Abstract
Serum-free light chain (SFLC) levels are useful for diagnosing nonsecretory myeloma and monitoring response in light-chain–only disease, especially in the presence of renal failure. As part of a tandem autotransplantation trial for newly diagnosed multiple myeloma, SFLC levels were measured at baseline, within 7 days of starting the first cycle, and before both the second induction cycle and the first transplantation. SFLC baseline levels higher than 75 mg/dL (top tertile) identified 33% of 301 patients with higher near-complete response rate (n-CR) to induction therapy (37% vs 20%, P = .002) yet inferior 24-month overall survival (OS: 76% vs 91%, P < .001) and event-free survival (EFS: 73% vs 90%, P < .001), retaining independent prognostic significance for both EFS (HR = 2.40, P = .008) and OS (HR = 2.43, P = .016). Baseline SFLC higher than 75 mg/dL was associated with light-chain–only secretion (P < .001), creatinine level 176.8 μM (2 mg/dL) or higher (P < .001), beta-2-microglobulin 297.5 nM/L (3.5 mg/L) or higher (P < .001), lactate dehydrogenase 190 U/L or higher (P < .001), and bone marrow plasmacytosis higher than 30% (P = .003). Additional independent adverse implications were conferred by top-tertile SFLC reductions before cycle 2 (OS: HR = 2.97, P = .003; EFS: HR = 2.56, P = .003) and before transplantation (OS: HR = 3.31, P = .001; EFS: HR = 2.65, P = .003). Unlike baseline and follow-up analyses of serum and urine M-proteins, high SFLC levels at baseline—reflecting more aggressive disease—and steeper reductions after therapy identified patients with inferior survival.
Introduction
Multiple myeloma (MM) is a prototypic monoclonal B-cell malignancy with a terminally differentiated plasma cell phenotype and monoclonal immunoglobulin secretion in the majority of cases.1 Approximately 30% secrete only light chains instead of complete immunoglobulin molecules comprising 2 heavy and 2 light chains that are assembled in the endoplasmic reticulum and secreted via the Golgi apparatus2 ; another 5% of patients have “nonsecretory” MM, although monoclonal plasma cells can be identified in the bone marrow or other sites with anti–light chain antibodies; very few patients have “nonproducing” MM, meaning absence of monoclonal cytoplasmic immunoglobulins but typical plasma cell morphologic, phenotypic, and gene expression features.3 Serial assessments of monoclonal protein levels in serum and daily urinary excretion have been used to monitor disease progression and response to therapy. In cases of light-chain–only–secreting MM with renal failure and in nonsecretory disease, such assays have not been helpful. The recently developed serum-free light chain (SFLC) assay has proved invaluable in case of light-chain-secreting MM with renal failure and in a proportion of patients otherwise deemed to have nonsecretory disease.4 SFLC levels have also been useful to predict progression from monoclonal gammopathy of undetermined significance (MGUS) to MM.5
In patients in whom both intact immunoglobulin and light chain are secreted, it is assumed that they originate in different MM plasma cell clones with different levels of differentiation.6 In fact, in the course of MM progression especially with multiple relapses, dedifferentiation is frequently observed, characterized by “Bence Jones escape,” cessation of M-protein secretion, or “high-grade transformation” (best captured by increased levels of serum lactate dehydrogenase [LDH]).7,8 The elusive MM stem cell is thought to have a pre–plasma cell phenotype incapable of immunoglobulin secretion,9 although others have shown that the terminally differentiated plasma cell retained self-renewal potential,10 consistent with the recent notion of stem cell plasticity.11 It is generally accepted that the aforementioned dedifferentiated phenotype encountered at advanced disease stages may represent an overgrowth of such cells initially present in a small MM subpopulation; alternatively, such cells may represent truly transformed cells developing under replicative stress in response to treatment, analogous to large cell transformation in non-Hodgkin lymphoma.
As the armamentarium of therapies for MM has been greatly expanded, increasing emphasis is placed on avoiding ineffective treatment modalities or, conversely, documenting that each cycle of treatment is maximally effective. The long half-lives of complete immunoglobulins have prevented their serial use for the early detection of sensitivity or resistance to therapy. In cases with both complete and light-chain–only immunoglobulin secretion, the shorter half-life of the latter lends itself to earlier recognition of treatment response. In this study, we examined (1) whether elevated baseline SFLC levels characterize a less differentiated and more aggressive MM subtype with negative consequences for survival and (2) whether serial SFLC analyses can distinguish early therapeutic response or resistance.
Patients, materials, and methods
The studies were approved by the institutional review board of the University of Arkansas for Medical Sciences, Little Rock, AR. Informed consent was obtained in accordance with the Declaration of Helsinki.
