Abstract
The study's objective was to identify HPA 1a–negative women and to offer them an intervention program aimed to reduce morbidity and mortality of neonatal alloimmune thrombocytopenia (NAIT). HPA 1 typing was performed in 100 448 pregnant women. The HPA 1a–negative women were screened for anti–HPA 1a. In immunized women, delivery was performed by Cesarean section 2 to 4 weeks prior to term, with platelets from HPA 1a–negative donors reserved for immediate transfusion if petechiae were present and/or if platelet count was less than 35 × 109/L. Of the women screened, 2.1% were HPA 1a negative, and anti–HPA 1a was detected in 10.6% of these. One hundred seventy pregnancies were managed according to the intervention program, resulting in 161 HPA 1a–positive children. Of these, 55 had severe thrombocytopenia (< 50 × 109/L), including 2 with intracranial hemorrhage (ICH). One woman with a twin pregnancy missed the follow-up and had one stillborn and one severely thrombocytopenic live child. In 15 previous prospective studies (136 814 women) there were 51 cases of severe NAIT (3 intrauterine deaths and 7 with ICH). Acknowledging the limitation of comparing with historic controls, implementation of our screening and intervention program seemed to reduce the number of cases of severe NAIT-related complications from 10 of 51 to 3 of 57.
Introduction
Neonatal alloimmune thrombocytopenia (NAIT) is a rare (1 of 1000 to 2000 pregnancies) but potentially serious condition. It most frequently occurs in women lacking the human platelet antigen 1a (HPA 1a). They may become immunized against HPA 1a during pregnancy if the fetus is HPA 1a positive.1 Maternal IgG antibodies against HPA 1a can traverse placenta and sensitize the fetal platelets, leaving the fetus thrombocytopenic and at risk of bleeding
In most cases of NAIT there is no clinical significant bleeding, but intracranial hemorrhage (ICH) has been reported to occur in 7% to 26%,2-5 with a fatal outcome in approximately one third of the ICH cases.4,5 The validity of these results can be questioned, as they are mainly based on retrospective studies.
Antenatal screening to identify women at risk of NAIT has previously been considered, but the main limitations have been uncertainty about what intervention should be applied to immunized women and the lack of reliable parameters to identify those fetuses most severely affected and in need of effective antenatal treatment.6 Another constraint is the cost of HPA 1a typing in a screening program, as only about 0.3% to 0.4% of the women are expected to be immunized.7,8 More recently, however, low-cost HPA 1a typing by flow cytometry has become available.9
The objective of the present study was to prospectively screen a large cohort of pregnant women for the HPA 1a phenotype in order to identify HPA 1a–negative women and to offer them an intervention program aimed to reduce the risk of severe NAIT-related complications defined as intrauterine death or ICH. Univariate analysis was used to compare our observed risk of severe NAIT-related complications with historic controls obtained from all hitherto published prospective studies assessing the frequency of severe NAIT-related complications.
In hemophilic neonates, serious ICH has been reported to occur during prolonged and complicated vaginal delivery10 and a consensus report from the American Society of Hematology11 does not recommend this route of delivery for pregnant women with idiopathic thrombocytopenic purpura (ITP) if fetal platelet count is very low. Although it is not known which route of delivery is optimal in NAIT, we chose to carry out elective Cesarean section (CS) 2 to 4 weeks prior to term to reduce risk of ICH during delivery and to allow having compatible platelets available at the time of delivery.
Preliminary data of the first 11 100 women included in this study have previously been published.12 In the present study we present the data from the total population of more than 100 000 pregnant women.
Patients, materials, and methods
Patients
Pregnant women were recruited consecutively from North Norway (December 1995 until March 2004) and from Health Regions South and East in the southern part of Norway (September 2001 until March 2004). No exclusion criteria were applied. All pregnant women had their HPA 1 allotype determined in the same sample that was used for RhD typing. HPA 1a–negative women were also HLA DRB3 genotyped.
A letter explaining their patients' increased risk of giving birth to a child with NAIT was sent to the general practitioners of the HPA 1a–negative women. After providing informed consent in accordance with the Declaration of Helsinki, these women were enrolled in the intervention program. The study was approved by the Regional Committee for Medical Research Ethics, North Norway (approval no. P-REK V 13/1995).
