Abstract
Relapse following remission induction chemotherapy remains a barrier to survival in approximately 20% of children suffering from acute lymphoblastic leukemia (ALL). To investigate the mechanism of relapse, 27 matched diagnosis and relapse ALL samples were analyzed for clonal populations using polymerase chain reaction (PCR)–based detection of multiple antigen receptor gene rearrangements. These clonal markers revealed the emergence of apparently new populations at relapse in 13 patients. More sensitive clone-specific PCR revealed that, in 8 cases, these “relapse clones” were present at diagnosis and a significant relationship existed between presence of the relapse clone at diagnosis and time to first relapse (P < .007). Furthermore, in cases where the relapse clone could be quantified, time to first relapse was dependent on the amount of the relapse clone at diagnosis (r = −0.84; P = .018). This observation, together with demonstrated differential chemosensitivity between subclones at diagnosis, argues against therapy-induced acquired resistance as the mechanism of relapse in the informative patients. Instead these data indicate that relapse in ALL patients may commonly involve selection of a minor intrinsically resistant subclone that is undetectable by routine PCR-based methods. Relapse prediction may be improved with strategies to detect minor potentially resistant subclones early during treatment, hence allowing intensification of therapy.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer in children and is one of the leading causes of childhood death from disease in developed countries.1,2 Recent advances in the treatment of childhood ALL with combination chemotherapy have resulted in initial remission rates of 97% to 99%, with overall long-term event-free survival rates of 80%.3,4 Despite the high initial remission rate, however, approximately 20% of children diagnosed with ALL will ultimately relapse, often with disease that is highly refractory to further therapy.3,5 Relapse therefore remains the major challenge in the successful treatment of this disease.
It is generally accepted that bone marrow relapse in ALL results from residual leukemic cells that have survived therapy. The level of minimal residual disease (MRD) at the end of induction chemotherapy is therefore an indicator of disease chemoresistance and has proven to be a powerful tool in predicting relapse in childhood ALL.6-10 MRD can be detected by highly sensitive techniques such as specialized flow cytometry directed at aberrant antigen expression or polymerase chain reaction (PCR) to detect antigen receptor (immunoglobulin and T-cell receptor) gene rearrangements.11-15 Using current real-time quantitative PCR (RQ-PCR) technology to assess clonal antigen receptor gene rearrangements, it is possible to determine MRD levels in most ALL patients at a sensitivity of 1 malignant cell among 104 to 105 cells.16-18
PCR-based MRD detection is widely used due to its excellent specificity and sensitivity. However, in some patients the profile of immunoglobulin (Ig) and T-cell receptor (TCR) gene rearrangements in the cell population detected at relapse differs from the profile found at diagnosis. A similar problem has been encountered when using flow cytometric methods, as antigen expression patterns may also change after therapy.19,20 Disappearance of clonal markers can potentially result in failure to detect MRD and the consequent incorrect stratification of potentially high-risk patients into a low-risk therapy group. However, for most patients at least one rearrangement remains stable between diagnosis and relapse. Therefore in most clinical protocols using MRD measured by RQ-PCR, it is recommended that at least 2 Ig or TCR rearrangements are followed for each patient.21-23
Alteration of clonal marker profiles between diagnosis and relapse is well documented,24-29 but the process by which this takes place is unclear. Various reports indicate differing clonal populations at diagnosis and relapse in up to 45% of patients.18,27,29,30 Leukemic clones emerging at relapse with apparently new rearrangements (referred to herein as the “relapse clone”) are often chemoresistant. Several recent studies have used these apparently new rearrangements to trace the relapse clone back to a minor population present at diagnosis, and in some cases relative resistance of this population to remission induction chemotherapy has been observed.18,27,31-33 Still, it is not clear at what point resistance may arise. Relapse in these cases could be due to either (1) acquisition of a resistant phenotype in response to therapy and subsequent selection or (2) selection of an inherently resistant subclone initially undetected at diagnosis but nevertheless present at very low levels.
To further investigate the mechanism of ALL relapse, we used target detection PCR to assess a large number of antigen receptor gene rearrangements in matched diagnosis and relapse bone marrow samples from 27 childhood ALL patients. The relapse samples of a subset of patients exhibited new rearrangements not initially detected at diagnosis. More sensitive RQ-PCR assays revealed that the apparently new clones were present in a high proportion of patient samples at diagnosis. The results demonstrate, for the first time, a significant relationship between the quantity of relapse clone present at diagnosis and clinical outcome. Together, our observations provide new evidence in support of clonal expansion of an intrinsically resistant subclone as a mechanism of relapse in ALL and raise important considerations for the clinical management of ALL patients.
Patients, materials, and methods
Patients and samples
Patient characteristics are listed in Table 1. Matched diagnosis and relapse bone marrow samples were initially available from 5 pediatric B-cell precursor ALL (pre B-ALL) patients diagnosed between 1993 and 1997 and treated according to the Australian and New Zealand Children's Cancer Study Group (ANZCCSG) Study VI protocol.34 These patients (cohort A: A1-A5) had relapsed despite having negative end-of-therapy MRD results, as determined using a single Ig gene rearrangement. A further set of matched diagnosis and relapse bone marrow samples were subsequently obtained from a cohort of 22 pediatric pre B-ALL or T-cell ALL patients (cohort B), selected solely on the basis of availability of clinical specimens and data. Eighteen of these patients (B1-B18) were diagnosed between 1984 and 2000 and treated according to Children's Cancer Group protocols.35 Patients B19-B22 were diagnosed in 2003 and received therapy on an intensive multiagent regimen based on the Berlin-Frankfurt-Munster BFM95 protocol.12 Unless otherwise indicated, patients recorded as surviving were followed for a minimum of 3 years after relapse. Additional samples were available for patient B2 at second relapse and for patient B20 at multiple points during treatment. For cohort A, DNA was extracted from bone marrow slides according to published methods.36 For patients B1-B22, DNA was extracted from fresh bone marrow specimens using either TRIZOL Reagent (Invitrogen, Victoria, Australia) or the Nucleobond AXG kit (Macherey-Nagel, Duren, Germany). For all cases involved in this study, protocols were approved by the institutional ethics committees at the relevant centers and informed parental or patient consent was obtained, in accordance with the Declaration of Helsinki, for sample collection following diagnosis.