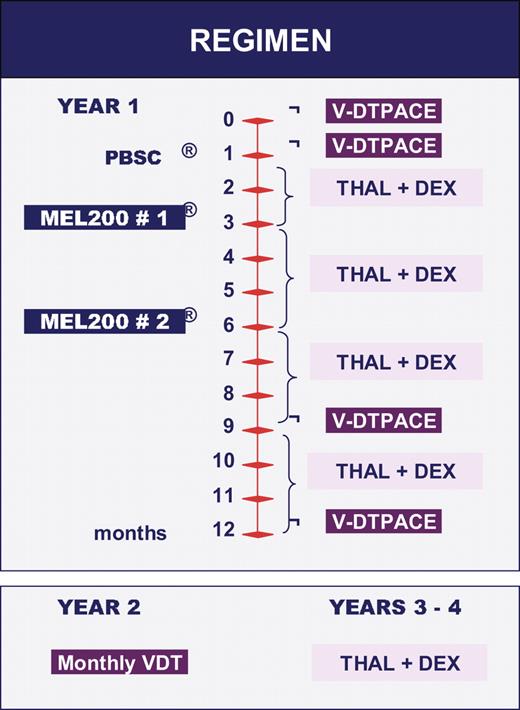
Three hundred three newly diagnosed patients were enrolled into our current tandem autotransplantation trial, Total Therapy 3 (TT3), using combination therapy with VTD-PACE (Bortezomib, Thalidomide, Dexamethasone, Cisplatin, Doxorubicin, Cytophosphamide, Etoposide) as induction before and consolidation therapy after melphalan-based high-dose therapies (Figure 1). For MM staging, M-protein measurements were performed that included serum and urine electrophereses, nephelometric analyses of serum immunoglobulin levels, and SFLC determination. In addition, serum levels were determined of beta-2-microglobulin (B2M), C-reactive protein (CRP), and lactate dehydrogenase (LDH). Bone marrow aspirate and biopsy specimens were procured to estimate the percentage infiltration by plasma cells along with analysis of DNA/cytoplasmic light chain expression by flow cytometry.3 Cytogenetic abnormalities (CAs) were detected by metaphase analysis of Giemsa-banded chromosomes in typically 20 cells.12
Such studies were performed at baseline before initiation of therapy and periodically, usually monthly, after therapy in order to determine onset of response, classified according to Bladé et al as complete response (CR) and partial response (PR)13 ; near-complete (n-CR) implied absence of monoclonal protein band on standard electropheresis of serum and urine, although immunofixation detected an M-protein band. For the purpose of some of the analyses presented here, we consider the proportion of patients achieving at least n-CR (≥ n-CR) that includes those achieving CR status. SFLC analyses were performed at least weekly during the first cycle of induction therapy, before initiation of the second cycle and before transplantation, and then monthly.
Of the 303 patients enrolled, 32 had had one cycle of prior systemic therapy as permitted by protocol eligibility criteria. Ninety-two percent had follow-up SFLC data around day 7 (range, days 5-9) of the first cycle; 97%, before the initiation of the second cycle (median, 3 days; range, 1 to 18 days); and 94%, before first transplantation (median, 5 days; range, 1 to 20 days).
Results are reported as of February 2007, with a median follow-up of 21 months (range, 5.1 months to 35.6 months). Event-free survival (EFS) and overall survival (OS) were both counted from initiation of therapy; events for the former were progressions, relapses, and deaths of any cause and, for the latter, deaths of any cause. The Kaplan-Meier method14 was used to estimate EFS and OS, with comparisons using the log-rank test. Tertiles were determined on values of the involved (kappa or lambda) SFLC level at baseline, or percentagereduction at selected time points (day 7 of the first cycle, before cycle 2, and before transplantation). We also applied methodology reported by Dispenzieri et al for patients with AL amyloidosis, evaluating baseline SFLC levels as a continuous variable or dichotomized by median pretreatment values, as well as normalization of SFLC levels and kappa-to-lambda SFLC ratio at baseline and following initiation of treatment.15 A similar approach was used in the evaluation of baseline and posttreatment standard serum and urine M-protein values. Multivariate models (MVs) of prognostic factors were examined using logistic and Cox regression analyses.16 The chi-square test was used to compare baseline patient characteristics. The dichotomized standard prognostic factors used in these analyses were those reported as part of the International Staging System (ISS) for MM.17
Results
Inferior event-free and overall survival with baseline SFLC levels of 75 mg/dL or higher
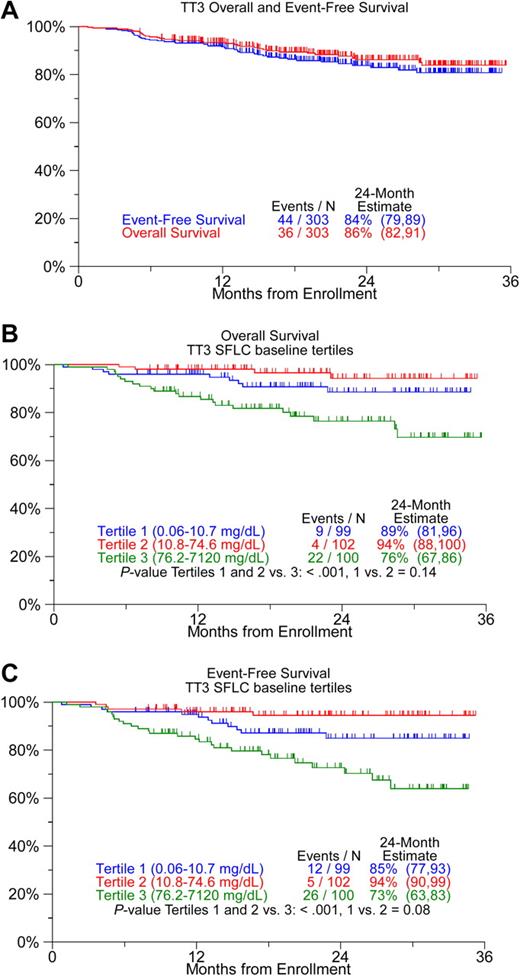
Two years after initiation of treatment, 83% of 303 patients enrolled in TT3 remain event free and 86% are alive (Figure 2A). Baseline SFLC levels were available for 301 patients and ranged from 0.06 to 7120 mg/dL (median, 31.6 mg/dL). Baseline SFLC levels were not lower among patients with prior treatment than among those without prior therapy (mean, 219 mg/dL vs161 mg/dL, P = .43), justifying inclusion of both groups in the analyses. Baseline levels of SFLC were significantly associated with OS when examined as log-transformed (P = .005) and as dichotomized variables (based on median values, P = .011) and also with EFS (P = .002 and P = .007, respectively). Top-tertile SFLC levels higher than 75 mg/dL were associated with inferior OS and EFS (Figure 2B,C) and were stronger predictors of outcome than the other SFLC measures listed above. Baseline concentrations of standard serum and urine M-protein did not identify prognostic subgroups (data not shown).