Intervention program
Approximately every fourth week during pregnancy, a blood sample was examined for anti–HPA 1a antibodies and quantified when present. No further follow-up was offered to the women who did not develop anti–HPA 1a. Immunized women were referred to the Department of Obstetrics and Gynecology at 1 of the 3 university hospitals (Ullevål University Hospital, Rikshospitalet-Radiumhospitalet Medical Center, or University Hospital of North Norway) for clinical follow-up. Delivery was performed by CS 2 to 4 weeks prior to term. The reason for variation in timing of CS was in most cases due to logistics (patient's travel to hospital, public holidays, scheduling of staff and operating facility for CS, and preparation of platelet concentrates from HPA 1a–negative donors) but in some women with very high anti–HPA 1a levels CS was advanced. The number of clinical visits before CS was decided by the obstetrician.
One or 2 days before CS, HPA 1a–negative platelets compatible with the plasma from the immunized mother were harvested by platelet apheresis. The panel of HPA 1a–negative donors comprised around 200, of whom most were also typed for the HLA class I A and B antigens. If the platelet count was less than 35 × 109/L and/or if the neonate had petechiae, platelets were transfused immediately at a dosage of 60 × 109 to 120 × 109.
Genomic platelet typing of the neonate was performed in samples from cord blood or from buccal swabs. In 2 neonates we did not obtain any material for platelet genotyping, but as their fathers were HPA 1aa, we inferred that the neonates both were HPA 1ab.
Laboratory analyses
The initial HPA 1a typing was carried out by flow cytometry,9 enzyme-linked immunosorbent assay (ELISA),13 or polymerase chain reaction (PCR).14 For the confirmatory typing we used real-time PCR with melting curve analysis15 or 5′ nuclease assay.14
The HLA DRB3 typing was performed by sequencing the HLA DRB3 gene when present. For the PCR, we used intron-located amplification primers previously described by Kotsch et al.16
Screening for anti–HPA 1a antibodies was performed by flow cytometry or by using the technique of monoclonal antibody immobilization of platelet antigen (MAIPA).17 Quantification of anti–HPA 1a in all samples from immunized women was carried out by a modified MAIPA test.12,18 The cross-match between donor platelets and plasma from the pregnant women was carried out by flow cytometry.19 Platelet typing of the neonates was performed by PCR as previously described.14,15
Literature search
PubMed20 was used to search for 2 types of prospective studies: (1) studies on NAIT and (2) studies of platelet counts in cohorts of neonates where the reason for thrombocytopenia was explored when platelet counts were low. For the search we used combinations of the following MeSH terms: “antigens, human platelet/immunology,” “infant, newborn,” and “thrombocytopenia/immunology.” Reference lists of review papers and congressional proceedings were also used to identify these 2 types of studies. In the identified studies, we focused on the total number of cases with severe NAIT defined as intrauterine death or severe thrombocytopenia in the fetus or newborn (ie, platelet counts < 50 × 109/L before delivery, immediately after delivery, or during the first 3 days in the postnatal period).
Statistics
Comparison of numbers was carried out by using χ2 test for trend, Fisher exact test, or by calculation of relative risk (RR) with 95% confidence interval (CI) wherever appropriate. The main analysis was a comparison with the historic controls based on univariate analysis using severe NAIT-related complications (ie, number of cases of intrauterine deaths and ICH) as outcome variable (numerator) relative to the number of cases with severe NAIT (ie, the number of neonates with platelet count < 50 × 109/L; denominator). P values less than .05 were considered significant.
Results
Demographics
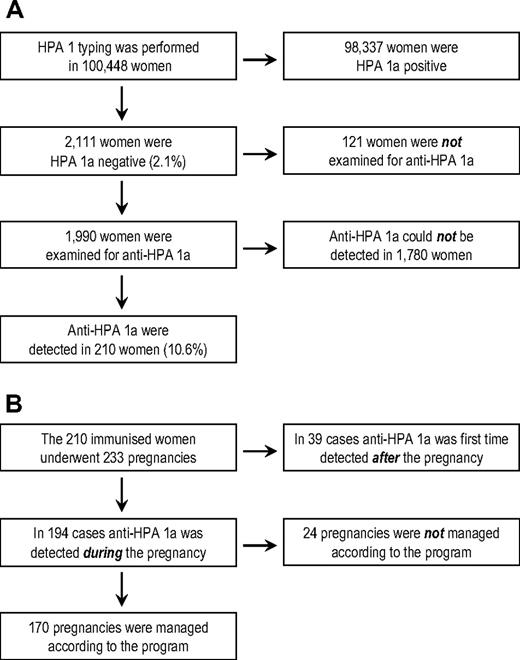
A total of 100 448 women were included in the study (Figure 1A). Two thousand one hundred eleven were found to be HPA 1a negative (2.1%). Of these, 1990 were examined for antibodies against HPA 1a. In 210 women (10.6%), anti–HPA 1a antibodies were detected, and these women completed 233 pregnancies during the study (Figure 1B). In 39 cases, anti–HPA 1a was first-time detected in a sample obtained approximately 6 weeks postpartum. Of the remaining 194 pregnancies, 170 (168 singleton and 2 twin pregnancies) were managed according to the intervention program, representing 154 women. Hence, 16 women were included twice during the study. The median age of these 154 women at the time of delivery was 31.3 years (range, 20.3 to 43.3 y) and a summary of their obstetric history is given in Table 1. The median gestational week in which CS was carried out was 37+1 (range, weeks 31+4 to 40+5).