Patient characteristics
| Patient ID . | Sex . | Immunophenotype . | WBC count, ×109/L* . | Age at diagnosis, y . | Time to relapse,† m . | Current status . |
|---|---|---|---|---|---|---|
| A1 | F | B | 31 | 2.5 | 43 | A |
| A2 | F | B | 305 | 4.9 | 31 | A |
| A3 | M | B | 9 | 8.1 | 25 | D |
| A4 | F | B | 64 | 1.9 | 43 | D |
| A5 | F | B | 7 | 3.9 | 47 | D |
| B1 | M | B | 73 | 6.6 | 52 | A |
| B2 | M | T | 510 | 3.5 | 5 | D |
| B3 | F | B | 17 | 16.3 | 6 | D |
| B4 | M | T | 9 | 12.2 | 24 | D |
| B5 | M | T | 157 | 11.6 | 29 | D |
| B6 | M | B | 6 | 6.6 | 8 | D |
| B7 | F | B | 7 | 14.3 | 14 | D |
| B8 | F | B | 9 | 11.0 | 37 | D |
| B9 | M | T | 201 | 2.8 | 31 | D |
| B10 | M | B | 12 | 8.8 | 58 | A‡ |
| B11 | M | B | 3 | 9.7 | 59 | A‡ |
| B12 | M | B | 28 | 3.1 | 46 | A |
| B13 | F | B | 5 | 9.4 | 26 | A‡ |
| B14 | F | B | 450 | 0.2 | 16 | A |
| B15 | F | B | 318 | 0.5 | 6 | D |
| B16 | M | B | 9 | 7.7 | 41 | A |
| B17 | M | B | 71 | 0.5 | 8 | D |
| B18 | M | B | 26 | 3.4 | 31 | D |
| B19 | M | B | 98 | 1.9 | 23 | D |
| B20 | M | B | 5 | 3.9 | 18 | A‡ |
| B21 | M | B | 3 | 7.7 | 16 | D |
| B22 | M | B | 5 | 3.6 | 15 | D |
| Patient ID . | Sex . | Immunophenotype . | WBC count, ×109/L* . | Age at diagnosis, y . | Time to relapse,† m . | Current status . |
|---|---|---|---|---|---|---|
| A1 | F | B | 31 | 2.5 | 43 | A |
| A2 | F | B | 305 | 4.9 | 31 | A |
| A3 | M | B | 9 | 8.1 | 25 | D |
| A4 | F | B | 64 | 1.9 | 43 | D |
| A5 | F | B | 7 | 3.9 | 47 | D |
| B1 | M | B | 73 | 6.6 | 52 | A |
| B2 | M | T | 510 | 3.5 | 5 | D |
| B3 | F | B | 17 | 16.3 | 6 | D |
| B4 | M | T | 9 | 12.2 | 24 | D |
| B5 | M | T | 157 | 11.6 | 29 | D |
| B6 | M | B | 6 | 6.6 | 8 | D |
| B7 | F | B | 7 | 14.3 | 14 | D |
| B8 | F | B | 9 | 11.0 | 37 | D |
| B9 | M | T | 201 | 2.8 | 31 | D |
| B10 | M | B | 12 | 8.8 | 58 | A‡ |
| B11 | M | B | 3 | 9.7 | 59 | A‡ |
| B12 | M | B | 28 | 3.1 | 46 | A |
| B13 | F | B | 5 | 9.4 | 26 | A‡ |
| B14 | F | B | 450 | 0.2 | 16 | A |
| B15 | F | B | 318 | 0.5 | 6 | D |
| B16 | M | B | 9 | 7.7 | 41 | A |
| B17 | M | B | 71 | 0.5 | 8 | D |
| B18 | M | B | 26 | 3.4 | 31 | D |
| B19 | M | B | 98 | 1.9 | 23 | D |
| B20 | M | B | 5 | 3.9 | 18 | A‡ |
| B21 | M | B | 3 | 7.7 | 16 | D |
| B22 | M | B | 5 | 3.6 | 15 | D |
A indicates alive at last follow-up; D, deceased.
White blood cell count at diagnosis.
Time to first relapse.
Last follow-up at 10 to 26 months after relapse.