Kaplan-Meier survival plots. (A) All patients, and (B,C) according to tertiles of baseline levels of serum-free light chain (SFLC). Overall survival and event-free survival were inferior among patients with top-tertile SFLC baseline levels.
Kaplan-Meier survival plots. (A) All patients, and (B,C) according to tertiles of baseline levels of serum-free light chain (SFLC). Overall survival and event-free survival were inferior among patients with top-tertile SFLC baseline levels.
Top-tertile baseline SFLC levels higher than 75 mg/dL are associated with more aggressive disease features
In light of the prognostic implications of baseline SFLC levels, we examined whether associations existed with traditional variables known to affect clinical outcome. Higher proportions of patients with baseline SFLC higher than 75 mg/dL were observed in case of ISS stage III, with elevations of B2M, LDH, and creatinine, bone marrow plasmacytosis higher than 30%; and in case of light-chain-only MM, a strong trend existed for the association of high SFLC levels with the presence of CA (Table 1).
Comparison of patient characteristics according to baseline SFLC levels
| Factor . | SFLC of 75 mg/dL or less (%) . | SFLC higher than 75 mg/dL (%) . | P . |
|---|---|---|---|
| IgG isotype | 135/200 (68) | 37/97 (38) | < .001 |
| Light chain only | 15/200 (8) | 34/97 (35) | < .001 |
| B2M of 3.5 mg/L or higher | 68/201 (34) | 65/99 (66) | < .001 |
| BMPC higher than 30% | 93/174 (53) | 66/91 (73) | .003 |
| Creatinine of 2.0 mg/dL or higher | 2/201 (1) | 20/100 (20) | < .001 |
| LDH of 190 U/L or higher | 39/201 (19) | 41/100 (41) | < .001 |
| ISS stage 1 | 113/201 (56) | 27/99 (27) | < .001* |
| ISS stage 2 | 68/201 (34) | 29/99 (29) | < .001* |
| ISS stage 3 | 20/201 (10) | 43/99 (43) | < .001* |
| CAs | 58/200 (29) | 40/100 (40) | .055 |
| Factor . | SFLC of 75 mg/dL or less (%) . | SFLC higher than 75 mg/dL (%) . | P . |
|---|---|---|---|
| IgG isotype | 135/200 (68) | 37/97 (38) | < .001 |
| Light chain only | 15/200 (8) | 34/97 (35) | < .001 |
| B2M of 3.5 mg/L or higher | 68/201 (34) | 65/99 (66) | < .001 |
| BMPC higher than 30% | 93/174 (53) | 66/91 (73) | .003 |
| Creatinine of 2.0 mg/dL or higher | 2/201 (1) | 20/100 (20) | < .001 |
| LDH of 190 U/L or higher | 39/201 (19) | 41/100 (41) | < .001 |
| ISS stage 1 | 113/201 (56) | 27/99 (27) | < .001* |
| ISS stage 2 | 68/201 (34) | 29/99 (29) | < .001* |
| ISS stage 3 | 20/201 (10) | 43/99 (43) | < .001* |
| CAs | 58/200 (29) | 40/100 (40) | .055 |
n/N (%): n indicates number with factor; N, number with valid data for factor.
Chi-square P for trend.