The obstetric history of the 154 women whose pregnancies were managed according to the intervention program
| No. and outcome of previous pregnancies . | No. of women . | ||||
|---|---|---|---|---|---|
| Pregnancies . | Deliveries . | Induced abortions . | Spontaneous abortions . | Extrauterine pregnancies . | |
| 0 | 14 | 40 | 125 | 112 | 153 |
| 1 | 71 | 82 | 24 | 30 | 1 |
| 2 | 38 | 25 | 5 | 8 | 0 |
| 3 | 20 | 6 | 0 | 3 | 0 |
| 4 or more | 11 | 1 | 0 | 1 | 0 |
| No. and outcome of previous pregnancies . | No. of women . | ||||
|---|---|---|---|---|---|
| Pregnancies . | Deliveries . | Induced abortions . | Spontaneous abortions . | Extrauterine pregnancies . | |
| 0 | 14 | 40 | 125 | 112 | 153 |
| 1 | 71 | 82 | 24 | 30 | 1 |
| 2 | 38 | 25 | 5 | 8 | 0 |
| 3 | 20 | 6 | 0 | 3 | 0 |
| 4 or more | 11 | 1 | 0 | 1 | 0 |
For the 16 women who were enrolled twice, we have given the obstetric history for only the first time they were enrolled.
Noncompliance
Twenty-four pregnancies were not managed according to the intervention program. The reasons for this are given in Table 2. One HPA 1a–negative woman was not followed up due to a misunderstanding. Consequently, no samples from this woman were obtained during pregnancy, and her anti–HPA 1a antibody status remained unknown. She had a twin pregnancy and delivered by acute CS in week 31. One of the twins had a platelet count of 45 × 109/L at the time of birth, whereas the other was stillborn. ICH could be neither excluded nor confirmed due to autolysis of the fetal brain.
An overview of the 24 pregnancies not managed according to the intervention program
| No. of pregnancies . | Reasons for not being followed up according to the intervention program . | Outcome . |
|---|---|---|
| 6 | Spontaneous abortion* | — |
| 2 | Induced abortion | — |
| 2 | Caesarean section at other hospitalswhere essential data are missing | No signs of bleeding in the newborns |
| 12 | Vaginal delivery† | No signs of bleeding in the newborns |
| 1 | Unknown | Unknown |
| 1 | Misunderstanding | A twin pregnancy, delivery by acute Caesarean section in week 31. One of the twins had a platelet count of 45×109/L at the time of birth, whereas the other was stillborn. ICH could be neither excluded nor confirmed due to autolysis of the fetal brain. |
| No. of pregnancies . | Reasons for not being followed up according to the intervention program . | Outcome . |
|---|---|---|
| 6 | Spontaneous abortion* | — |
| 2 | Induced abortion | — |
| 2 | Caesarean section at other hospitalswhere essential data are missing | No signs of bleeding in the newborns |
| 12 | Vaginal delivery† | No signs of bleeding in the newborns |
| 1 | Unknown | Unknown |
| 1 | Misunderstanding | A twin pregnancy, delivery by acute Caesarean section in week 31. One of the twins had a platelet count of 45×109/L at the time of birth, whereas the other was stillborn. ICH could be neither excluded nor confirmed due to autolysis of the fetal brain. |
— indicates not applicable.
It is not known if any of these cases were caused by NAIT.
Three women gave birth before the antibody result was available. Four women had spontaneous delivery before elective Caesarean section. Four women chose vaginal delivery because anti–HPA 1a had only been found in 1 sample early in pregnancy. One woman moved to another health region that had not adopted the screening program.