Identification of clonal antigen receptor gene rearrangements
Clonal rearrangements of the immunoglobulin heavy chain (IgH), immunoglobulin light chain kappa (Igκ), T-cell receptor gamma (TCRγ), or T-cell receptor delta (TCRδ) genes were determined using PCR analysis. The target detection for cohort A patients, for whom limited amounts of DNA were available, involved testing for only a limited subset of Ig and TCR rearrangements using primers to the CDR3 region of IgH and TCRγ genes.37 For patients B1-B22, a total of 31 PCR primer sets, including 13 IgH (6 variable to joining (V-J) region and 7 diversity to joining (D-J) region), 5 Igκ-κ del (kappa deletion element), 7 TCRγ, and 6 TCRδ reactions, were used to detect gene rearrangements using primers and conditions as previously described.23,38 Homogeneity of PCR products was determined using heteroduplex analysis.39 Monoclonal products were directly sequenced after ExoSAP-IT clean-up (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), whereas oligoclonal products were further amplified by PCR using internal primers. Sequences were compared against germ line sequences in the National Center for Biotechnology Information (NCBI) database40 to identify and characterize each rearrangement.
RQ-PCR analysis
The quality of DNA obtained from patient material was verified by RQ-PCR analysis of β-actin gene sequences using Human ACTB Endogenous Control Kit (Applied Biosystems, Foster City, CA). RQ-PCR was used to detect and quantitate apparently new relapse rearrangements in diagnostic samples. Consensus fluorogenic TaqMan probes designed to bind consensus sequences within the V, D, or J regions were used together with a forward and reverse primer, one of which was designed on a patient-specific basis to span the unique N region of each rearrangement to ensure assay specificity.17 Primers were designed with the aid of Primer Express software v1.5 (Applied Biosystems). With the exception of the extremely short N region in the rearrangement marking the relapse clone of patient B1, it was possible to design a sensitive RQ-PCR assay in all cases. Data were collected using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) and analyzed as described previously.17 DNA from the relapse samples was diluted with normal bone marrow DNA to generate standard curves in an informative range. Assays were quantitative to 10−5 or 10−4 leukemic cells, as defined by European Study Group for MRD analysis in ALL guidelines.41
Statistical analysis
Correlations were determined using Pearson correlation coefficient (r), and a Fisher r-to-z transformation was carried out to calculate a probability level (P value). Mean time to first relapse was compared between groups using Student unpaired t test. Relapse-free survival analysis was performed according to the method of Kaplan and Meier, and comparisons of outcome between subgroups were performed by the log-rank test for univariate comparisons, using 2-tailed tests.
Results
Patient characteristics and clonal antigen receptor gene rearrangements
The ALL patients assessed in this study included a total of 23 with pre B-ALL and 4 with T-ALL (Table 1) and with time to relapse ranging from 5 to 59 months with a median of 29.9 months, a characteristic similar to larger cohorts studied previously.42,43 Their ages at diagnosis ranged from 2.5 months to 16.3 years, with a median of 4.9 years, and 17 of the 27 patients were male. Of the 27 children, 12 relapsed while still on therapy.
An initial investigation was conducted on 5 patients (A1-A5) treated on the ANZCCSG ALL Study VI who had previously been analyzed as part of an MRD study that involved screening for a limited number of IgH and TCRγ rearrangements.34 For each of these patients a single clonal IgH marker had been identified in the leukemic sample at diagnosis. Monitoring for this marker throughout treatment had failed to predict relapse in all 5 cases, with the diagnosis clone being undetectable in the corresponding relapse sample. To investigate this further, matched diagnosis and relapse marrow samples from these patients were screened again for IgH and TCRγ rearrangements. In each case, the original PCR target evident at diagnosis was replaced by an apparently new clonal rearrangement at relapse (Table 2).
Rearrangements detected at diagnosis and relapse in cohort A patients
| Patient ID . | Diagnosis . | Relapse . |
|---|---|---|
| A1 | VH2-26/D6-6/J5 | VH2-48/D1-26/J4 |
| A2 | VH2-26/D3-10/J6 | VH1-69/D3-10/J6* |
| A3 | VH2-26/D3-22/J4 | VH2-5/D3-3/J4* |
| A4 | VH2-26/J6 | VH2-5/D3-22/J4 |
| A5 | VH2-26/J4b | VH3-15/D2-15/J4 |
| Patient ID . | Diagnosis . | Relapse . |
|---|---|---|
| A1 | VH2-26/D6-6/J5 | VH2-48/D1-26/J4 |
| A2 | VH2-26/D3-10/J6 | VH1-69/D3-10/J6* |
| A3 | VH2-26/D3-22/J4 | VH2-5/D3-3/J4* |
| A4 | VH2-26/J6 | VH2-5/D3-22/J4 |
| A5 | VH2-26/J4b | VH3-15/D2-15/J4 |
Rearrangement later detected at diagnosis via specific RQ-PCR (see Table 4).
Because limited amounts of DNA precluded a more extensive analysis of these patients, a more comprehensive screen was conducted on matched diagnosis and relapse samples from a larger group of 22 patients (cohort B) using consensus primers for 31 potential Ig and TCR rearrangements. In all cases, the relapse DNA sample used was from the first clinical relapse of the respective patient, with the exception of patient B20 who suffered a central nervous system (CNS) relapse where the sample from the second clinical relapse was used for this analysis. The profile of Ig and TCR clonal markers detected in these patient samples is listed in Table 3. At least one clonal immunoglobulin or T-cell receptor rearrangement was detected in all patient specimens at diagnosis (IgH 81% of patients, Igκ 41%, TCRγ 73%, TCRδ 59%). As anticipated, mature IgH rearrangements were detected only in B-lineage ALL patient samples (Table 3). Four patients had more than 2 allelic rearrangements present at diagnosis for TCRγ (B1, B5), TCRδ (B4), or IgH (B17) genes, suggesting the presence of more than one clone (Table 3).
Rearrangements detected at diagnosis and relapse in cohort B patients
| Patient ID and marker . | Rearrangement detected . | ||
|---|---|---|---|
| Diagnosis only . | Relapse only . | Both diagnosis and relapse . | |
| B-lineage ALL | |||
| B1 | |||
| Ig | — | VκInt/κdel | Vκ3–20/κdel |
| TCR | Vγ9/Jγ2* | — | Vγ3/Jγ2, Vγ9/Jγ2 |
| B3 | |||
| Ig | — | — | VH3–30/D2–21/J4; Vκ1–8/κdel |
| TCR | — | — | Vγ2/Jγ2, Vγ3/Jγ2 |
| B6 | |||
| Ig | — | VH4–39/D5–5/J4† | VH3–33/D5–5/J4 |
| TCR | — | — | Vγ2/Jγ1 |
| B7 | |||
| Ig | VH6–1/D6–13/J1 | VH6–1/D6–13/J2† | Vκ4–1/κdel |
| TCR | Vδ2/Dδ3 | — | — |
| B8 | |||
| Ig | VH3–13/D1–7/D1–26/J6 | VκInt/κdel | — |
| TCR | — | — | Vγ3/Jγ2 |
| B10 | |||
| Ig | VH2–5/D1–26/J4 | — | VH3–33/D2–2/J5 |
| TCR | Vδ2/Dδ3, Dδ2/Dδ3 | — | — |
| B11 | |||
| Ig | — | — | VH1–2/D2–2/J4; VH4–59/D2–2/J6 |
| TCR | Vγ9/Jγ2 | — | Vδ2/Dδ3 |
| B12 | |||
| Ig | VH3–13/D2–8/J5; DH2–8/J5 | — | |
| B13 | |||
| Ig | VH3–33/D3–16/D2–27/J3; Vκ1–5/κdel | — | Vκ3–20/κdel |
| TCR | — | — | Vγ2/Jγ1, Vγ3/Jγ1; Vδ2/Dδ3 |
| B14 | |||
| Ig | DH3–9/J3 | — | DH5–12/J4 |
| TCR | Vδ2/Dδ3 | Vγ4Jγ1, Vγ11/Jγ1† | — |
| B15 | |||
| TCR | — | — | Vγ11/Jγ1 |
| B16 | |||
| Ig | — | Vκ1–8/κdel, Vκ7–3/κdel† | VH3–30/D6–13/J6 |
| TCR | Vδ2/Jδ1 | — | — |
| B17 | |||
| Ig | VH1–46/D2–15/J5 | — | VH3–48/D2–15/J5; VH6–1/D3–10/J1; VκInt/κdel |
| TCR | Vγ11/Jγ1* | — | Vγ11/Jγ1; Vδ2/Dδ3 |
| B18 | |||
| Ig | — | — | VH1–3/D6–13/J6 |
| TCR | — | — | Vδ2/Dδ3 |
| B19 | |||
| Ig | VH5–51/D3–3/J4 | VH6–1/D2–8/J6† | DH2–8/J6; VH3–30/D6–6/J4 |
| TCR | — | Vγ11/Jγ2; Vδ2/Dδ3, Dδ2/Dδ3 | — |
| B20 | |||
| Ig | VH2–26/J6 | VH3–23/D6–6/J5† | Vκ7–3 |
| TCR | — | — | Vγ4/Jγ2 |
| B21 | |||
| Ig | — | — | VH1–2/D3–3/J6 |
| B22 | |||
| Ig | VH3–13/D2–8/JH1 | — | VH6–1/D6–25/J4; Vκ1/κdel |
| TCR | — | — | Vγ4/Jγ1, Vγ11/Jγ1; Dδ2/Dδ3, Vδ2/Dδ3 |
| T-cell ALL | |||
| B2 | |||
| TCR | — | — | Vγ2/Jγ2‡, Vγ3/Jγ2‡; Vδ1/Jδ1‡ |
| B4 | |||
| TCR | — | — | Vγ2/Jγ1, Vγ3/Jγ2; Vδ2/Jδ1, Vδ2/Dδ3, Dδ2/Jδ1 |
| B5 | |||
| Ig | DH4–23/J4 | — | — |
| TCR | Vγ3/Jγ2, Vγ9/Jγ1, Vγ10/Jγ2; Vδ2/Dδ3 | — | Vγ9/Jγ2 |
| B9 | |||
| Ig | DH4–23/J4 | — | — |
| TCR | — | — | Vγ2/Jγ2 |
| Totals | 26 | 13 | 50 |
| Patient ID and marker . | Rearrangement detected . | ||
|---|---|---|---|
| Diagnosis only . | Relapse only . | Both diagnosis and relapse . | |
| B-lineage ALL | |||
| B1 | |||
| Ig | — | VκInt/κdel | Vκ3–20/κdel |
| TCR | Vγ9/Jγ2* | — | Vγ3/Jγ2, Vγ9/Jγ2 |
| B3 | |||
| Ig | — | — | VH3–30/D2–21/J4; Vκ1–8/κdel |
| TCR | — | — | Vγ2/Jγ2, Vγ3/Jγ2 |
| B6 | |||
| Ig | — | VH4–39/D5–5/J4† | VH3–33/D5–5/J4 |
| TCR | — | — | Vγ2/Jγ1 |
| B7 | |||
| Ig | VH6–1/D6–13/J1 | VH6–1/D6–13/J2† | Vκ4–1/κdel |
| TCR | Vδ2/Dδ3 | — | — |
| B8 | |||
| Ig | VH3–13/D1–7/D1–26/J6 | VκInt/κdel | — |
| TCR | — | — | Vγ3/Jγ2 |
| B10 | |||
| Ig | VH2–5/D1–26/J4 | — | VH3–33/D2–2/J5 |
| TCR | Vδ2/Dδ3, Dδ2/Dδ3 | — | — |
| B11 | |||
| Ig | — | — | VH1–2/D2–2/J4; VH4–59/D2–2/J6 |
| TCR | Vγ9/Jγ2 | — | Vδ2/Dδ3 |
| B12 | |||
| Ig | VH3–13/D2–8/J5; DH2–8/J5 | — | |
| B13 | |||
| Ig | VH3–33/D3–16/D2–27/J3; Vκ1–5/κdel | — | Vκ3–20/κdel |
| TCR | — | — | Vγ2/Jγ1, Vγ3/Jγ1; Vδ2/Dδ3 |
| B14 | |||
| Ig | DH3–9/J3 | — | DH5–12/J4 |
| TCR | Vδ2/Dδ3 | Vγ4Jγ1, Vγ11/Jγ1† | — |
| B15 | |||
| TCR | — | — | Vγ11/Jγ1 |
| B16 | |||
| Ig | — | Vκ1–8/κdel, Vκ7–3/κdel† | VH3–30/D6–13/J6 |
| TCR | Vδ2/Jδ1 | — | — |
| B17 | |||
| Ig | VH1–46/D2–15/J5 | — | VH3–48/D2–15/J5; VH6–1/D3–10/J1; VκInt/κdel |
| TCR | Vγ11/Jγ1* | — | Vγ11/Jγ1; Vδ2/Dδ3 |
| B18 | |||
| Ig | — | — | VH1–3/D6–13/J6 |
| TCR | — | — | Vδ2/Dδ3 |
| B19 | |||
| Ig | VH5–51/D3–3/J4 | VH6–1/D2–8/J6† | DH2–8/J6; VH3–30/D6–6/J4 |
| TCR | — | Vγ11/Jγ2; Vδ2/Dδ3, Dδ2/Dδ3 | — |
| B20 | |||
| Ig | VH2–26/J6 | VH3–23/D6–6/J5† | Vκ7–3 |
| TCR | — | — | Vγ4/Jγ2 |
| B21 | |||
| Ig | — | — | VH1–2/D3–3/J6 |
| B22 | |||
| Ig | VH3–13/D2–8/JH1 | — | VH6–1/D6–25/J4; Vκ1/κdel |
| TCR | — | — | Vγ4/Jγ1, Vγ11/Jγ1; Dδ2/Dδ3, Vδ2/Dδ3 |
| T-cell ALL | |||
| B2 | |||
| TCR | — | — | Vγ2/Jγ2‡, Vγ3/Jγ2‡; Vδ1/Jδ1‡ |
| B4 | |||
| TCR | — | — | Vγ2/Jγ1, Vγ3/Jγ2; Vδ2/Jδ1, Vδ2/Dδ3, Dδ2/Jδ1 |
| B5 | |||
| Ig | DH4–23/J4 | — | — |
| TCR | Vγ3/Jγ2, Vγ9/Jγ1, Vγ10/Jγ2; Vδ2/Dδ3 | — | Vγ9/Jγ2 |
| B9 | |||
| Ig | DH4–23/J4 | — | — |
| TCR | — | — | Vγ2/Jγ2 |
| Totals | 26 | 13 | 50 |
Unrelated to Vγ rearrangement detected at relapse.
Rearrangement later detected at diagnosis via specific RQ-PCR (see Table 4).
Marker detected at diagnosis, relapse 1, and relapse 2.
— indicates not applicable.
An altered rearrangement pattern between diagnosis and relapse ALL samples was observed for 16 (80%) of these 22 patients, for a total of 39 (44%) of the 89 rearrangements detected. Nevertheless, in 21 patients at least 1 marker was conserved between diagnosis and relapse, eliminating the possibility that the leukemia present atrelapse was unrelated to the initial disease. No rearrangements were detected in the relapse sample of patient B12, indicating loss of both diagnosis rearrangements (Table 3). For pre B-ALL patients, Igκ rearrangements were the most stable (87%), with stability rates of 56%, 81%, and 54% seen for IgH, TCRγ, and TCRδ markers, respectively.
Detection of the relapse clone as a subclone present at diagnosis
It was evident from the above analysis that in the 5 patients of cohort A (Table 2) and in 8 patients of cohort B (Table 3, “Relapse Only”), one or more relapse rearrangements were not detected in the corresponding diagnosis samples by conventional PCR with consensus primers, which typically has a detection limit of between 1% and 10% of cells.23 To determine whether the newly identified relapse rearrangements were present at low levels at the time of diagnosis in these 13 patients, more sensitive leukemia-specific RQ-PCR assays were developed for one or more of the identified relapse markers in 12 patients; a sensitive and specific assay could not be developed for the thirteenth patient (B1). In 8 of these 12 informative patients (67%), a clone-specific relapse marker was identified in the corresponding patient sample taken at the time of diagnosis at levels between 10−5 and 10−1 (Table 4). Thus in 8 patients, the relapse clone was indeed present at diagnosis. The relapse samples of patients A1, A4, A5, B8, B14, B16, and B19 also contained new rearrangements that could not be detected at diagnosis using specific RQ-PCR (Table 4) and which may have arisen via ongoing rearrangements between diagnosis and relapse.
Detection of relapse markers at diagnosis
| ID . | Relapse rearrangement . | Quantity of relapse clone at diagnosis . |
|---|---|---|
| A1 | VH2-48/D1-26/J4 | ND |
| A2 | VH1-69/D3-10/J6 | 1.0 × 10−5 |
| A3 | VH2-5/D3-3/J4 | 1.0 × 10−4 |
| A4 | VH2-5/D3-22/J4 | ND |
| A5 | VH3-15/D2-15/J4 | ND |
| B1 | VκInt/κdel | NS |
| B6 | VH4-39/D5-5/J4 | 8.7 × 10−3 |
| B7 | VH6-1/D6-13/J2 | 1.2 × 10−1 |
| B8 | VκInt/κdel | ND |
| B14 | Vγ4/Jγ1; Vγ11/Jγ1 | ND; 9.0 × 10−4 |
| B16 | Vκ1/κdel; Vκ7/κdel | ND; 1.01 × 10−5* |
| B19 | VH6-1/D2-8/J6; Vγ11/Jγ2; Vδ2/Dδ3; Dδ2/Dδ3 | 4.5 × 10−4; ND; NS; NS |
| B20 | VH3-23/D6-6/J5 | 9.0 × 10−3 |
| ID . | Relapse rearrangement . | Quantity of relapse clone at diagnosis . |
|---|---|---|
| A1 | VH2-48/D1-26/J4 | ND |
| A2 | VH1-69/D3-10/J6 | 1.0 × 10−5 |
| A3 | VH2-5/D3-3/J4 | 1.0 × 10−4 |
| A4 | VH2-5/D3-22/J4 | ND |
| A5 | VH3-15/D2-15/J4 | ND |
| B1 | VκInt/κdel | NS |
| B6 | VH4-39/D5-5/J4 | 8.7 × 10−3 |
| B7 | VH6-1/D6-13/J2 | 1.2 × 10−1 |
| B8 | VκInt/κdel | ND |
| B14 | Vγ4/Jγ1; Vγ11/Jγ1 | ND; 9.0 × 10−4 |
| B16 | Vκ1/κdel; Vκ7/κdel | ND; 1.01 × 10−5* |
| B19 | VH6-1/D2-8/J6; Vγ11/Jγ2; Vδ2/Dδ3; Dδ2/Dδ3 | 4.5 × 10−4; ND; NS; NS |
| B20 | VH3-23/D6-6/J5 | 9.0 × 10−3 |
Approximate level.
ND indicates not detectable; NS, a specific assay could not be developed.
Presence of related subclones at diagnosis
The finding that at least one marker was conserved between the diagnosis and relapse samples for all but one patient (Table 3) indicated that the predominant leukemic clone present at relapse was part of the clonal population present at diagnosis, despite alterations in clonal profile. In 3 cases in which major and minor leukemic clones coexisted at diagnosis, further investigation of the IgH rearrangements confirmed the relationship between these clones. Thus, for patients A2, B6, and B19, sequence analysis of the IgH rearrangement revealed that the minor and major clones at diagnosis were related via ongoing recombination events, with common DH-JH stems being present in each case (Figure 1). For example, the rearrangement detected in patient A2 at relapse [VH(1-69)-N1-DH(3–10)-JH6] shared common N and D-J sequences with the major population at diagnosis [VH(2-26)-N1-DH(3-10)-JH6], indicating VH-VH gene replacement. The more distal location of the VH(1-69) gene segment within the IgH locus indicates a more recent origin for the relapse clone, which might explain the small proportion of the relapse clone at presentation.
Differential therapeutic response of ALL subclones in patient B20
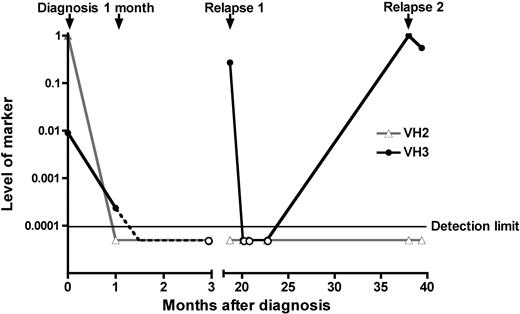
The process by which a minor subclone at diagnosis could expand to become the major population present at relapse was investigated using sensitive RQ-PCR to monitor the levels of clonal rearrangements during treatment in patient B20 (Figure 2). The level of the relapse clone at diagnosis, together with adequate marker sensitivity and the availability of samples obtained at multiple points during treatment, enabled comparison of the therapeutic response of the leukemic subclones in this patient. The major clone detected at diagnosis harbored a VH2-J rearrangement (which had been used to monitor MRD in this patient), whereas the minor clone exhibited a VH3-DH6-J rearrangement. The major diagnosis clone reduced rapidly during remission induction such that this rearrangement was undetectable after 1 month of therapy (Figure 2, VH2). As the limit of detection of this VH2 RQ-PCR assay is 10−4, this represents a reduction of this clone in the realm of 1000-fold. However, the relapse clone, while initially present at lower levels, showed only 30- to 40-fold reduction over the same time period and was still detectable after remission induction (Figure 2, VH3). Both the VH2 and VH3 markers were below detectable levels after 3 months of therapy, with VH2 remaining undetectable for the duration of follow-up. In contrast, the VH3 marker reappeared in the bone marrow at subclinical levels at the time of CNS relapse (18 months) and again at second relapse (38 months), indicating selection and persistence of a relatively resistant subclone of the oligoclonal leukemic population at clinical diagnosis.