Inferior outcome in patients with top-tertile decline in SFLC levels before cycle 2 and before transplantation
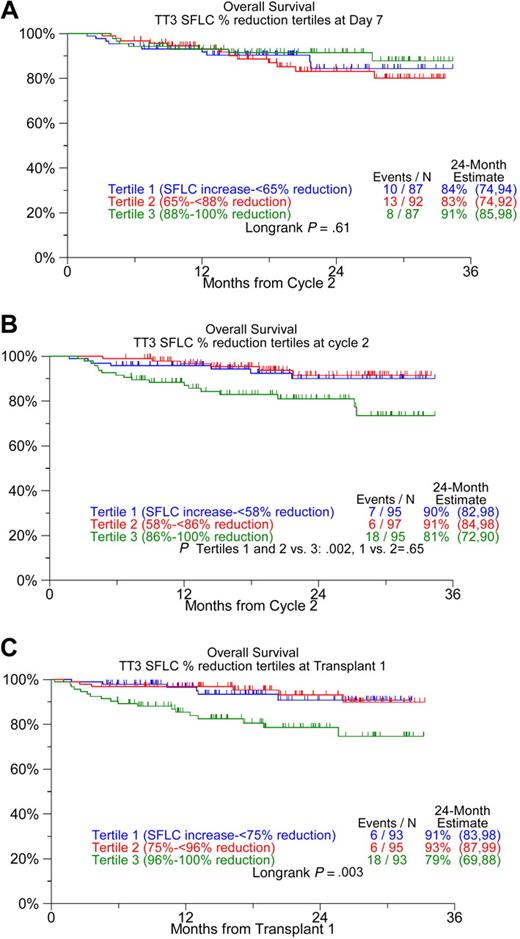
Serial SFLC measurements were available in 92% of patients for day 7 (range, days 5-9) of the first course, in 94% immediately before the second cycle of induction therapy, and in 94% before first transplantation. When examined according to SFLC percentage reduction tertiles for the 3 follow-up time points, OS was inferior among the top-tertile SFLC percentage reduction groups before cycle 2 and before transplantation; thus, 24-month survival estimates were 81% and 79% among the top-tertile SFLC reduction group as opposed to 91% and 92% for the remainder (Figure 3). Similar findings pertained to EFS (data not shown). No such survival implications were noted for tertile reductions in serum and urine M-protein values (data not shown).
Kaplan-Meier plots of overall survival from landmarks of SFLC reduction. (A) By day 7 of the first induction cycle, (B) before cycle 2, and (C) before transplantation. Top-tertile SFLC reductions are associated with inferior survival when considered before cycle 2 and before transplantation, unadjusted for SFLC baseline values.
Kaplan-Meier plots of overall survival from landmarks of SFLC reduction. (A) By day 7 of the first induction cycle, (B) before cycle 2, and (C) before transplantation. Top-tertile SFLC reductions are associated with inferior survival when considered before cycle 2 and before transplantation, unadjusted for SFLC baseline values.
Univariate and multivariate analyses of features predicting event-free and overall survival
Univariately significant baseline factors associated with inferior EFS and OS included advanced age of 65 years or older, presence of CA, advanced ISS stage, as well as serum elevations of B2M, CRP, LDH, creatinine, and SFLC (Table 2). Among these baseline variables, CA and high levels of LDH and SFLC retained independent prognostic significance for both EFS and OS (Table 3). Among follow-up parameters considered (Table 2), top-tertile SFLC reduction at cycle 2 and before transplantation both were also significant independent adverse parameters for EFS or OS (Tables 4–5), after adjustment for LDH and CA (Tables 4-5). Serum and urine M-protein baseline levels or their posttreatment reduction failed to influence clinical outcome.
Univariate analysis of baseline and follow-up parameters associated with event-free and overall survival
| Univariate . | Overall survival . | Event-free survival . | |||
|---|---|---|---|---|---|
| Variable . | n/N (%) . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Female | 109/301 (36) | 1.39 (0.71-2.71) | .340 | 1.22 (0.66-2.24) | .529 |
| Age, 65 y or older | 83/301 (28) | 1.86 (0.94-3.66) | .072 | 1.87 (1.02-3.45) | .044 |
| IgA isotype | 72/297 (24) | 1.47 (0.70-3.08) | .303 | 1.09 (0.53-2.21) | .816 |
| IgG isotype | 172/297 (58) | 0.88 (0.45-1.73) | .708 | 1.16 (0.62-2.16) | .646 |
| Light chain only | 49/297 (16) | 0.63 (0.22-1.79) | .387 | 0.62 (0.25-1.59) | .324 |
| Bone marrow plasma cells higher than 30% | 161/267 (60) | 1.09 (0.54-2.20) | .802 | 1.38 (0.72-2.63) | .328 |
| Albumin lower than 3.5 g/dL | 75/301 (25) | 1.93 (0.96-3.89) | .067 | 1.80 (0.95-3.41) | .073 |
| Hb lower than 10 g/dL | 92/301 (31) | 2.01 (1.03-3.91) | .040 | 2.11 (1.16-3.85) | .015 |
| Creatinine of 2.0 mg/dL or higher | 22/301 (7) | 3.23 (1.41-7.39) | .006 | 3.01 (1.39-6.48) | .005 |
| LDH of 190 U/L or higher | 80/301 (27) | 4.07 (2.09-7.94) | < .001 | 3.22 (1.77-5.87) | < .001 |
| B2M of 3.5 mg/L or higher | 133/300 (44) | 2.54 (1.25-5.13) | .010 | 2.48 (1.32-4.67) | .005 |
| CRP 8 mg/L or higher | 99/300 (33) | 2.07 (1.06-4.01) | .032 | 2.11 (1.16-3.84) | .014 |
| ISS stage 2, reference stage 1 | 97/300 (32) | 0.51 (0.21-1.23) | < .001 | 0.74 (0.36-1.52) | < .001 |
| ISS stage 3, reference stage 1 | 63/300 (21) | 4.11 (2.09-8.05) | < .001 | 3.40 (1.85-6.24) | < .001 |
| CAs | 98/300 (33) | 3.44 (1.75-6.78) | < .001 | 2.96 (1.62-5.41) | < .001 |
| Baseline K/L ratio abnormal | 279/301 (93) | 1.44 (0.35-6.02) | .614 | 1.82 (0.44-7.52) | .409 |
| Baseline SFLC higher than 75 mg/dL, top tertile | 100/301 (33) | 3.66 (1.85-7.28) | < .001 | 3.34 (1.81-6.16) | < .001 |
| Top-tertile % SFLC reduction, day 7* | N = 266 | 0.87 (0.56-1.35) | .532 | 0.98 (0.66-1.45) | .915 |
| Baseline SFLC more than 75 mg/dL, top-tertile % SFLC reduction, day 7* | N = 90 | 0.75 (0.43-1.30) | .307 | 0.85 (0.52-1.39) | .508 |
| Top-tertile % SFLC reduction, before cycle 2* | 95/287 (33) | 2.97 (1.45-6.06) | .003 | 2.56 (1.36-4.81) | .003 |
| Baseline SFLC higher than 75 mg/dL, top-tertile % SFLC reduction, before cycle 2* | 31/94 (33) | 2.20 (0.91-5.29) | .079 | 1.94 (0.87-4.34) | .106 |
| Top-tertile % SFLC reduction, before transplantation† | 92/280 (33) | 3.31 (1.59-6.88) | .001 | 2.65 (1.39-5.05) | .003 |
| Baseline SFLC higher than 75 mg/dL, top-tertile % SFLC reduction, before transplantation† | 30/90 (33) | 1.80 (0.73-4.43) | .204 | 1.27 (0.54-2.99) | .580 |
| Serum-M, with serum M of 1 g/dL or higher | N = 210 | 0.95 (0.73-1.24) | .698 | 1.01 (0.81-1.27) | .908 |
| Serum-M higher than median, with serum-M of 1 g/dL or higher | 103/210 (49) | 0.77 (0.33-1.84) | .560 | 0.95 (0.45-2.03) | .899 |
| Top-tertile % serum-M reduction, day 7* | N = 170 | 1.09 (0.58-2.06) | .787 | 1.27 (0.74-2.20) | .390 |
| Top-tertile % serum-M reduction, before cycle 2* | N = 196 | 0.88 (0.50-1.56) | .666 | 0.90 (0.55-1.48) | .690 |
| Top-tertile % serum-M reduction, before transplantation† | N = 198 | 1.28 (0.73-2.27) | .388 | 1.22 (0.75-2.01) | .420 |
| Urine-M, with urine M of 200 mg or higher/24 h | N = 117 | 1.01 (1.00-1.02) | .046 | 1.01 (1.00-1.01) | .227 |
| Urine-M higher than median, with urine-M of 200 mg/24 h or higher | 58/117 (50) | 2.30 (0.80-6.64) | .122 | 1.34 (0.56-3.20) | .503 |
| Top-tertile % urine-M reduction, day 7* | N = 68 | 0.54 (0.18-1.59) | .260 | 0.83 (0.39-1.75) | .627 |
| Top-tertile % urine-M reduction, before cycle 2* | N = 103 | 1.06 (0.33-3.45) | .923 | 1.29 (0.46-3.62) | .628 |
| Top-tertile % urine-M reduction, before transplantation† | N = 108 | 0.70 (0.23-2.16) | .540 | 0.85 (0.32-2.29) | .755 |
| Univariate . | Overall survival . | Event-free survival . | |||
|---|---|---|---|---|---|
| Variable . | n/N (%) . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Female | 109/301 (36) | 1.39 (0.71-2.71) | .340 | 1.22 (0.66-2.24) | .529 |
| Age, 65 y or older | 83/301 (28) | 1.86 (0.94-3.66) | .072 | 1.87 (1.02-3.45) | .044 |
| IgA isotype | 72/297 (24) | 1.47 (0.70-3.08) | .303 | 1.09 (0.53-2.21) | .816 |
| IgG isotype | 172/297 (58) | 0.88 (0.45-1.73) | .708 | 1.16 (0.62-2.16) | .646 |
| Light chain only | 49/297 (16) | 0.63 (0.22-1.79) | .387 | 0.62 (0.25-1.59) | .324 |
| Bone marrow plasma cells higher than 30% | 161/267 (60) | 1.09 (0.54-2.20) | .802 | 1.38 (0.72-2.63) | .328 |
| Albumin lower than 3.5 g/dL | 75/301 (25) | 1.93 (0.96-3.89) | .067 | 1.80 (0.95-3.41) | .073 |
| Hb lower than 10 g/dL | 92/301 (31) | 2.01 (1.03-3.91) | .040 | 2.11 (1.16-3.85) | .015 |
| Creatinine of 2.0 mg/dL or higher | 22/301 (7) | 3.23 (1.41-7.39) | .006 | 3.01 (1.39-6.48) | .005 |
| LDH of 190 U/L or higher | 80/301 (27) | 4.07 (2.09-7.94) | < .001 | 3.22 (1.77-5.87) | < .001 |
| B2M of 3.5 mg/L or higher | 133/300 (44) | 2.54 (1.25-5.13) | .010 | 2.48 (1.32-4.67) | .005 |
| CRP 8 mg/L or higher | 99/300 (33) | 2.07 (1.06-4.01) | .032 | 2.11 (1.16-3.84) | .014 |
| ISS stage 2, reference stage 1 | 97/300 (32) | 0.51 (0.21-1.23) | < .001 | 0.74 (0.36-1.52) | < .001 |
| ISS stage 3, reference stage 1 | 63/300 (21) | 4.11 (2.09-8.05) | < .001 | 3.40 (1.85-6.24) | < .001 |
| CAs | 98/300 (33) | 3.44 (1.75-6.78) | < .001 | 2.96 (1.62-5.41) | < .001 |
| Baseline K/L ratio abnormal | 279/301 (93) | 1.44 (0.35-6.02) | .614 | 1.82 (0.44-7.52) | .409 |
| Baseline SFLC higher than 75 mg/dL, top tertile | 100/301 (33) | 3.66 (1.85-7.28) | < .001 | 3.34 (1.81-6.16) | < .001 |
| Top-tertile % SFLC reduction, day 7* | N = 266 | 0.87 (0.56-1.35) | .532 | 0.98 (0.66-1.45) | .915 |
| Baseline SFLC more than 75 mg/dL, top-tertile % SFLC reduction, day 7* | N = 90 | 0.75 (0.43-1.30) | .307 | 0.85 (0.52-1.39) | .508 |
| Top-tertile % SFLC reduction, before cycle 2* | 95/287 (33) | 2.97 (1.45-6.06) | .003 | 2.56 (1.36-4.81) | .003 |
| Baseline SFLC higher than 75 mg/dL, top-tertile % SFLC reduction, before cycle 2* | 31/94 (33) | 2.20 (0.91-5.29) | .079 | 1.94 (0.87-4.34) | .106 |
| Top-tertile % SFLC reduction, before transplantation† | 92/280 (33) | 3.31 (1.59-6.88) | .001 | 2.65 (1.39-5.05) | .003 |
| Baseline SFLC higher than 75 mg/dL, top-tertile % SFLC reduction, before transplantation† | 30/90 (33) | 1.80 (0.73-4.43) | .204 | 1.27 (0.54-2.99) | .580 |
| Serum-M, with serum M of 1 g/dL or higher | N = 210 | 0.95 (0.73-1.24) | .698 | 1.01 (0.81-1.27) | .908 |
| Serum-M higher than median, with serum-M of 1 g/dL or higher | 103/210 (49) | 0.77 (0.33-1.84) | .560 | 0.95 (0.45-2.03) | .899 |
| Top-tertile % serum-M reduction, day 7* | N = 170 | 1.09 (0.58-2.06) | .787 | 1.27 (0.74-2.20) | .390 |
| Top-tertile % serum-M reduction, before cycle 2* | N = 196 | 0.88 (0.50-1.56) | .666 | 0.90 (0.55-1.48) | .690 |
| Top-tertile % serum-M reduction, before transplantation† | N = 198 | 1.28 (0.73-2.27) | .388 | 1.22 (0.75-2.01) | .420 |
| Urine-M, with urine M of 200 mg or higher/24 h | N = 117 | 1.01 (1.00-1.02) | .046 | 1.01 (1.00-1.01) | .227 |
| Urine-M higher than median, with urine-M of 200 mg/24 h or higher | 58/117 (50) | 2.30 (0.80-6.64) | .122 | 1.34 (0.56-3.20) | .503 |
| Top-tertile % urine-M reduction, day 7* | N = 68 | 0.54 (0.18-1.59) | .260 | 0.83 (0.39-1.75) | .627 |
| Top-tertile % urine-M reduction, before cycle 2* | N = 103 | 1.06 (0.33-3.45) | .923 | 1.29 (0.46-3.62) | .628 |
| Top-tertile % urine-M reduction, before transplantation† | N = 108 | 0.70 (0.23-2.16) | .540 | 0.85 (0.32-2.29) | .755 |
P value from Wald chi-square test in Cox regression.
HR indicates hazard ratio; 95% CI, 95% confidence interval.
Overall and event-free survival calculated from induction cycle 2 date.
Overall and event-free survival calculated from transplantation 1 date.
Multivariate analysis of baseline parameters associated with event-free and overall survival
| Variable . | n/N (%) . | HR (95% CI) . | P . |
|---|---|---|---|
| Overall survival | |||
| Baseline SFLC higher than 75 mg/dL, top tertile | 100/300 (33) | 2.43 (1.18-5.01) | .016 |
| LDH of 190 U/L or higher | 79/300 (26) | 2.59 (1.27-5.28) | .009 |
| CAs | 98/300 (33) | 2.43 (1.21-4.89) | .013 |
| Event-free survival | |||
| Baseline SFLC higher than 75 mg/dL, top tertile | 100/300 (33) | 2.40 (1.26-4.57) | .008 |
| LDH of 190 U/L or higher | 79/300 (26) | 2.10 (1.11-3.99) | .023 |
| CAs | 98/300 (33) | 2.21 (1.19-4.13) | .012 |
| Variable . | n/N (%) . | HR (95% CI) . | P . |
|---|---|---|---|
| Overall survival | |||
| Baseline SFLC higher than 75 mg/dL, top tertile | 100/300 (33) | 2.43 (1.18-5.01) | .016 |
| LDH of 190 U/L or higher | 79/300 (26) | 2.59 (1.27-5.28) | .009 |
| CAs | 98/300 (33) | 2.43 (1.21-4.89) | .013 |
| Event-free survival | |||
| Baseline SFLC higher than 75 mg/dL, top tertile | 100/300 (33) | 2.40 (1.26-4.57) | .008 |
| LDH of 190 U/L or higher | 79/300 (26) | 2.10 (1.11-3.99) | .023 |
| CAs | 98/300 (33) | 2.21 (1.19-4.13) | .012 |
P value from Wald chi-square test in Cox regression analysis; multivariate model uses stepwise selection with entry level of P ≤ .1; variable remains in case of P ≤ .05.
HR indicates hazard ratio; 95% CI, 95% confidence interval.
Multivariate analysis of baseline parameters and before cycle 2 percentage SFLC reduction associated with event-free and overall survival
| Variable . | n/N (%) . | HR (95% CI) . | P . |
|---|---|---|---|
| Overall survival from cycle 2 | |||
| Top-tertile % SFLC reduction, before cycle 2 | 95/286 (33) | 2.15 (1.03-4.47) | .041 |
| LDH of 190 U/L or higher | 74/286 (26) | 2.96 (1.41-6.22) | .004 |
| CAs | 94/286 (33) | 3.01 (1.40-6.45) | .005 |
| Event-free survival from cycle 2 | |||
| Top-tertile % SFLC reduction, before cycle 2 | 95/286 (33) | 1.96 (1.03-3.74) | .041 |
| LDH of 190 U/L or higher | 74/286 (26) | 2.38 (1.23-4.60) | .010 |
| CAs | 94/286 (33) | 2.62 (1.35-5.07) | .004 |
| Variable . | n/N (%) . | HR (95% CI) . | P . |
|---|---|---|---|
| Overall survival from cycle 2 | |||
| Top-tertile % SFLC reduction, before cycle 2 | 95/286 (33) | 2.15 (1.03-4.47) | .041 |
| LDH of 190 U/L or higher | 74/286 (26) | 2.96 (1.41-6.22) | .004 |
| CAs | 94/286 (33) | 3.01 (1.40-6.45) | .005 |
| Event-free survival from cycle 2 | |||
| Top-tertile % SFLC reduction, before cycle 2 | 95/286 (33) | 1.96 (1.03-3.74) | .041 |
| LDH of 190 U/L or higher | 74/286 (26) | 2.38 (1.23-4.60) | .010 |
| CAs | 94/286 (33) | 2.62 (1.35-5.07) | .004 |
P value from Wald chi-square test in Cox regression analysis. Multivariate model uses stepwise selection with entry level .1 and variable remains if meets the .05 level.
HR indicates hazard ratio; 95% CI, 95% confidence interval.
Multivariate analysis of baseline parameters and pretransplantation percentage SFLC reduction associated with event-free and overall survival
| Variable . | n/N (%) . | HR (95% CI) . | P . |
|---|---|---|---|
| Overall survival from transplantation 1 | |||
| Top-tertile % SFLC reduction, before transplantation | 93/279 (33) | 2.24 (1.03-4.87) | .042 |
| LDH of 190 U/L or higher | 70/279 (25) | 2.58 (1.18-5.65) | .018 |
| CAs | 92/279 (33) | 2.90 (1.34-6.27) | .007 |
| Event-free survival from transplantation 1 | |||
| Top-tertile % SFLC reduction, before transplantation | 93/279 (33) | 2.01 (1.02-3.97) | .045 |
| LDH of 190 U/L or higher | 70/279 (25) | 2.12 (1.06-4.24) | .034 |
| CAs | 92/279 (33) | 2.52 (1.30-4.91) | .007 |
| Variable . | n/N (%) . | HR (95% CI) . | P . |
|---|---|---|---|
| Overall survival from transplantation 1 | |||
| Top-tertile % SFLC reduction, before transplantation | 93/279 (33) | 2.24 (1.03-4.87) | .042 |
| LDH of 190 U/L or higher | 70/279 (25) | 2.58 (1.18-5.65) | .018 |
| CAs | 92/279 (33) | 2.90 (1.34-6.27) | .007 |
| Event-free survival from transplantation 1 | |||
| Top-tertile % SFLC reduction, before transplantation | 93/279 (33) | 2.01 (1.02-3.97) | .045 |
| LDH of 190 U/L or higher | 70/279 (25) | 2.12 (1.06-4.24) | .034 |
| CAs | 92/279 (33) | 2.52 (1.30-4.91) | .007 |
P value from Wald chi-square test in Cox regression analysis. Multivariate model uses stepwise selection with entry level .1 and variable remains if meets the .05 level.