Platelet counts
Eleven neonates were typed HPA 1bb, all of whom had platelet counts at birth greater than 150 × 109/L. The platelet counts of all 161 HPA 1a–positive neonates are given in Table 3. Severe thrombocytopenia (platelet count < 50 × 109/L) was found in 55 neonates, whereas 30 suffered from mild to moderate thrombocytopenia (platelet count between 50 × 109/L and 150 × 109/L). The remaining 76 neonates had normal platelet counts. Fifty neonates had 1 or more platelet transfusions, and 19 neonates were, in addition, treated with intravenous gamma globulin. Sixteen of 154 women had previously given birth to a child with NAIT, including 1 woman with a previous stillbirth that was most probably caused by NAIT. The platelet counts of the 14 HPA 1a–positive neonates delivered by these women are given in Table 3. There was no significant difference between the number of neonates with severe thrombocytopenia born of women who previously had a pregnancy complicated by symptomatic NAIT and those that did not have such a history (P = .24). There was no significant difference between the number of neonates with severe thrombocytopenia in primigravida and multigravida (P = .56; Table 3).
Number of HPA 1a–positive children with platelet counts within specified ranges
| . | Platelet count, ×109/L . | |||||
|---|---|---|---|---|---|---|
| ≥150 . | <150 and ≥100 . | <100 and ≥50 . | <50 and ≥35 . | <35 and ≥10 . | <10 . | |
| All pregnancies | 76 | 12 | 18 | 10 | 29*† | 16 |
| The 14 cases with previous NAIT | 4 | 1 | 2 | 2 | 3 | 2 |
| Primigravida only | 7 | 1 | 0 | 0 | 5† | 1 |
| . | Platelet count, ×109/L . | |||||
|---|---|---|---|---|---|---|
| ≥150 . | <150 and ≥100 . | <100 and ≥50 . | <50 and ≥35 . | <35 and ≥10 . | <10 . | |
| All pregnancies | 76 | 12 | 18 | 10 | 29*† | 16 |
| The 14 cases with previous NAIT | 4 | 1 | 2 | 2 | 3 | 2 |
| Primigravida only | 7 | 1 | 0 | 0 | 5† | 1 |
One newborn with a large intracranial hemorrhage. The platelet count in cord blood immediately after CS was 13×109/L. The mother was gravida 2, para 0.
One newborn with a small, clinically insignificant intracranial hemorrhage. The platelet count in cord blood immediately after CS was 26×109/L. The mother was primigravida.
Clinical outcome
ICH was detected in 2 HPA 1a–positive children. The mother of one of these children was primigravida and had CS performed in gestational week 38. At the time of delivery, the antibody level was moderately elevated (20 IU/mL). As the platelet count immediately after birth was 26 × 109/L, a platelet transfusion was given. A few days after birth, cerebral ultrasonographic examination revealed a small grade 1 bleeding under the right ventricular horn. At the age of 5 years, no clinical sequelae were observed.
The mother of the other child with ICH was gravida 2, para 0. Anti–HPA 1a antibody level decreased from greater than 150 IU/mL early in pregnancy to 41 IU/mL in gestational week 29. At ultrasonographic examination at gestational week 34+0, an ICH was suspected. The mother was treated with corticosteroids for fetal lung maturation. The next day, a girl (weight, 2320 g; Apgar scores, 9 and 10 at 1 and 5 minutes, respectively) was delivered by CS. As the platelet count immediately after birth was 13 × 109/L and there were widespread petechiae, a platelet transfusion was given. Magnetic resonance brain imaging revealed a large bleeding (6 × 3 × 5 cm in size) in the left temporal region. Almost normal psychomotoric development without seizures or signs of hydrocephalus was observed during the first 6 months. At the age of 7 months, the child developed epilepsy with daily seizures. The long-term prognosis is unknown, as the child was born in September 2004.
HLA DRB3*0101 typing
HLA DRB3*0101 typing was carried out in 198 of the 210 immunized women and 18 were found to be HLA DRB3*0101 negative. Of the 154 women who followed the screening and intervention program, 150 were typed for the presence of HLA DRB3*0101, of whom 12 were HLA DRB3*0101 negative. Only 2 of these 12 women gave birth to thrombocytopenic children (platelet counts, 59 × 109/L and 94 × 109/L).
Adverse effects of the program
Thirty-seven (21.5%) of the neonates suffered from adverse effects associated with the intervention program and were treated at the neonatal intensive care unit. Pulmonary maladaption (wet lung syndrome) was the primary diagnosis in 28 neonates, whereas the remaining neonates had intensive care treatment due to prematurity, hypoglycemia, or because they were small for gestational age. Most of the neonates were treated for just 1 day. Those with pulmonary maladaption had extra oxygen supply and/or nasal CPAP (continuous positive airways pressure).
The proportion of neonates who suffered from adverse effects was significantly higher among neonates delivered before week 37 compared with children delivered in week 37 or later (24 of 75 vs 13 of 97; P = .005). Twenty of the 37 neonates who suffered from adverse effects were thrombocytopenic at birth, but only 11 of these had severe thrombocytopenia. No sequelae were observed in any of the 37 neonates who suffered from adverse effects associated with the intervention program.
Comparison with previous prospective studies
The literature search revealed 16 prospective studies.7,8,21-34 One of these studies was excluded from our comparison because it also included retrospective data.30 In 6 studies, the platelet count was assessed in a cohort of neonates, and for those neonates with low platelet counts, the reason for thrombocytopenia was explored.24,25,28,29,31,32 In the 15 prospective studies (Table 4) that included a total of 136 814 women, severe NAIT was observed in 51 cases and severe complications were found in 10 of these: 7 neonates with ICH and 3 cases of intrauterine death. As a comparison, there were 57 cases with severe NAIT in our study (including the twin pregnancy of the woman who did not follow the intervention program), but severe NAIT-related complications were only observed in 3 of these cases: 2 neonates with ICH and 1 stillbirth. If the comparison with historic controls was extended to encompass calculation of RR based on the crude data (ie, 10/57 versus 3/51), we found that RR of serious complications related to severe NAIT was 0.27 (95% CI: 0.08 to 0.92; P = .034).
A comparison of the number of cases with severe complications to severe NAIT in 15 previous prospective studies that have examined the frequency of NAIT and the present study
| Author, reference . | Total no. of pregnant women . | No. of neonates with severe NAIT . | No. of severe complications . | |
|---|---|---|---|---|
| Intracranial hemorrhage . | Intrauterine deaths . | |||
| Mueller-Eckhardt et al21 | 1211 | 0 | 0 | 0 |
| Reznikoff-Etievant et al22 | 860 | 0 | 0 | 0 |
| Blanchette et al23 | 5000 | 1 | 1 | 0 |
| Burrows et al24 * | 15 471 | 10 | 2 | 1 |
| Panzer et al25 * | 923 | 0 | 0 | 0 |
| Doughty et al26 † | 3473 | 2 | 0 | 0 |
| Durand-Zaleski et al27 † | 2066 | 2 | 0 | 0 |
| Dreyfus et al28 *† | 5632 | 4 | 1‡ | 0 |
| de Moerloose et al29 *† | 8388 | 3 | 0 | 1‡ |
| Williamson et al8 † | 24 417 | 10 | 1 | 1‡ |
| Sainio et al31 * | 4489 | 3 | 0 | 0 |
| Uhrynowska et al32 * | 26 275 | 5 | 1 | 0 |
| Davoren et al33 | 4090 | 3 | 0 | 0 |
| Maslanka et al34 † | 8013 | 3 | 1 | 0 |
| Turner et al7 | 26 506 | 5 | 0 | 0 |
| All hitherto published studies | 136 814 | 51 | 7 | 3 |
| The present study | 100 448 | 57§ | 2 | 1§ |
| Author, reference . | Total no. of pregnant women . | No. of neonates with severe NAIT . | No. of severe complications . | |
|---|---|---|---|---|
| Intracranial hemorrhage . | Intrauterine deaths . | |||
| Mueller-Eckhardt et al21 | 1211 | 0 | 0 | 0 |
| Reznikoff-Etievant et al22 | 860 | 0 | 0 | 0 |
| Blanchette et al23 | 5000 | 1 | 1 | 0 |
| Burrows et al24 * | 15 471 | 10 | 2 | 1 |
| Panzer et al25 * | 923 | 0 | 0 | 0 |
| Doughty et al26 † | 3473 | 2 | 0 | 0 |
| Durand-Zaleski et al27 † | 2066 | 2 | 0 | 0 |
| Dreyfus et al28 *† | 5632 | 4 | 1‡ | 0 |
| de Moerloose et al29 *† | 8388 | 3 | 0 | 1‡ |
| Williamson et al8 † | 24 417 | 10 | 1 | 1‡ |
| Sainio et al31 * | 4489 | 3 | 0 | 0 |
| Uhrynowska et al32 * | 26 275 | 5 | 1 | 0 |
| Davoren et al33 | 4090 | 3 | 0 | 0 |
| Maslanka et al34 † | 8013 | 3 | 1 | 0 |
| Turner et al7 | 26 506 | 5 | 0 | 0 |
| All hitherto published studies | 136 814 | 51 | 7 | 3 |
| The present study | 100 448 | 57§ | 2 | 1§ |
Studies examining the platelet counts in a cohort of neonates and for those neonates with low platelet counts the reason for thrombocytopenia was explored.
Studies that employed fetal blood sampling (FBS) as part of their strategy.
Complications associated with FBS. In addition, there was 1 case of acute Caesarean section due to bleeding after FBS but without any sequelae.27
Including 1 stillborn and 1 severely thrombocytopenic live child of born of a woman who mistakenly was not followed up according to the intervention program.
If the comparison was limited to the 6 studies in which platelet counts were obtained immediately after delivery,24,25,28,29,31,32 RR of serious complications was 0.22 (95% CI: 0.06 to 0.81; P = .021), whereas RR was 0.34 (95% CI: 0.08 to 1.42; P = .20) if a comparison was made with the studies that employed HPA 1a typing of the pregnant women7,8,21-23,26,27,33,34 (Table 4).
Discussion
The present study is unparalleled because it is by far the largest prospective study of NAIT and also because none of the previously published prospective studies have used an intervention program similar to ours. A direct comparison with previously published studies that have used different methodology and have been carried out over many years is therefore difficult and calls for a cautious interpretation of our data. Acknowledging these limitations, it is however noteworthy that the risk of serious complications to severe NAIT in our study was approximately 1 in 4 (RR = 0.27, 95% CI: 0.08 to 0.92; P = .034) compared with the 15 previously published prospective studies.7,8,21-31,33,34 Our comparison with historic controls could potentially have been biased by 2 studies: Durand-Zaleski et al27 only included women who were expecting their first child; and in the large Scottish study (Turner et al7 ), 3 women with a previous history of anti–HPA 1a NAIT were excluded from the data analysis. The latter study has not biased our results, as none of these 3 women gave birth to thrombocytopenic children (Stan Urbaniak, Academic Transfusion Medicine Unit, Department of Medicine and Therapeutics, University of Aberdeen, Scotland, United Kingdom; personal e-mail communication on September 22, 2006). Removal of the data by Durand-Zaleski et al from the comparison makes the difference between our results and the historic controls even larger (RR = 0.26, 95% CI: 0.08 to 0.88; P = .034). If we, on the contrary, assume that inclusion of multiparous women by Durand-Zaleski et al would have increased the number of severely thrombocytopenic children by, for example, 3 and that none of these suffered ICH or intrauterine death, the screening and intervention program would still be associated with a risk reduction to approximately 1 in 4 (RR = 0.28, 95% CI: 0.08 to 0.98; P = .039).
The comparison with historic controls was based on univariate analysis. Although a multivariate analysis could have adjusted the risk assessment for potential confounders, such analysis was not carried out for the following reasons: first, the main result of the present study was based on small numbers (ie, neonates with severe NAIT); and second, because necessary information about confounders was not uniformly reported in the previously published studies.7,8,21-31,33,34
Differences in diagnostic procedures between the present study and the other prospective studies could potentially have biased our results. The 2 most important diagnostic procedures were counting of platelets and ultrasonographic examination of the newborn's brain. Since both methods are considered standard procedures, we believe it is unlikely that differences in these procedures between studies have biased the comparison.
In our screening program, some HPA 1a–negative women were not anti–HPA 1a typed for reasons we do not know, but we can suggest at least 4 possible explanations: (1) the general practitioner (GP) did not inform the woman about the screening and intervention program, (2) the woman did not want to follow the screening and intervention program, (3) the woman may have moved to another health region not covered by the screening and intervention program, and (4) the woman was lost from further follow-up for other reasons. Although it would have been preferable to have this information, we find it unlikely that the HPA 1a–negative women who were not examined for anti–HPA 1a have significantly biased our results, as they represent less than 6% of all HPA 1a–negative women.
The 6 prospective studies24,25,28,29,31,32 in which platelet counts were obtained from the neonates immediately after delivery may reflect the natural history of NAIT better than the other prospective studies7,8,21-23,26,27,33,34 in which pregnant women were HPA 1a typed, because different interventions were employed in many of the latter studies. A comparison with the first group may therefore be more relevant in order to examine whether implementation of our screening and intervention program reduces the number of serious complications to severe NAIT. The results indicate that our screening and intervention strategy may reduce the risk of serious complications to severe NAIT to approximately one fourth compared with a situation without any screening.
It can be argued that the 6 prospective studies assessing platelet counts immediately after delivery24,25,28,29,31,32 do not reflect the natural history of NAIT but may have underestimated the number of serious complications related to severe NAIT. In these studies, the number of neonates with serious complications in the postnatal period may have been reduced because platelet counts were obtained in all neonates, and severely thrombocytopenic newborns would most probably have been transfused with platelets sooner than if no platelet counts of the newborns had been obtained. Moreover, the number of cases with NAIT-related intrauterine death may also have been underestimated if some cases of stillbirth had not been subjected to autopsy. Therefore, if these studies have underestimated the number of serious complications related to severe NAIT, our screening and intervention program would possibly result in an even greater reduction in complications.
We have used a rather conservative approach for intervention in immunized women: CS a few weeks prior to term with compatible platelets to be transfused immediately in severely thrombocytopenic neonates. The rationale for our approach was 3-fold. First, we assumed that vaginal delivery in many cases will cause more trauma to the neonate compared with a planned CS carried out cautiously. Although it has never been documented in a randomized controlled trial (RCT) that CS is less traumatic for neonates in NAIT compared with vaginal delivery, it has been reported that trauma during vaginal delivery is a frequent cause of ICH in hemophilic neonates.10 In addition, vaginal delivery is not recommended in ITP if fetal platelet count is very low.11 Therefore, it is reasonable to believe that a prolonged and complicated operative vaginal delivery may also impose a considerable risk for serious fetal bleedings in severe NAIT. Second, by shortening the pregnancy, the fetus will be exposed to the harmful antibodies for a shorter period of time, which may reduce the risk of serious bleedings. Third, elective CS allows the blood bank to prepare compatible platelet concentrate for the neonate.
Many of the previously published prospective studies8,26-29,34 have used a more proactive strategy with FBS and intrauterine transfusions. We decided not to use such a strategy because of the increased risk of fetal death associated with FBS in pregnancies complicated by fetal thrombocytopenia.35 It is also noteworthy that serious complications of FBS were reported in 4 of the 6 prospective studies that used this procedure as part of their strategy.8,27-29 Our more conservative approach using CS a few weeks prior to term seemed to be associated with less NAIT-associated complications than more proactive strategies.
According to our results, which are based on an unselected population of immunized women, the obstetric history does not seem useful for prediction of the risk of severe thrombocytopenia in subsequent pregnancies (Table 3). First, there was no significant difference in the number of neonates with severe thrombocytopenia born of women with a previous history of NAIT compared with women without such a history. Second, there was no significant difference in the number of neonates with severe thrombocytopenia born of primigravida compared with multigravida. In this context, it should be mentioned that the women who gave birth to the 2 neonates with ICH in our study were gravida 2, para 0 and primigravida, respectively. These results are in accordance with another large prospective study from the United Kingdom (Williamson et al8 ), where 4 of 10 women giving birth to severely thrombocytopenic children were primigravida and where severe NAIT-related complications were observed in 2 of the primigravida (1 case of ICH and 1 case of intrauterine death).
In concordance with previous studies8,36 we found that the majority of the immunized women were HLA DRB3*0101 positive. Of the 12 women who did not carry this HLA type, all but2 gave birth to children with normal platelet counts. As these 2 thrombocytopenic neonates had only moderately reduced platelet counts, it seems as HLA DRB3*0101 typing can be used as a tool for identifying risk of severe NAIT.
In a large-scale screening program, the benefits should always be balanced with potentially adverse effects of the program. Thirty-seven neonates suffered from adverse effects associated with the intervention program and required treatment at the neonatal intensive care unit. As this was clearly related to the time of delivery, it is likely that the number of neonates with adverse effects would have been smaller if all neonates had been delivered later than week 37. A consequence of postponed delivery, however, would be prolonged fetal exposure to antiplatelet antibodies and this may have increased the number of newborns with serious complications to severe NAIT. Although adverse effects of early delivery are undesirable, we would like to emphasize that none of the neonates suffered any sequelae.
Maternal complications to elective CS are not very frequent and should be compared with complications related to planned vaginal delivery.37 However, there are increased costs associated with elective CS compared with planned vaginal delivery and we have calculated the average costs per CS, including possible additional costs associated with treatment of neonates with pulmonary maladaption, to be approximately US $4800.38 This calculation is part of a comprehensive cost-effectiveness analysis where we show that the screening and intervention program applied to a population of 100 000 pregnant women would save 210 to 230 quality-adjusted life years and, at the same time, reduce health care costs by approximately US $2.2 million.38
An important weakness of the present study is the absence of a control group consisting of planned vaginal deliveries. If we had conducted an RCT, the GPs should have been willing and able to explain to the pregnant women the implications of being included in the trial and the consequences of being randomized to one or the other study arm. Since we intended to include approximately 100 000 women, we expected that a little more than 2000 would be HPA 1a negative. As the population basis was around 2.8 million, each GP would in fact recruit very few or no women to the study during the study period. Hence, we regarded it as unrealistic to expect that more than 1000 GPs, dispersed over half of Norway, would recruit more than 2000 patients to an RCT. More importantly, however, we found it ethically difficult to carry out an RCT. This would imply that some women with very high anti–HPA 1a levels had to deliver vaginally and we considered that there would be a considerable risk of ICH in the respective neonates, particularly in prolonged and complicated deliveries. In a large prospective study by Williamson et al,8 36% of the immunized women delivered by CS. This percentage was surprisingly high considering that only 15% of an unselected population in the same geographic area would deliver by CS. Although their study8 was only observationally aimed at describing the natural history of NAIT, it is possible that the obstetricians in pregnancies with high antibody levels, or a previous history of NAIT, decided to carry out a CS on less-stringent indications than if the woman had not been immunized nor had any history of NAIT. Moreover, if we had conducted a controlled trial, the women randomized to the arm with no treatment could easily be worried by knowing that they were immunized without any intervention being instituted. At many obstetric centers, women have a large influence on their mode of delivery and some centers accept to do CS solely based on maternal request. Accordingly, we do not consider CS as a disproportionate intervention for immunized women.
Acknowledging the limitation of comparing with historic controls, our data suggest that a program consisting of HPA 1a typing and screening for anti–HPA 1a antibodies in HPA 1a–negative women, together with a close clinical follow-up in the last trimester of immunized women and timed delivery by CS 2 to 4 weeks prior to term with compatible platelets available for the infant, may reduce mortality and serious morbidity associated with severe NAIT.
Acknowledgments
We are grateful to Dr John Torgils Vaage (Institute of Immunology, Rikshospitalet-Radiumhospitalet Medical Center) for help with the HLA-typing, to Prof Egil Arnesen (Institute of Community Medicine, University of Tromsø) for the statistical help in planning the study, and to Drs Leif Svenningsen and Håkon Wergeland for fruitful discussions during planning of the study and collection of data at Department of Obstetrics and Gynecology, Ullevål University Hospital. Helene Pedersen is acknowledged for her contribution to the acquisition of data from the northern part of Norway. We also want to thank Prof Leiv Sandvik (Institute for Oral Biology, University of Oslo) for his sound statistical advice during the last phase of preparation of the manuscript. Many thanks for the tremendous work several staff members have carried out at the Departments of Immunology and Transfusion Medicine at Ullevål University Hospital and University Hospital of North Norway, and at the Departments of Obstetrics and Gynecology, and Pediatrics at Ullevål University Hospital, University Hospital of North Norway and Rikshospitalet-Radiumhospitalet Medical Center. Finally, special thanks for the enthusiasm and support provided by the general practitioners, the midwives, and the staff at the blood banks in the regions where the screening and intervention program was implemented.
This work was supported by grants from the Norwegian Government (the Ministry of Health and Care Services).
Authorship
Contribution: J.K.-K. was involved in planning the study and was responsible for organizing the study in the southern part of Norway, the analysis and interpretation of data, and drafting and writing of the manuscript. A.H., B.S., and M.K.K. participated in the planning of the study; the acquisition, analysis, and interpretation of data; and drafting and writing of the manuscript. E.G. and G.T. automated the platelet typing, organized the work flow in the laboratory analyzing the samples from the southern part of Norway, and critically reviewed the paper. I.R. and R.H. established the method for HLA DRB3 sequencing and critically reviewed the manuscript. B.A., P.Ø., and L.B.D. participated in discussions of the study design, acquisition of data from the northern part of Norway, and critical revision of the manuscript. J.P., R.L., H.H., G.H., and M.G. participated in discussions of the study design, acquisition of data from the southern part of Norway, and critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jens Kjeldsen-Kragh, Department of Immunology and Transfusion Medicine, Ullevål University Hospital, 0407 Oslo, Norway; e-mail: jens.kjeldsen-kragh@medisin.uio.no.