Therapeutic response of leukemic clones in patient B20. The relative levels of IgH rearrangements marking either the major diagnosis (VH2, ▴) or relapse (VH3, ●) clones were analyzed by RQ-PCR in diagnostic, follow-up, and relapse bone marrow samples. The presence of either marker could be detected at levels above 10−4. The patient suffered a CNS relapse (relapse 1) and a second bone marrow relapse. Data points below the detection limit line are arbitrarily placed and indicate points at which VH2 (▵) or VH3 (○) rearrangements were undetectable. Dotted line indicates extrapolated level of clone between 1 and 3 months.
Therapeutic response of leukemic clones in patient B20. The relative levels of IgH rearrangements marking either the major diagnosis (VH2, ▴) or relapse (VH3, ●) clones were analyzed by RQ-PCR in diagnostic, follow-up, and relapse bone marrow samples. The presence of either marker could be detected at levels above 10−4. The patient suffered a CNS relapse (relapse 1) and a second bone marrow relapse. Data points below the detection limit line are arbitrarily placed and indicate points at which VH2 (▵) or VH3 (○) rearrangements were undetectable. Dotted line indicates extrapolated level of clone between 1 and 3 months.
Presence and quantity of relapse clone at diagnosis correlate with patient outcome
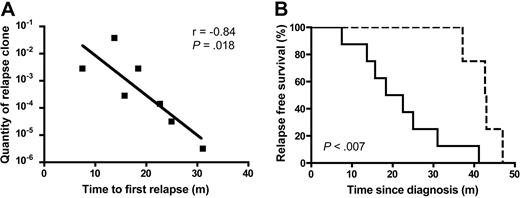
To gain insight into the biology of ALL relapse, we examined the relationship between the quantity of the relapse clone at diagnosis and patient outcome among the 12 informative patients in Table 4. Of the 8 patients for whom the relapse clone was detected as a minor clone in their leukemia at diagnosis, the level of the relapse clone at diagnosis could be accurately quantified for 7 of these patients, with the exception of patient B16 (Table 4). Notably, a significant inverse correlation was observed between the time to first relapse and the amount of the relapse clone present at diagnosis, with high levels predicting a shorter remission (r = −0.84, P = .018; Figure 3A). For example, the leukemic populations of patients B6, B7, and B20 had the highest levels of the relapse clone at diagnosis and these patients had the shortest relapse-free survival (a mean of 13 months compared with 24 months for the remaining 4 informative patients). The leukemic populations of patients A2 and A3 had the lowest levels of the relapse clone at diagnosis along with the longest relapse-free survival among these 7 informative patients. While the relapse clone of patient B16 was detectable but at levels too low to allow accurate quantitation, it is interesting to note that this patient had the longest relapse-free survival among the 8 patients for whom the relapse clone was detected in the diagnosis sample. Furthermore, for the 4 patients in whom no relapse clone could be detected in the diagnosis marrow sample, the time to first relapse was significantly longer compared with the 8 patients in whom the relapse clone was detected in the diagnosis sample (mean 22 months, range 8 to 41 months; and mean 42.5 months, range 37 to 47 months, respectively; P = .004). Kaplan-Meier survival analysis confirmed a significant relationship between detection of the relapse clone at diagnosis and relapse-free survival (P < .007; Figure 3B).
Impact of presence of relapse clone at diagnosis on patient outcome. (A) Correlation between quantity of relapse clone present at diagnosis (expressed as a proportion of the quantity present at relapse) and time to first relapse. (B) Relapse-free survival according to detection of the relapse clone at diagnosis (solid line, relapse clone detected, n = 8; dotted line, relapse clone not detected, n = 4).
Impact of presence of relapse clone at diagnosis on patient outcome. (A) Correlation between quantity of relapse clone present at diagnosis (expressed as a proportion of the quantity present at relapse) and time to first relapse. (B) Relapse-free survival according to detection of the relapse clone at diagnosis (solid line, relapse clone detected, n = 8; dotted line, relapse clone not detected, n = 4).
Discussion
The antigen receptor rearrangements in matched diagnostic and relapse bone marrow samples from 27 ALL patients were used to study the mechanism of relapse in childhood ALL. For a subset of patients, new rearrangements were detected at relapse. While antigen receptor rearrangements themselves can be used solely to determine clonality, in this case the new rearrangements provided useful specific markers of the cell populations responsible for relapse. This study is the first to investigate the biologic significance of the presence and quantity of the relapse clone at diagnosis and its relationship to clinical outcome. We found that relapse may result from the selection of a relatively resistant subclone within the original leukemic population that is undetectable using routine methods. For the informative patients in this study, we observed that the amount of this subclone at diagnosis directly impacts on patient outcome. This research highlights the need to identify patients with resistant or persistent subclones early during treatment, so these patients can be stratified to alternative therapeutic protocols.
Two hypotheses can be proposed to explain the mechanism behind expansion of leukemic cell populations at relapse following remission induction chemotherapy. On the one hand, relapse could arise from induction of resistance during chemotherapy, presumably via acquisition of genetic mutations after diagnosis. Such an event would be expected to occur within a single cell of the residual leukemic population and the time to relapse following this change would be determined by the timing of the change and the resulting phenotype. Alternatively, relapse could arise through selection and further expansion of an already relatively resistant subpopulation present at diagnosis. If the level of this subpopulation could be determined, it is reasonable to predict that the quantity of this clone would influence the length of clinical remission. The findings of this study strongly support the latter hypothesis. Firstly, in concordance with a number of recent studies we found that relapse may indeed arise after expansion of a minor subpopulation of leukemic cells present at diagnosis.18,27,31-33 A second key piece of evidence supporting the existence of an inherently resistant subclone at diagnosis, rather than the alternative possibility of a small number of cells surviving initial therapy and acquiring further alterations during treatment or remission, is the differential chemosensitivity between subclones present at diagnosis, as exemplified by patient B20. Differential responses of leukemic subclones to remission induction therapy were observed previously in a small number of TEL/AML1-positive32,44 or -negative18,31 B-ALL patients. Thirdly, and most significantly, the results presented here demonstrate for the first time that, for a number of patients with a range of remission durations, the time to relapse depends on the initial amount of the relapse clone present at diagnosis. In the subcohort of informative cases, a strong inverse correlation was observed, where patients with the lowest level of the relapse clone at diagnosis had the longest interval to relapse, whereas those with the highest levels suffered earlier relapses.
Taken together, these data indicate that the biologic characteristics necessary for relapse may already be present in the relapse-causing clone at the time of diagnosis. Further support for a model of selection rather than acquisition of resistance comes from our observations in a previous study of ALL diagnostic bone marrow specimens propagated in nonobese diabetic–severe combined immunodeficiency (NOD/SCID) mice. Where a new clonal marker had emerged in a patient relapse sample that was undetectable at diagnosis using conventional PCR, this same rearrangement was also detected after tertiary transplantation in the mice, thus confirming the presence of this relapse clone in the diagnosis specimen and its subsequent selection.45 This powerful experimental model could be used in the future to further characterize the relatively resistant clone identified in patient B20 and other similar cases. Further studies including a similar assessment of the diagnosis and early treatment time points in patients who remained in continuous complete remission and were subsequently cured, but whose leukemias contained detectable subclones at diagnosis, would also complement the data reported herein. Such subclones would be expected not to show differential sensitivity to treatment. In addition, it is notable that the correlation between level of the relapse clone at diagnosis and time to relapse was observed despite differing risk classifications and/or treatment protocols, and it will be of great interest to confirm these findings in a larger cohort of uniformly treated patients and to make comparisons between treatment groups.
For a subset of patients, the level of the relapse clone at diagnosis predicted the timing of relapse from as early as 8 months out to 41 months after diagnosis. However, in the 4 patients for whom the relapse clone could not be detected by sensitive RQ-PCR in the diagnosis sample, relapse occurred at intervals between 37 and 47 months after diagnosis. It is not clear whether relapse in these 4 cases was due to expansion of an existing clone of intermediate resistance or of extremely low number or due to acquisition of resistance after therapy. However, given the strong correlation observed between the level of the relapse clone in the diagnosis sample and the time to relapse, and the overlap in the range of time to relapse between the 2 groups, it is also possible that the relapse clone of these 4 patients was present at diagnosis but at levels below the detection limits of the technique used. Thus, for a proportion of patients, the likelihood of relapse would appear to be predetermined early following diagnosis and a major challenge will be the development of techniques to detect these low-level clones during the early treatment phase so their chemosensitivities can be monitored. Indeed, had the minor (relapse) clone of patient B20 been identified at diagnosis and monitored after remission induction, a positive determination for MRD would have led to stratification of this patient into a high-risk therapy group. This study therefore highlights the need to identify as many antigen receptor rearrangements at diagnosis as practicable, to enhance the likelihood of detecting any small, relatively resistant subpopulation during the course of the disease. Current MRD technology using specific RQ-PCR can routinely detect 1 leukemic cell in 104 or 105 cells and this study reveals that a small population of leukemic cells responsible for relapse could be present at levels below this threshold, perhaps by an order of magnitude, thus revealing limitations in assay sensitivity as another potential source of false-negative results for postinduction MRD.
In conclusion, clonal profiles of antigen receptor rearrangements were used to study the mechanism of relapse in ALL. In a number of cases where distinct rearrangements marked the relapse population, we were able to address the biologic significance of detecting potentially resistant leukemic subclones at the time of diagnosis. The direct relationship between the amount of the relapse clone present at diagnosis and time to relapse suggests that improvements in monitoring for such populations at the time of disease presentation may be a useful adjunct to current treatment regimens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laura Piras for coordination of patient bone marrow sample procurement and storage, Professor Jacques van Dongen for advice on MRD methodology, and Dr Catherine Cole for critical review of the manuscript.
Children's Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children's Hospital, Randwick.
This work was supported by the National Health and Medical Research Council, The Cancer Council New South Wales, The Leukaemia Foundation, the Anthony Rothe Memorial Trust, and The Children's Leukaemia and Cancer Research Foundation, WA.
Authorship
Contribution: S.C. designed and performed research, collected and analyzed data, and revised the manuscript; M.J.H. collected and analyzed data and drafted the manuscript; E.K. performed research and collected and analyzed data; A.H.B. and R.S. collected and analyzed data and revised the manuscript; A.Y.B. performed research and analyzed data; N.C.V. and J.G. performed research; L.D.P. provided vital specimens and performed clinical analysis; D.L.B. was involved in study design, provided vital specimens, and revised the manuscript; G.M.M. provided vital specimens, performed clinical analysis, and revised the manuscript; U.R.K. was involved in study design, analyzed data, and revised the manuscript; and M.H. and M.D.N. designed research, analyzed data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. D. Norris, Children's Cancer Institute Australia for Medical Research, PO Box 81 (High St), Randwick NSW 2031, Australia; e-mail: mnorris@ccia.unsw.edu.au.