HR indicates hazard ratio; 95% CI, 95% confidence interval.
Multivariate analyses of features associated with near-complete response (n-CR) rate before transplantation
The frequency of n-CR to induction therapy was higher (37% vs 20%, P = .002) when baseline SFLC levels exceeded 75 mg/dL. Independently significant baseline parameters associated with n-CR before first transplantation included non-IgG isotype and light-chain-only disease (Table 6). Excluding these disease-type indicators from the multivariate model, high LDH and top-tertile baseline SFLC levels were significantly associated with higher n-CR rates.
Multivariate analysis of variables associated with near-complete response
| Variable . | N . | With factor (%) . | Without factor (%) . | OR (95% CI) . | P . |
|---|---|---|---|---|---|
| Including “disease type” variables* | |||||
| Non-IgG Isotype | 297 | 63/125 (50) | 13/172 (8) | 8.90 (4.30-18.38) | < .001 |
| Light-chain only | 297 | 31/49 (63) | 45/248 (18) | 2.37 (1.13-4.95) | .022 |
| Excluding “disease type” variables* | |||||
| Baseline SFLC higher than 75 mg/dL | 301 | 37/100 (37) | 41/201 (20) | 1.99 (1.15-3.44) | .014 |
| LDH of 190 U/L or more | 301 | 31/80 (39) | 47/221 (21) | 2.00 (1.13-3.55) | .017 |
| Variable . | N . | With factor (%) . | Without factor (%) . | OR (95% CI) . | P . |
|---|---|---|---|---|---|
| Including “disease type” variables* | |||||
| Non-IgG Isotype | 297 | 63/125 (50) | 13/172 (8) | 8.90 (4.30-18.38) | < .001 |
| Light-chain only | 297 | 31/49 (63) | 45/248 (18) | 2.37 (1.13-4.95) | .022 |
| Excluding “disease type” variables* | |||||
| Baseline SFLC higher than 75 mg/dL | 301 | 37/100 (37) | 41/201 (20) | 1.99 (1.15-3.44) | .014 |
| LDH of 190 U/L or more | 301 | 31/80 (39) | 47/221 (21) | 2.00 (1.13-3.55) | .017 |
P value from Wald chi-square test in logistic regression.
Multivariate model uses stepwise selection with entry level .1 and variable remains if meets the .05 level.
Multivariate model considered the following factors for stepwise regression: female sex, age of 65 years or older, albumin less than 3.5 g/dL, LDH of 190 U/L or higher, and baseline SFLC higher than 75 mg/dL.
OR indicates odds ratio; 95% CI, 95% confidence interval.
“Disease type” variables are IgG isotype, IgA isotype, and light-chain only.
Discussion
High baseline SFLC levels were a reflection of higher tumor burden (ISS stage, B2M, bone marrow plasmacytosis), higher degree of disease aggressiveness (LDH, CA), and light-chain-only MM with its greater propensity for renal failure (Table 1). These data are in agreement with findings reported by Rajkumar et al in MGUS, portending earlier progression to MM in patients with elevated SFLC levels.5 In the case of myeloma requiring therapy, high baseline SFLC levels conferred inferior EFS and OS despite being associated with higher n-CR rate (Figure 2B,C; Table 6). Top-tertile SFLC reductions before cycle 2 and before transplantation also were independent adverse features for EFS and OS (Figure 3; Tables 4–5).
The apparent paradox of high n-CR rates and inferior survival in case of high baseline SFLC levels and high SFLC reduction rates is reminiscent of the adverse implications for survival, despite higher CR rates, of LDH elevation, and presence of CA and of immunoglobulin A isotype.18 While associated with more rapid cell kill initially, these features of aggressive high-proliferative activity MM are likely associated with rapid disease regrowth between treatment cycles and thus account for early relapse and death. We recently demonstrated, in the context of comprehensive data including gene expression profiling, that CR uniquely benefited a small subgroup of 13% with very high-risk myeloma that could be identified only by molecular genetic studies.19 The current data are also consistent with observations of favorable clinical outcome, despite slower onset and lower rates of CR, among patients with genetic features of a MGUS-like MM20 and those with a documented prior history of this benign precursor condition.21 Our data support the incorporation of SFLC assays into the work-up of newly diagnosed patients with MM at baseline and early after induction therapy to help identify those at high risk. Studies are in progress to determine whether serial SFLC analyses in remission may permit earlier detection of (dedifferentiated) relapse than is currently possible with the use of serial serum and urine M-protein examinations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by National Institutes of Health grant CA55819.
National Institutes of Health
Authorship
Contribution: F.R. and B.B. conceptualized the work and supervised studies; V.B. and J.C. analyzed data; F.R., K.H., M.P.-R., E.A., M.Z., G.T., A.M., Y.A., and B.B. enrolled patients and ensured high-quality clinical research data; J.E. and J.D.S. provided critical discussions; B.B. and F.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bart Barlogie, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, Little Rock, AR 72205; e-mail: barlogiebart@uams.edu.