, selective blockade of VEGFR-2 function may optimally reverse the immunosuppression effects of VEGF while preserving the positive effects of VEGFR-1 in immunocyte mobilization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank ImClone for providing the MF1 and DC101 antibodies, and Genentech for providing rhVEGF and receptor-specific mutants. The authors also thank James O. Price for technical assistance.
This work was supported by grants RO1 CA76321 (D.P.C.) and RO1 CA100562 (M.M.D.) from the National Cancer Institute.
Authorship
Contribution: Y.H. designed and performed the research. X.C. performed some of the research. L.Y. discussed the results and provided helpful suggestions. S.N. performed parts of the research. M.D. discussed the results and provided helpful suggestions. D.C. designed the experiments and supervised their conduct, wrote the paper, and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David P. Carbone, 685 PRB Vanderbilt-Ingram Comprehensive Cancer Center, Vanderbilt University, 2200 Pierce Avenue, Nashville, TN 37232-6838; e-mail: d.carbone@vanderbilt.edu.

![Figure 1. Immature myeloid cells (Gr1+CD11b+) were increased in spleen and peripheral blood of VEGF-pump mice. The 8- to 10-week-old Balb/c mice were given a continuous infusion of PBS, rhVEGF-A (50 ng/hr), VEGFR2-sel (KDR-sel, 50 ng/hr), or VEGFR1-sel (Flt-sel, 50 ng/hr) over a period of 28 days. (A) Splenocytes were analyzed by FACS for the expression of Gr1 and CD11b. The representative FACS plots were shown. The first plot is the isotype control. (B) The percentage of Gr1+CD11b+ cells in spleens. (C) The 8- to 10-week-old Balb/c mice given a continuous infusion of PBS treated with rat IgG or infusions of rhVEGF-A (50 ng/hr) were treated with rat IgG, anti-R1 (MF1), or anti-R2 (DC101), 800 μg every 3 days over a period of 28 days. The percentage of Gr1+CD11b+ cells in spleens is shown. The data (mean ± standard error of the mean [SEM]; n ≥ 5) are repeated 2 times. (D) Red blood cells from murine peripheral blood were lysed and then Gr1+CD11b+ cell population was analyzed by FACS. The representative FACS plots are shown. The first plot is the isotype control. (E) The percentage of Gr1+CD11b+ cells in peripheral blood. All of the other data (mean ± SEM, n ≥ 3) repeat 3 times. *P < .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2007-01-065714/4/m_zh80130703250001.jpeg?Expires=1769106512&Signature=j4CnMWd4hmqwgCZrR69tg0zoRX3ar38s8U-KVR2CyiDov7KtQe166JurikP7kZWQ2H6MWotrR04mD7Gk~anv6c6EnU-KvkfH-M2ZTh5dUCmRXCUQTr7mr9Egupgh0ac9ldO1rlga9-4WHT2SflF-EOQbSuTHbH0hb2qtZ~qeqju6u2Ai7NswDjWo826SYxZgcGW3cHZzaB9jWFUUBEB1PmEdKkgGJ~E5Pb4c~wlzuwnlyUWxNq0n~UgI-wIhk1y091be6lTatZ6TGrsLTFRd2kThxE8MCdZ~ikVLBmm6-x2LUK5lR950NcIWR863nXDNHeiI5PCJWknWQwfrrGSqDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal