Abstract
Mobilized peripheral blood hematopoietic stem cells (PBSCs) demonstrate accelerated engraftment compared with bone marrow; however, mechanisms responsible for enhanced engraftment remain unknown. PBSCs mobilized by GROβ (GROβΔ4/CXCL2Δ4) or the combination of GROβΔ4 plus granulocyte colony-stimulating factor (G-CSF) restore neutrophil and platelet recovery faster than G-CSF–mobilized PBSCs. To determine mechanisms responsible for faster hematopoietic recovery, we characterized immunophenotype and function of the GROβ-mobilized grafts. PBSCs mobilized by GROβΔ4 alone or with G-CSF contained significantly more Sca-1+-c-kit+-lineage− (SKL) cells and more primitive CD34−-SKL cells compared with cells mobilized by G-CSF and demonstrated superior competitive long-term repopulation activity, which continued to increase in secondary and tertiary recipients. GROβΔ4-mobilized SKL cells adhered better to VCAM-1+ endothelial cells compared with G-CSF–mobilized cells. GROβΔ4-mobilized PBSCs did not migrate well to the chemokine stromal derived factor (SDF)-1α in vitro that was associated with higher CD26 expression. However, GROβΔ4-mobilized SKL and c-Kit+ lineage− (KL) cells homed more efficiently to marrow in vivo, which was not affected by selective CXCR4 and CD26 antagonists. These data suggest that GROβΔ4-mobilized PBSCs are superior in reconstituting long-term hematopoiesis, which results from differential mobilization of early stem cells with enhanced homing and long-term repopulating capacity. In addition, homing and engraftment of GROβΔ4-mobilized cells is less dependent on the SDF-1α/CXCR4 axis.
Introduction
Granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood hematopoietic stem cells (PBSCs) are widely used for transplantation1,2 and restore platelet and neutrophil levels faster than bone marrow or cord blood cells.2-4 However, 10% to 30% of patients and volunteers mobilize poorly to G-CSF, resulting in collection of suboptimal numbers of PBSCs for transplantation,5,6 highlighting the need for more efficacious mobilizing regimens/agents. Single administration of the chemokines GROβ (CXCL2) or GROβΔ4 (CXCL2Δ4) rapidly mobilize hematopoietic stem and progenitor cells (HSPCs), including long-term repopulating cells (LTRC), to an equivalent degree as G-CSF when used alone and there is synergy when combined with G-CSF.7,8 Neutrophil and platelet recoveries in mice receiving GROβΔ4-mobilized PBSCs are significantly accelerated compared with mice receiving G-CSF–mobilized cells,7 suggesting that GROβΔ4-mobilized PBSCs engraft more efficiently
Differences in engraftment using HSPCs from different sources may reflect stem cell number, cell cycle status,9,10 presence of facilitating cells,11,12 or intrinsic differences in homing properties or proliferative capacity.13,14 Hematopoietic stem cells (HSCs) require an appropriate marrow niche to differentiate and self renew15,16 and only HSCs homing to marrow contribute to long-term repopulation.17,18 The SDF-1α/CXCR4 axis is involved in HSPC trafficking.13,14,19-21 SDF-1α can attract HSCs that express CXCR4 to the marrow microenvironment,14,20 and enhanced in vitro transmigration of G-CSF–mobilized CD34+ cells to SDF-1α is associated with hematopoietic recovery.22 However, blocking Gαi-coupled receptors, which includes CXCR4, does not affect homing of Sca-1+-lin−-Rhlow cells23 and marrow engraftment of CXCR4−/− Sca-1+-c-kit+-lineage− (SKL) cells is equivalent to CXCR4+/+ SKL cells,24 suggesting that the SDF-1α/CXCR4 axis is not absolutely required for HSPC homing and engraftment. Furthermore, marrow HPCs rendered unresponsive to SDF-1α home efficiently, provided that the α4-integrin/VCAM-1 pathway is intact.25 In the absence of α4-integrin or VCAM-1, blocking CXCR4 reduces homing, indicating a dominant role for the α4-integrin/VCAM-1 pathways in progenitor cell homing.
To determine mechanisms responsible for enhanced hematopoietic repopulation by GROβΔ4-mobilized HSC, we examined in vitro migration to SDF-1α, adhesion, static expression of adhesion molecules, expression of the cell surface exopeptidase CD26, CXCR4 expression, apoptosis, cell cycle, in vivo marrow homing capacity, and competitive LTRC capacity of the mobilized graft. Our results indicate that compared with G-CSF, GROβΔ4 mobilizes more primitive HSCs characterized by enhanced adhesion to endothelial cells, more competitive repopulating capacity, and more efficient marrow homing that is less dependent on the SDF1/CXCR4 axis.
Materials and methods
Animals
SPF female BALB/c and C57Bl/6 mice, 6-8 weeks of age, were purchased from Harlan Sprague-Dawley, Indianapolis, IN. B6.SJL-PtrcAPep3B/BoyJ (B6.BoyJ) mice were bred at Indiana University. Indiana University School of Medicine Institutional Animal Care and Use Committee (IACUC) approved all experimental procedures.
Reagents
Endotoxin-free human GROβ was purchased from R&D Systems (Minneapolis, MN). Recombinant human GROβΔ4 was produced and purified as previously described.7 G-CSF was from Amgen (Thousand Oaks, CA). Recombinant murine stem cell factor was a gift from Dr Karl Nocka, UCB Research, Cambridge, MA. Recombinant murine stem cell factor was purchased from BioVision, Palo Alto, CA. MegaCult-C reagents were obtained from Stem Cell Technologies (Vancouver, BC, Canada).
Peripheral blood cell mobilization
Mobilized peripheral blood was collected 15 minutes after subcutaneous injection of 2.5 mg/kg GROβ or GROβΔ4 or 16 hours after 4 days of subcutaneous administration (50 μg/kg per day, twice daily) of G-CSF. In the combination setting, G-CSF was administered for 4 days, GROβ or GROβΔ4 was administered 16 hours after the last dose of G-CSF, and peripheral blood was collected 15 minutes later. These regimens induce optimal HSPC mobilization.7,8 Mononuclear cells (PBMCs) were isolated as previously described.7,8 Total colony forming units (CFUs)/mL blood were calculated as follows: (%CFU in PBMC) × (PBMC/mL blood) × 100−1.
In vitro migration
Mobilized PBMCs were blocked with antimouse CD16/CD32(FcγIII/II) (BD Biosciences, San Diego, CA), stained with phycoerythrin (PE)-conjugated antimouse Ly-6G (Gr-1) and Ly-6C (RB6-8C5), CD45R/B220 (RA-6B2), CD3e (145-2C11), TER119 (TER-119), and CD11b (M1/70; BD Biosciences), and incubated with anti-PE magnetic beads (Miltenyi Biotech, Auburn, CA). Cells passed through a magnetic column were collected and stained with FITC-anti-c-kit antibody (2B8; BD Biosciences). PE− KL cells were isolated using fluorescence-activated cell sorter (FACS) (Figure 6A) and KL cells subjected to in vitro migration to rmSDF-1α (R&D Systems) for 4 hours. Input and migrated cells were collected, washed, and CFU enumerated in 1% methylcellulose/IMDM containing 30% fetal bovine serum, 1 U/mL rhEpo, 10 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor, and 50 ng/mL recombinant murine stem cell factor. Percent migration was calculated as (total CFU/lower chamber ÷ total input CFU × 100). SKL cell migration was determined using lin− cells and input cells and migrated cells stained with anti-c-kit and anti-Sca-1 (E13-161.7; BD Biosciences) antibodies. Migration was calculated as (migrated SKL cells ÷ SKL input cells × 100). Total granulocyte macrophage colonies (CFU-GM) and granulocyte-erythrocyte-megakaryocyte-macrophage (GEMM)/mL in mobilized blood migrating to SDF-1α was calculated as: (CFU/mL blood) × (percent migration to SDF-1α) × 1/100. In some experiments, linneg cells were pre-incubated with 10 μM AMD3100 (AnorMED, Langley, BC, Canada).
PBSC transplantation in mice
BALB/c mice received 875 cGy total body irradiation (lethal dose [LD]100 [radiation dose lethal to 100% of mice]) from a Gammacel-40 irradiator (Nordon International, Kanata, ON, Canada) in 2 sessions, 6 hours apart. Irradiated mice received mobilized PBMCs via the tail vein in 0.2 mL endotoxin-free saline (Baxter Healthcare, Deerfield, IL). Neutrophil (ANC) and platelet (PLT) counts were monitored using a Hemavet Mascot hematology analyzer (CDC Technologies, Oxford, CT).
Competitive transplants
Peripheral blood mononuclear cells from C57BL/6 (CD45.2) mice mobilized by GROβΔ4 or G-CSF were mixed with 5 × 105 competitor marrow cells from Boy J mice (CD45.1) at ratios of 4:1, 3:1, or 2:1 and transplanted into lethally irradiated C57BL/6 mice (1050-Gy split dose the day before transplantation). Peripheral blood was sampled monthly and the proportion of CD45.1 and CD45.2 cells determined by flow cytometry.
Flow cytometry
Lin−-PBMCs were blocked with anti-FcγIII/II and stained with antibodies against CXCR4 (2B11; BD Biosciences) or CD11a (2D7), CD62L (MEL-14), CD49d (9C10), CD49e (5H10-27; BD Biosciences) with anti-c-kit (2B8, BD Biosciences) with or without anti-Sca-1. Total CD34−-SKL cells/mL blood was determined by staining lin− cells with anti-CD34 (RAM34; BD Biosciences), Sca-1, and c-kit and calculated using the following formula: (percent CD34− SKL cells in lin− fraction) × (percent lin− cells in PBMC) × (PBMC/mL blood) × 10−4. Total SKL or KL cells/mL blood was similarly calculated. Mobilized PBMC were stained with CD8a-PE (53-6.7; BD Biosciences) and TCR-FITC (H57-597; BD Biosciences) for determination of facilitating cells. Lin− cells prestained with APC-c-kit and PE-Cy7-Streptavidin-biotin-Sca-1 were stained with FITC–Annexin-V (BD Bioscience). Intracellular CXCR4 in mobilized KL or SKL cells was determined by staining lin− cells with FITC-Sca-1, APC-c-kit, and a saturating amount of nonconjugated antimouse CXCR4 sufficient to block surface CXCR4 (2B11; BD Biosciences). Cells were fixed with 1% paraformaldehyde, permeabilized by 0.25% Triton-X/0.5% bovine serum albumin/phosphate-buffered saline (BSA/PBS), and stained with PE-CXCR4 (2B11, BD Biosciences) to examine intracellular CXCR4.
Adhesion assays
Mouse endothelial C166 cells (3 × 104; ATCC, Manassas, VA), which express VCAM-126 and support proliferation of primitive hematopoietic cells,27 were seeded on replicate plates precoated with 5 μg/mL human fibronectin (BD Biosciences) 48 hours before adhesion assays. Mobilized PBMCs in 0.5% bovine serum albumin/RPMI-1640 (4 × 104 cells/well) were seeded into wells coated with C166 cells. Cells were briefly centrifuged to facilitate adhesion, incubated for 30 minutes at 37°C, washed, and nonadherent cells collected. Input cells and nonadherent KL and SKL cells were quantitated and percent adhesion determined by subtracting percent nonadherent cells from 100.
Statistics
Data are expressed as mean plus or minus a standard error of the mean (SEM) and statistical significance was evaluated using the 2-tailed Student t test in Microsoft Excel (Microsoft, Seattle WA). Synergy in response to G-CSF plus GROβ/GROβΔ4 was compared with G-CSF or GROβ/GROβΔ4 used alone by ANOVA with Bonferroni correction for multiple comparisons using GraphPad InStat3 (Graphpad Software, San Diego, CA). Hematologic recovery for mice that underwent transplantation with PBMCs mobilized by G-CSF, GROβ, and GROβΔ4 alone and with G-CSF were compared by nonlinear regression analysis as described.7
Results
Accelerated hematologic recovery in mice receiving GROβ or GROβΔ4-mobilized PBMCs
We previously reported that GROβΔ4-mobilized PBMCs restore neutrophil and platelet recovery faster in mice than G-CSF–mobilized PBMCs.7 In separate studies, we reported that GROβ mobilized fewer total WBCs but a greater number of early HSPCs into peripheral blood than G-CSF, and that compared with G-CSF–mobilized PBSCs, the PBSCs mobilized by GROβ showed a stronger competitive trend at least when used at a 2:1 ratio.28 To more precisely understand the faster engraftment observed with the more clinically relevant GROβΔ4 ligand, we analyzed in depth the phenotypic and functional composition of the chemokine-mobilized graft that produced faster engraftment and directly compared PBSCs mobilized by full-length GROβ to evaluate the role of N-terminal truncation. Comparison of PBMCs mobilized by full-length and truncated GROβ showed similar engraftment and accelerated neutrophil and platelet recovery compared with G-CSF (Table 1), demonstrating that the effect of GROβΔ4 is not a consequence of N-terminal truncation. Neutrophil recovery was significantly improved further using PBMCs mobilized by either chemokine in combination with G-CSF (13 days, P < .05 vs 15 and 17 days for GROβ/GROβΔ4 and G-CSF used alone, respectively), whereas, 80% platelet recovery using combination-mobilized PBMCs (29 days, P < .05) was slower than observed with cells mobilized by GROβ or GROβΔ4 alone (23 days) but still faster than by G-CSF (39 days). The reasons for slower platelet recovery with G-CSF or GROβ/GROβΔ4 with G-CSF are not clear, but may reflect graft composition. It has been shown that G-CSF mobilizes CD14+ monocytes with suppressive activity, at least for T-cell proliferation.29
Neutrophil and platelet recovery in mice that underwent transplantation with mobilized PBSCs
| Mobilizer . | Days to more than 500 ANCs . | Days to PLT Recovery . | ||
|---|---|---|---|---|
| 20% . | 40% . | 80% . | ||
| PBS | Over 95% mortality day 14 | — | — | — |
| G-CSF | 17 | 20 | 26 | 39 |
| GROβ | 15* | 16* | 18* | 23* |
| GROβΔ4 | 15* | 16* | 18* | 23* |
| GROβ + G-CSF | 13* | 16* | 20* | 29* |
| GROβΔ4 + G-CSF | 13* | 16* | 20* | 29* |
| Mobilizer . | Days to more than 500 ANCs . | Days to PLT Recovery . | ||
|---|---|---|---|---|
| 20% . | 40% . | 80% . | ||
| PBS | Over 95% mortality day 14 | — | — | — |
| G-CSF | 17 | 20 | 26 | 39 |
| GROβ | 15* | 16* | 18* | 23* |
| GROβΔ4 | 15* | 16* | 18* | 23* |
| GROβ + G-CSF | 13* | 16* | 20* | 29* |
| GROβΔ4 + G-CSF | 13* | 16* | 20* | 29* |
Lethally irradiated mice underwent transplantation with 2 × 106 PBMCs mobilized by G-CSF, GROβ, GROβΔ4, or G-CSF plus GROβ/GROβΔ4. Hematologic recovery in recipient mice was evaluated by enumeration of neutrophil (ANC) and platelet (PLT) count on alternate days using a Hemavet Mascot hematology analyzer equipped with veterinary software (CDC Technologies, Oxford, CT). The rates of hematologic recovery for G-CSF, GROβ, and GROβΔ4 alone and in combination with G-CSF were determined by nonlinear regression analysis as previously described.7
—indicates over 95% mortality by day 14.
P < .05 compared with G-CSF.
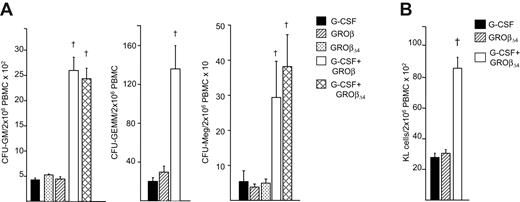
While total HSPCs in hematopoietic grafts influence recovery,30 GROβ, GROβΔ4, and G-CSF–mobilized grafts contained equivalent total CFU-GM, CFU-GEMM, and CFU-megakaryocyte (Meg) (Figure 1A), as well as immunophenotypically defined KL cells (Figure 1B),7 making it unlikely that accelerated recoveries were due to the number of transplanted HPCs. PBMCs mobilized by GROβ/GROβΔ4 plus G-CSF contained 6 to 8-fold more CFU-GM, 5- to 7-fold more CFU-GEMM, 8 to 12-fold more CFU-Meg, and 3- to 4-fold more KL cells (Figures 1A,B), which probably contributed to accelerated recovery.
Mobilization of HPC in mice treated with GROβ or GROβΔ4 alone or in combination with G-CSF. (A) CFU-GM, CFU-GEMM, and CFU-Meg/2 × 106 PBMC graft mobilized with G-CSF, GROβ, GROβΔ4, or the combination of G-CSF plus either GROβ or GROβΔ4. Data are an average of 5 experiments for CFU-GM and 2 to 4 experiments for CFU-GEMM and CFU-Meg from 3 mice/group, each assayed individually. †Synergy compared with G-CSF or GROβ/GROβΔ4 (P < .05), as determined using ANOVA with Bonferroni multiple comparison test. (B) C-kit+, lineage− (KL) cells/2 × 106 PBMC graft mobilized by G-CSF, GROβΔ4 or the combination of G-CSF plus GROβΔ4. Data are expressed as mean (± SEM) from 6 replicates of 10 mice per group in 2 experiments. †Synergy compared with G-CSF or GROβΔ4, P < .05, determined using ANOVA with Bonferroni multiple comparison test.
Mobilization of HPC in mice treated with GROβ or GROβΔ4 alone or in combination with G-CSF. (A) CFU-GM, CFU-GEMM, and CFU-Meg/2 × 106 PBMC graft mobilized with G-CSF, GROβ, GROβΔ4, or the combination of G-CSF plus either GROβ or GROβΔ4. Data are an average of 5 experiments for CFU-GM and 2 to 4 experiments for CFU-GEMM and CFU-Meg from 3 mice/group, each assayed individually. †Synergy compared with G-CSF or GROβ/GROβΔ4 (P < .05), as determined using ANOVA with Bonferroni multiple comparison test. (B) C-kit+, lineage− (KL) cells/2 × 106 PBMC graft mobilized by G-CSF, GROβΔ4 or the combination of G-CSF plus GROβΔ4. Data are expressed as mean (± SEM) from 6 replicates of 10 mice per group in 2 experiments. †Synergy compared with G-CSF or GROβΔ4, P < .05, determined using ANOVA with Bonferroni multiple comparison test.
GROβΔ4-mobilized LTRCs are more competitive than G-CSF–mobilized LTRCs
HPCs provide short-term protection; however, HSCs are required for long-term repopulation. Because hematopoietic recovery occurs faster with GROβΔ4-mobilized PBMCs, we assessed their long-term repopulating ability using limiting dilution competitive repopulation. Lethally irradiated C57BL/6 mice underwent transplantation with 0.5 × 106 congenic B6.BoyJ (CD45.1) marrow competitor cells plus 2 × 106 (4:1), 1.5 × 106 (3:1), and 1 × 106 (2:1) CD45.2 PBMCs mobilized by GROβΔ4 and/or G-CSF. All ratios of mobilized donor/recipient competitor cells showed significant chimerism (> 10-fold) compared with cells from mice mobilized by saline. Little difference in chimerism was seen between mice receiving GROβΔ4 or G-CSF–mobilized PBMCs at the highest ratio, whereas enhanced chimerism was observed using PBMCs mobilized by the combination of both compounds (Figure 2A). Because this graft contains more HPCs, enhanced chimerism is also likely because of more LTRCs. At ratios of 3:1 and 2:1, PBMCs mobilized by GROβΔ4 alone or with G-CSF produced significantly higher chimerism than G-CSF–mobilized PBMCs, suggesting that these PBMCs contain more LTRCs or LTRCs with enhanced repopulating ability/self-renewal.
Competitive repopulation in mice that underwent transplantation with GROβΔ4 and/or G-CSF mobilized PBMCs. (A) One-half million marrow cells from Boy J mice (CD45.1) were mixed with 1.0, 1.5, or 2 × 106 PBMCs from C57BL/6 mice (CD45.2) mobilized with GROβΔ4 or G-CSF (ratio: 2:1, 3:1, and 4:1, respectively) and transplanted into lethally irradiated C57BL/6 mice. Donor and recipient chimerism in recipient blood was analyzed at 6 months after transplantation. The percentage of CD45.2 cells (PBMC-derived cells) is shown. The results are an average from 6 to 8 recipients. *P < .05 compared with G-CSF; †P < .05 compared with GROβΔ4, analyzed by ANOVA with Bonferroni correction. (B) Each individual chimeric mouse in panel A was killed and 106 bone marrow cells transplanted in noncompetitive fashion into lethally irradiated C57BL6 mice. Chimerism in peripheral blood was analyzed and the percentage CD45.2 cells at 6 months after transplantation is shown. Data are the average of 3 to 6 mice with cells from each mouse transplanted into 2 recipients. *P < .05 compared with primary transplantation and G-CSF; †P < .05 compared with GROβΔ4 alone, analyzed by ANOVA with Bonferroni correction. (C) Marrow cells from the secondary recipients were transplanted in a similar manner and chimerism in peripheral blood was analyzed. *P < .05 compared with primary transplantation and G-CSF; †P < .05 compared with GROβΔ4 alone, analyzed by ANOVA with Bonferroni correction. Data are expressed as means (±SEM).
Competitive repopulation in mice that underwent transplantation with GROβΔ4 and/or G-CSF mobilized PBMCs. (A) One-half million marrow cells from Boy J mice (CD45.1) were mixed with 1.0, 1.5, or 2 × 106 PBMCs from C57BL/6 mice (CD45.2) mobilized with GROβΔ4 or G-CSF (ratio: 2:1, 3:1, and 4:1, respectively) and transplanted into lethally irradiated C57BL/6 mice. Donor and recipient chimerism in recipient blood was analyzed at 6 months after transplantation. The percentage of CD45.2 cells (PBMC-derived cells) is shown. The results are an average from 6 to 8 recipients. *P < .05 compared with G-CSF; †P < .05 compared with GROβΔ4, analyzed by ANOVA with Bonferroni correction. (B) Each individual chimeric mouse in panel A was killed and 106 bone marrow cells transplanted in noncompetitive fashion into lethally irradiated C57BL6 mice. Chimerism in peripheral blood was analyzed and the percentage CD45.2 cells at 6 months after transplantation is shown. Data are the average of 3 to 6 mice with cells from each mouse transplanted into 2 recipients. *P < .05 compared with primary transplantation and G-CSF; †P < .05 compared with GROβΔ4 alone, analyzed by ANOVA with Bonferroni correction. (C) Marrow cells from the secondary recipients were transplanted in a similar manner and chimerism in peripheral blood was analyzed. *P < .05 compared with primary transplantation and G-CSF; †P < .05 compared with GROβΔ4 alone, analyzed by ANOVA with Bonferroni correction. Data are expressed as means (±SEM).
To evaluate if GROβ-mobilized HSCs have greater intrinsic repopulating/self-renewal activity, at 6 months after transplantation marrow from competitively engrafted mice was transplanted into secondary recipients in noncompetitive fashion (Figure 2B). All secondary mice survived less than 6 months, with significant levels of chimerism, demonstrating durable engraftment of primary mice. Secondary mice engrafted with marrow from mice receiving G-CSF–mobilized PBMCs showed the same level of chimerism in peripheral blood as the donor mice. In contrast, mice engrafted with HSCs from mice that underwent transplantation with GROβΔ4 or GROβΔ4 plus G-CSF–mobilized cells showed significantly higher chimerism than observed in the primary mice at all dilutions and significantly higher than mice that received marrow from mice that originally underwent transplantation with G-CSF–mobilized PBMCs. When marrow cells from the secondary recipients were transplanted into the tertiary recipients, chimerism was again significantly higher in mice receiving marrow reconstituted from GROβΔ4 or GROβΔ4 plus G-CSF–mobilized cells compared with G-CSF–mobilized cells (Figure 2C). The reasons for enhanced competitiveness in secondary transplants and the continued enhanced competitiveness on tertiary transplantation displayed by HSCs from mice receiving GROβΔ4-mobilized grafts is not clear, but probably represents mobilization of earlier and more competitive HSCs, which is consistent with the enhanced hematopoietic recovery in mice that received GROβΔ4–mobilized PBMCs.
Mobilization of SKL cells and CD34− SKL cells by GROβΔ4 and G-CSF
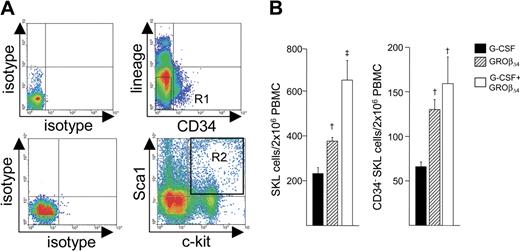
To further define HSCs mobilized by GROβ alone or with G-CSF, we quantitated and compared the SKL cell population (Figure 3A) that contains mouse LTRCs.31,32 We have previously shown that G-CSF and GROβΔ4 each mobilized significant numbers of SKL and CD34−SKL cells.28 However, although no significant difference in total SKL cells/mL blood was observed between GROβΔ4 or G-CSF, fewer PBMCs are obtained from GROβΔ4-mobilized blood than G-CSF–mobilized blood; therefore, the GROβΔ4-mobilized PBMCs contained 30% more SKL cells, which is numerically meaningful (1.3-fold ± 0.1-fold; P < .05; Figure 3B). GROβΔ4 also mobilized significantly more CD34−SKL cells/mL blood (1.5-fold ± 0.1-fold; P < .05; not shown), and the GROβΔ4-mobilized PBMC graft used for transplantation contained significantly more CD34−SKL cells (2.0-fold ± 0.2-fold; P < .05; Figure 3C), which contains one of the most primitive HSC populations in vivo.31,32 GROβΔ4 plus G-CSF synergistically mobilized SKL cells, similar to what is observed for CFU-GM8,28 (not shown), and the G-CSF plus GROβΔ4-mobilized graft contained significantly more SKL and CD34−SKL cells compared with G-CSF (Figure 3C). These results suggest that GROβ-mobilized grafts, either alone or with G-CSF, contain significantly more primitive long-term repopulating HSCs than G-CSF–mobilized grafts. CD8+, TCR−, or CD8+ TCR+-facilitating cells can accelerate HSC engraftment in the allogeneic settings.12 There was no significant change in CD8a+ TCR− cells (0.5% ± 0.02%, 0.5% ± 0.03%, and 0.5% ± 0.03%) and CD8a+ TCR+ cells (7.9% ± 0.4%, 8.2% ± 1.2%, and 6.3% ± 2.8%) between G-CSF, GROβΔ4, and G-CSF plus GROβΔ4-mobilized cells.
Mobilization of SKL and CD34− SKL cells in mice mobilized with G-CSF or GRO. (A) Lin− PBMC were stained with anti-c-kit, anti-Sca-1, and anti-CD34. The upper plots show isotype (left) and CD34 and lineage marker staining (right). The R1 gate represents CD34− lin− cells. The bottom left plots show the isotype (left) and c-kit and Sca-1 staining in CD34− lin− cells (right) in the R1 gate. The R2 gate represents c-kit+ Sca-1+ cells. The plot shown is representative data acquiring approximately 0.5 × 103 SKL events in the CD34− lin− cell fraction. (B) SKL cells (left panel) and CD34− SKL cells (right panel) in 2 × 106 PBMCs. Data are expressed as mean (± SEM) from 6 replicates of 10 mice per group in 2 experiments. †P < .05 compared with G-CSF; ‡Synergy compared with G-CSF or GROβΔ4, P < .05, determined using ANOVA with Bonferroni multiple comparison test.
Mobilization of SKL and CD34− SKL cells in mice mobilized with G-CSF or GRO. (A) Lin− PBMC were stained with anti-c-kit, anti-Sca-1, and anti-CD34. The upper plots show isotype (left) and CD34 and lineage marker staining (right). The R1 gate represents CD34− lin− cells. The bottom left plots show the isotype (left) and c-kit and Sca-1 staining in CD34− lin− cells (right) in the R1 gate. The R2 gate represents c-kit+ Sca-1+ cells. The plot shown is representative data acquiring approximately 0.5 × 103 SKL events in the CD34− lin− cell fraction. (B) SKL cells (left panel) and CD34− SKL cells (right panel) in 2 × 106 PBMCs. Data are expressed as mean (± SEM) from 6 replicates of 10 mice per group in 2 experiments. †P < .05 compared with G-CSF; ‡Synergy compared with G-CSF or GROβΔ4, P < .05, determined using ANOVA with Bonferroni multiple comparison test.
Reduced apoptosis in GROβΔ4 plus G-CSF–mobilized SKL cells
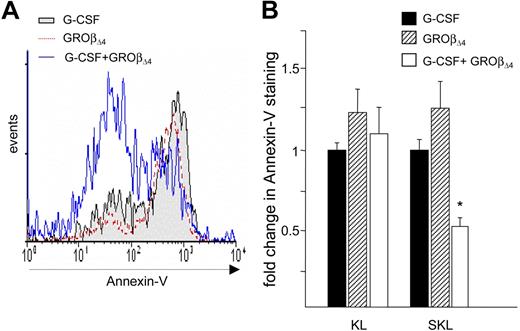
Stem cell survival contributes to homing and engraftment efficiency.33,34 G-CSF–mobilized CD34+ cells express higher levels of caspase-3, -4, and -8 with elevated Annexin-V binding, compared with marrow CD34+ cells.35 We therefore quantitated apoptosis in the KL and SKL cell populations mobilized by GROβΔ4 or G-CSF to determine whether differences in survival contribute to engraftment. Annexin-V staining was equivalent in GROβΔ4 and G-CSF–mobilized KL and SKL cells (Figure 4A,B), suggesting that enhanced engraftment of GROβΔ4-mobilized PBMCs is unrelated to apoptosis. However, Annexin-V staining was significantly reduced in SKL cells mobilized by GROβΔ4 plus G-CSF (Figure 4A,B), suggesting that enhanced survival probably contributes to accelerated hematopoietic recovery.
Annexin-V on SKL cells mobilized by G-CSF or GRO. (A) Representative Annexin-V staining on mobilized SKL cells. (B) Fold change Annexin-V mean fluorescence intensity on KL or SKL cells, expressed as mean (± SEM) from 2 independent experiments performed in triplicate using 10 mice per group per experiment. *P < .01 compared with G-CSF and GROβΔ4.
Annexin-V on SKL cells mobilized by G-CSF or GRO. (A) Representative Annexin-V staining on mobilized SKL cells. (B) Fold change Annexin-V mean fluorescence intensity on KL or SKL cells, expressed as mean (± SEM) from 2 independent experiments performed in triplicate using 10 mice per group per experiment. *P < .01 compared with G-CSF and GROβΔ4.
GROβΔ4-mobilized cells adhere better to VCAM-1+ endothelial cells
HSC trafficking is regulated by adhesion molecules, including leukocyte function associated antigen 1 (LFA-1),36 very late antigen-4 (VLA-4),36-40 and VLA-5.36 L-selectin expression in CD34+ cells predicts rapid platelet recovery in patients undergoing transplantation with G-CSF–mobilized cells.41 Firm adhesion to the vascular endothelial cells is the first step in homing, followed by transendothelial migration.42 We compared adhesion of G-CSF- and GROβΔ4-mobilized KL and SKL cells to VCAM-1+ C166 endothelial cells that support proliferation of HSPCs26,27 under static conditions to determine whether adhesion differences are involved in enhanced engraftment of GROβΔ4-mobilized HSCs. Adhesion of GROβΔ4-mobilized KL and SKL cells to C166 cells was greater than G-CSF–mobilized cells (Figure 5A, left panel). GROβΔ4-mobilized HSPC adhesion was 2- to 4-fold greater than cells mobilized by G-CSF (Figure 5A, right panel), consistent with enhanced adhesion to immobilized recombinant VCAM-1, as we reported previously.28 Cells mobilized by GROβΔ4 plus G-CSF also demonstrated enhanced adhesion (not shown). However, despite enhanced adhesion to C166 cells, static expression of CD11a (αL-integrin, LFA-1), CD49d (α4-integrin), CD49e (α5-integrin), and CD62L (L-selectin, LCECAM-1) was equivalent in SKL cells mobilized by GROβΔ4 or G-CSF (Figure 5B), suggesting that adhesion molecule activation rather than expression is probably responsible for enhanced adhesion of GROβΔ4-mobilized cells.
Adhesion to C166 endothelial cells, expression of adhesion molecules, and DNA staining for mobilized SKL and/or KL cells. (A) Percent adhesion of mobilized SKL and KL cells to C166 endothelial cells from one of 3 experiments with similar results (left panel). The right panel shows mean fold increase in adhesion (± SEM) compared with G-CSF from 3 experiments. *P < .05. (B) Expression of CD11a, CD49d, CD49e, and CD62L on SKL cells mobilized by G-CSF and/or GROβΔ4. Mean fluorescence intensity (± SEM) of each molecule and isotype staining are shown for 3 replicates of 10 mice per group. (C) DNA histogram of KL cells mobilized by G-CSF or GROβΔ4. PBMCs were stained with antilineage antibodies (PE), c-kit (FITC), and 7-AAD. Cell cycle distribution was quantitated using ModFIT Software (Becton Dickinson).
Adhesion to C166 endothelial cells, expression of adhesion molecules, and DNA staining for mobilized SKL and/or KL cells. (A) Percent adhesion of mobilized SKL and KL cells to C166 endothelial cells from one of 3 experiments with similar results (left panel). The right panel shows mean fold increase in adhesion (± SEM) compared with G-CSF from 3 experiments. *P < .05. (B) Expression of CD11a, CD49d, CD49e, and CD62L on SKL cells mobilized by G-CSF and/or GROβΔ4. Mean fluorescence intensity (± SEM) of each molecule and isotype staining are shown for 3 replicates of 10 mice per group. (C) DNA histogram of KL cells mobilized by G-CSF or GROβΔ4. PBMCs were stained with antilineage antibodies (PE), c-kit (FITC), and 7-AAD. Cell cycle distribution was quantitated using ModFIT Software (Becton Dickinson).
Although cell cycle state can influence engraftment and homing of HSCs,9,10 cell cycle distribution determined by DNA staining was equivalent in KL cells mobilized by either GROβΔ4 or G-CSF, with less than 90% of both cell populations falling in G0/G1 (Figure 5C), which is similar to nonhuman primate CD34+ cells mobilized by AMD3100.43 Similarly, cell cycle distribution of cells mobilized by GROβΔ4 plus G-CSF was identical to cells mobilized by either agent alone (not shown).
In vitro migration of CFU and SKL cells mobilized by GROβΔ4 to SDF-1α is lower than that of G-CSF–mobilized cells
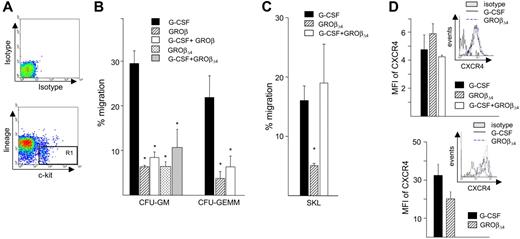
The SDF-1α/CXCR4 migration axis is believed to play a major role in HSPC homing.13,14,20 Enhanced in vitro migration to SDF-1α by G-CSF–mobilized CD34+ cells is associated with hematopoietic recovery.22 We quantitated transmigration of mobilized HPCs to SDF-1α in vitro to determine whether the enhanced engraftment of GROβΔ4-mobilized PBMCs is attributable to enhanced migration capability. Migration of CFU-GM and CFU-GEMM in the sorted KL cell fraction mobilized by GROβ/GROβΔ4 alone or with G-CSF to recombinant murine SDF-1α (Figure 6B) was significantly lower compared with G-CSF. Migration of GROβΔ4-mobilized CD34−-SKL cells was also significantly lower (Figure 6C). Despite reduced migration, surface CXCR4 expression on GROβΔ4-mobilized SKL (Figure 6D, top) and KL cells (not shown) were equivalent. Similarly, surface CXCR4 on G-CSF plus GROβΔ4-mobilized SKL or KL cells was not different from cells mobilized by G-CSF or GROβΔ4 alone (Figure 6D). We examined intracellular CXCR4 level because human CD34+CXCR4− cells harbor intracellular CXCR4 that can restore NOD/SCID repopulation potential44 and found that intracellular CXCR4 expression in SKL (Figure 6D, bottom) and KL cells (not shown) mobilized by GROβΔ4 were not statistically different from expression in G-CSF–mobilized cells.
In vitro migration of mobilized CFU, KL and SKL cells. (A) Mobilized PBMCs were depleted of lin+ cells, stained with FITC-conjugated anti-c-kit and c-kit+ lin− cells (shown in R1) isolated by FACS, and subjected to in vitro migration to 100 ng/mL of rmSDF-1α. (B) Percent CFU-GM or GEMM migration in KL mobilized cells in response to recombinant murine SDF-1α. Data are expressed as mean (± SEM) from 2 experiments with 3 mice per group. *P < .05 compared with G-CSF. (C) Migration of CD34-SKL cells in mobilized peripheral blood in response to 100 ng/mL recombinant murine SDF-1α. Data are expressed as mean (± SEM) from 2 experiments with 20 mice per group. *P < .05 compared with G-CSF. (D) Expression of cell surface (left) and intracellular CXCR4 (right) for SKL cells mobilized by G-CSF and GROβΔ4. The insert shows one representative plot; the bar graph represents mean (± SEM) from 3 replicate experiments with 10 mice per group.
In vitro migration of mobilized CFU, KL and SKL cells. (A) Mobilized PBMCs were depleted of lin+ cells, stained with FITC-conjugated anti-c-kit and c-kit+ lin− cells (shown in R1) isolated by FACS, and subjected to in vitro migration to 100 ng/mL of rmSDF-1α. (B) Percent CFU-GM or GEMM migration in KL mobilized cells in response to recombinant murine SDF-1α. Data are expressed as mean (± SEM) from 2 experiments with 3 mice per group. *P < .05 compared with G-CSF. (C) Migration of CD34-SKL cells in mobilized peripheral blood in response to 100 ng/mL recombinant murine SDF-1α. Data are expressed as mean (± SEM) from 2 experiments with 20 mice per group. *P < .05 compared with G-CSF. (D) Expression of cell surface (left) and intracellular CXCR4 (right) for SKL cells mobilized by G-CSF and GROβΔ4. The insert shows one representative plot; the bar graph represents mean (± SEM) from 3 replicate experiments with 10 mice per group.
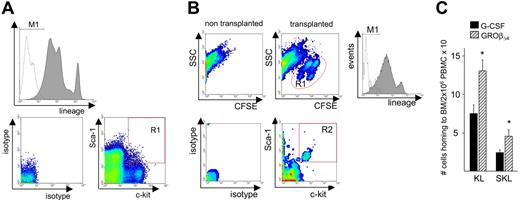
Enhanced homing of GROβΔ4-mobilized SKL cells to marrow
HSC homing can be regulated by a hierarchy of pathways including the SDF-1α/CXCR4 and α4-integrin/VCAM-1 pathways.25 Because neutrophil and platelet recovery is faster in recipients that underwent transplantation with GROβΔ4-mobilized PBMCs, whereas in vitro migration of mobilized HPCs to SDF-1α was significantly lower but their adhesion to endothelial cells was higher, we evaluated the in vivo marrow homing capacity of CFSE-labeled mobilized KL and SKL cells (Figure 7 and Table 2). Although GROβΔ4-mobilized SKL and KL cells showed reduced migration to SDF-1α in vitro, their marrow homing in vivo was significantly higher than G-CSF–mobilized SKL cells (Table 2). Moreover, the total number of SKL cells homing to recipient marrow in vivo in the 2 × 106 GROβΔ4-mobilized PBMC graft was significantly higher than in an equivalent G-CSF–mobilized PBMC graft (Figure 7C). Homing of SKL cells mobilized by G-CSF or GROβΔ4 was significantly higher than KL cells (Table 2). Despite superior homing of SKL cells, there was no significant difference in surface CXCR4 expression between mobilized KL and SKL cells (mean fluorescent intensity [MFI] = 4.7 ± 0.5 and 4.8 ± 1, respectively, for G-CSF and MFI = 5.4 ± 0.8 and 5.9 ± 0.7 for GROβΔ4, respectively).
In vivo marrow homing of mobilized KL and SKL cells. (A) The top histogram shows lineage markers (PE) and isotype staining of donor PBMNCs before transplantation. The bottom 2 plots show Sca-1 (PE-Cy7) vs c-kit (APC; right) and their isotype staining (left) in lin− cells. M1: lin− cells; R1: Sca-1+, c-kit+ cells in lin− cells. (B) PBMCs mobilized by G-CSF or GROβΔ4 were stained with CFSE and 4 × 107 cells were transplanted into lethally irradiated syngeneic recipients. Recipient marrow cells were harvested 16 hours after transplantation. The top plots show CFSE staining in nontransplanted recipient (left) and transplanted recipient marrow (right) stained with lineage markers (PE), anti-Sca-1 (PE-Cy7), and anti-c-kit (APC) antibodies. The R1 gate represents CFSE+ cells. The middle histogram represents lineage markers (PE) and isotype staining of CFSE+ cells. The bottom plots shows Sca-1 (PE-Cy7) vs c-kit (APC) staining and its isotype staining in the CFSE+ lin− population. Gate R2 are Sca-1+ c-kit+ cells in CFSE+ lin− cells. (C) Total KL or SKL cells in 2 × 106 PBMCs homing to recipient marrow. Data are expressed as mean (± SEM) from 3 experiments using 30 donors and 3 recipients per group per experiment. *P < .05 compared with G-CSF.
In vivo marrow homing of mobilized KL and SKL cells. (A) The top histogram shows lineage markers (PE) and isotype staining of donor PBMNCs before transplantation. The bottom 2 plots show Sca-1 (PE-Cy7) vs c-kit (APC; right) and their isotype staining (left) in lin− cells. M1: lin− cells; R1: Sca-1+, c-kit+ cells in lin− cells. (B) PBMCs mobilized by G-CSF or GROβΔ4 were stained with CFSE and 4 × 107 cells were transplanted into lethally irradiated syngeneic recipients. Recipient marrow cells were harvested 16 hours after transplantation. The top plots show CFSE staining in nontransplanted recipient (left) and transplanted recipient marrow (right) stained with lineage markers (PE), anti-Sca-1 (PE-Cy7), and anti-c-kit (APC) antibodies. The R1 gate represents CFSE+ cells. The middle histogram represents lineage markers (PE) and isotype staining of CFSE+ cells. The bottom plots shows Sca-1 (PE-Cy7) vs c-kit (APC) staining and its isotype staining in the CFSE+ lin− population. Gate R2 are Sca-1+ c-kit+ cells in CFSE+ lin− cells. (C) Total KL or SKL cells in 2 × 106 PBMCs homing to recipient marrow. Data are expressed as mean (± SEM) from 3 experiments using 30 donors and 3 recipients per group per experiment. *P < .05 compared with G-CSF.
Homing of KL and SKL cells mobilized by G-CSF or GROβΔ4
| Mobilizer . | G-CSF . | GROβΔ4 . |
|---|---|---|
| KL cells transplanted | 57 927 ± 5473 | 61 349 ± 4766 |
| (38 436-85 256) | (46 087-85 112) | |
| KL cells recovered | 377 ± 101 | 589 ± 121 |
| (137-927) | (253-1225) | |
| KL cells homed to the recipient marrow | 1256 ± 335 | 1964 ± 408 |
| (457-3090) | (844-4084) | |
| % homing of KL cells‡ | 2.5 ± 1.5 | 3.6 ± 0.8 |
| SKL cells transplanted | 8999 ± 3021 | 7615 ± 1153 |
| (2124-10 762) | (3584-12 494) | |
| SKL cells recovered | 128 ± 21 | 235 ± 38* |
| (61-246) | (111-389) | |
| SKL cells homed to the recipient marrow | 426 ± 70 | 783 ± 127* |
| (204-820) | (369-1296) | |
| % homing of SKL cells‡ | 7.1 ± 1.1† | 11.5 ± 1.8*† |
| Mobilizer . | G-CSF . | GROβΔ4 . |
|---|---|---|
| KL cells transplanted | 57 927 ± 5473 | 61 349 ± 4766 |
| (38 436-85 256) | (46 087-85 112) | |
| KL cells recovered | 377 ± 101 | 589 ± 121 |
| (137-927) | (253-1225) | |
| KL cells homed to the recipient marrow | 1256 ± 335 | 1964 ± 408 |
| (457-3090) | (844-4084) | |
| % homing of KL cells‡ | 2.5 ± 1.5 | 3.6 ± 0.8 |
| SKL cells transplanted | 8999 ± 3021 | 7615 ± 1153 |
| (2124-10 762) | (3584-12 494) | |
| SKL cells recovered | 128 ± 21 | 235 ± 38* |
| (61-246) | (111-389) | |
| SKL cells homed to the recipient marrow | 426 ± 70 | 783 ± 127* |
| (204-820) | (369-1296) | |
| % homing of SKL cells‡ | 7.1 ± 1.1† | 11.5 ± 1.8*† |
Mobilized PBMCs were stained with 5 μM 5-carboxyfluorescein diacetate succinimidyl ester (CFSE) (and 6-CFSE) (Molecular Probes, Eugene, OR) for 10 minutes, as described.59,60 Total KL or SKL cells homed to marrow were calculated as (% CFSE+ KL or SKL cells in recipient marrow) × 100−1 × (% CFSE+ cells in recipient marrow) × 100−1 × (total nucleated recipient marrow cells recovered) × (100/30), assuming marrow cells from 2 femurs, 2 tibias, 2 humerus, and the pelvis (total 8 bones) represents 30% of total body marrow.61 Percent homing was determined as (KL or SKL cells homed to the marrow) ÷ (KL or SKL cells transplanted) × 100 in each recipient and the data were averaged.
The absolute numbers (mean ± SEM, with range in parentheses) of KL or SKL cells recovered from 2 femurs, tibias, humerus, and pelvis of the recipients are shown from 3 experiments with 30 donors and 3 recipients /group/experiment. The number of SKL cells was determined by gating Sca-1+ fraction on KL cells in each experiment.
P < .05 compared with G-CSF.
P < .05 compared with KL cells.
Data are calculated as the average of % homing for each of 9 mice.
The dissociation between in vitro migration to SDF-1α and in vivo homing of SKL cells mobilized with GROβΔ4 suggested that their homing may be less dependent on the SDF-1α/CXCR4 axis. To directly address this possibility, we used the selective CXCR4 antagonist AMD3100, which inhibits homing and migration of mouse HSPC and Diprotin A, a selective inhibitor for the cell surface peptidase CD26 that increases marrow stem cell homing and engraftment by blocking CD26-mediated cleavage of SDF1.13 Consistent with published findings, treatment of normal bone marrow cells and G-CSF–mobilized SKL cells with AMD3100 significantly inhibited in vitro migration to SDF-1α and in vivo marrow homing of SKL cells; however, in vivo homing of GROβΔ4-mobilized SKL cells was unaffected by pretreatment with AMD3100, despite significant reduction in migration to SDF-1α in vitro (Table 3). The lack of inhibition of homing of GROβΔ4-mobilized SKL by AMD3100 was not caused by altered CXCR4 expression. In fact, CXCR4 expression on GROβΔ4-mobilized SKL cells was higher than on normal marrow SKL cells (MFI: 30.7 ± 8.8 vs 13.1 ± 0.4, respectively). Similarly, the CD26 inhibitor Diprotin A significantly enhanced homing of G-CSF–mobilized SKL cells as reported,13 but had little effect on homing of GROβΔ4-mobilized cells (Table 4). However, CD26 expression on GROβΔ4-mobilized SKL cells was significantly higher than on G-CSF–mobilized cells and the number of CD26+SKL cells in the GROβΔ4-moblized graft was significantly higher than G-CSF (Table 4). While the higher CD26 expression on GROβΔ4-mobilized HSCs relative to G-CSF is consistent with their reduced migration to SDF1 in vitro, it is counterintuitive to enhanced in vivo homing and provides further evidence for the fact that in vivo homing of GROβΔ4-moblized SKL cells may not be primarily dependent on the SDF1/CXCR4 axis. Mobilization of CFU-GM by G-CSF was inhibited by Diprotin A as reported;13 however, mobilization by GROβΔ4 was unaffected (Table 4). Identical findings were observed in CD26-deficient mice. These data indicate that CD26 is not involved in the release of stem cells by GROβΔ4 (Table 4).
Homing, migration, and CXCR4 expression of marrow or GROβΔ4-mobilized-SKL cells pretreated with AMD3100
| Cells . | % Reduction in homing . | % Reduction in migration to SDF-1α . | Mean fluorescent intensity for CXCR4 . |
|---|---|---|---|
| Marrow SKL cells | 45 ± 6%* | 30 ± 3%* | 13.1 ± 0.4 |
| GROβΔ4 mobilized SKL cells | −2 ± 12% (NS) | 66 ± 6%* | 30.7 ± 8.8 |
| G-CSF mobilized SKL cells | 37 ± 9%* | ND | ND |
| Cells . | % Reduction in homing . | % Reduction in migration to SDF-1α . | Mean fluorescent intensity for CXCR4 . |
|---|---|---|---|
| Marrow SKL cells | 45 ± 6%* | 30 ± 3%* | 13.1 ± 0.4 |
| GROβΔ4 mobilized SKL cells | −2 ± 12% (NS) | 66 ± 6%* | 30.7 ± 8.8 |
| G-CSF mobilized SKL cells | 37 ± 9%* | ND | ND |
CFSE-stained PBMCs or lin− cells were pretreated with 10 μM AMD3100 or PBS at 37°C for 15 minutes, washed, and transplanted into lethally irradiated recipients or subjected to in vitro migration to 100 ng/mL of recombinant murine SDF-1α. After 4 hours, migrated cells and input cells were enumerated and stained for Sca-1 and c-kit to calculate percent migration of SKL cells. Homing was determined as described in “Materials and methods.” Percent reduction of migration or homing was compared with the cells treated with phosphate-buffered saline. Means (± SEM) fluorescence intensity for CXCR4 from 3 independent experiments are shown.
ND indicates not done.
P < .05 compared with phosphate-buffered saline (N = 3).
CD26 expression on mobilized SKL cells and the effect of CD26 inhibition on HSPC mobilization and homing
| Mobilizer . | G-CSF . | GROβΔ4 . | G-CSF+GROβΔ4 . |
|---|---|---|---|
| MFI of CD26 on SKL cells | 22 ± 1 | 50 ± 4* | 17 ± 3** |
| CD26 pos. SKL cells/mL blood | 761 ± 83 | 1060 ± 46* | 1332 ± 127* |
| CD26 pos. SKL cells/2×106 MNC | 140 ± 15 | 262 ± 11* | 283 ± 27* |
| % homing of KL cells | 2.4 ± 0.6 | 4.6 ± 0.7 | ND |
| % homing of KL cells treated with Diprotin A | 4.3 ± 0.6† | 5.4 ± 0.9 | ND |
| % homing of SKL cells | 8.2 ± 0.9 | 13.5 ± 1.8* | ND |
| % homing of SKL cells treated with Diprotin A | 12.5 ± 1.6† | 14.5 ± 2.1 | ND |
| CFU-GM/mL blood in control mice | 1560 ± 480 | 2142 ± 954 | 5105 ± 991 |
| CFU-GM/mL blood in mice treated with Diprotin A | 944 ± 202‡ | 2413 ± 91 | 5378 ± 1440 |
| CFU-GM/mL blood in CD26+/+ mice | 3460 ± 211 | 1650 ± 263 | 4820 ± 683 |
| CFU-GM/mL blood in CD26−/− mice | 2415 ± 374‡ | 1510 ± 437 | 2214 ± 124‡ |
| Mobilizer . | G-CSF . | GROβΔ4 . | G-CSF+GROβΔ4 . |
|---|---|---|---|
| MFI of CD26 on SKL cells | 22 ± 1 | 50 ± 4* | 17 ± 3** |
| CD26 pos. SKL cells/mL blood | 761 ± 83 | 1060 ± 46* | 1332 ± 127* |
| CD26 pos. SKL cells/2×106 MNC | 140 ± 15 | 262 ± 11* | 283 ± 27* |
| % homing of KL cells | 2.4 ± 0.6 | 4.6 ± 0.7 | ND |
| % homing of KL cells treated with Diprotin A | 4.3 ± 0.6† | 5.4 ± 0.9 | ND |
| % homing of SKL cells | 8.2 ± 0.9 | 13.5 ± 1.8* | ND |
| % homing of SKL cells treated with Diprotin A | 12.5 ± 1.6† | 14.5 ± 2.1 | ND |
| CFU-GM/mL blood in control mice | 1560 ± 480 | 2142 ± 954 | 5105 ± 991 |
| CFU-GM/mL blood in mice treated with Diprotin A | 944 ± 202‡ | 2413 ± 91 | 5378 ± 1440 |
| CFU-GM/mL blood in CD26+/+ mice | 3460 ± 211 | 1650 ± 263 | 4820 ± 683 |
| CFU-GM/mL blood in CD26−/− mice | 2415 ± 374‡ | 1510 ± 437 | 2214 ± 124‡ |
Means (± SEM) are shown. Lineage-depleted cells mobilized by G-CSF, GROβΔ4, or G-CSF+GROβΔ4 were stained with Sca-1-PE, c-kit-APC, and CD26-FITC (clone H194-112, BD Biosciences) and analyzed for CD26 expression on SKL cells by flow cytometry. Mean fluorescent intensity (MFI) of CD26 is shown. The number of CD26+-SKL cells in blood and in 2 × 106 mononuclear cells was calculated by the percentage of CD26-positive cells multiplied by the number of SKL cells/mL blood and SKL cells/2 × 106 mononuclear cells. To determine homing of mobilized HSPCs, cells were treated with or without 5 mM Diprotin A for 15 minutes and washed before transplantation as described.13 Homing of KL and SKL cells were determined as described in Table 2. CFU-GM mobilization was compared in mice treated with Diprotin A (Peptides International, Louisville, KY) 2 times/day for 4 days as reported62 and CD26−/− mice, kindly provided by Dr Hal E. Broxmeyer, Indiana University School of Medicine, with control mice.
ND indicates not done.
P < .05 compared with G-CSF.
P < .05 compared with GROβΔ4 (N = 4).
P < .05 compared with nontreated mice (N = 6).
P < .05 compared with control wild-type mice.
Discussion
GROβΔ4 mobilizes HSPCs when used alone and synergizes with G-CSF when used in combination. In mice, GROβΔ4-mobilized PBMCs restore neutrophil and platelet counts more rapidly and demonstrate more competitive LTRC ability than G-CSF–mobilized PBMCs. Compared with G-CSF, the GROβΔ4-mobilized PBSC graft contains significantly more SKL and primitive CD34−- SKL cells, demonstrates superior adhesion to VCAM-1+ endothelial cells, and shows enhanced marrow homing capacity that is less dependent on CXCR4. The combination of GROβ and G-CSF produces highly synergistic mobilization and further enhanced engraftment. Our findings provide functional evidence supporting the utility of GROβΔ4 as a rapid and effective HSC mobilizer and evidence that PBSCs mobilized by GROβΔ4, either used alone or with G-CSF, are a superior hematopoietic graft, at least in mice.
The absolute number of transplanted HSCs is critical to hematopoietic recovery.30 Although total CFU-GM and KL cells in GROβΔ4 and G-CSF–mobilized blood were nearly identical, the GROβΔ4-mobilized graft contained significantly more primitive LTR-SKL cells and CD34−-SKL cells31,32 and contributed to hematopoiesis at a significantly higher degree than G-CSF–mobilized HSC. One might expect that mature HSPCs rather than immature primitive HSCs would contribute to accelerated short-term hematopoietic recovery. GROβΔ4 may mobilize short-term repopulating cells with greater intrinsic proliferative capacity, although clonogenic assays show no obvious differences in colony size between GROβΔ4 and G-CSF–mobilized colony-forming cells (CFCs). Alternatively, GROβΔ4 may mobilize a population of cells that are not identified by our clonogenic or phenotypic analyses, such as high proliferative potential-colony forming cells (HPP-CFCs) that facilitate accelerated white blood cell recovery. However, our results are consistent with previous reports that primitive stem cells alone mediate rapid marrow recovery after transplantation17 and that the early phase of engraftment after transplantation is mediated by stem cells.30 PBSCs mobilized by GROβΔ4 plus G-CSF demonstrated greater engraftment and repopulating capacity and contained significantly more CFU-GM, KL, SKL, and CD34−-SKL cells, suggesting that both mature and immature HSPCs contribute to their faster engraftment and enhanced chimerism. Lower apoptosis of SKL cells mobilized by GROβΔ4 plus G-CSF probably also contributes to accelerated engraftment. While CD8+, TCR−, or CD8+ TCR+-facilitating cells accelerate HSC engraftment in the allogeneic settings12 and ours is a syngeneic transplant model, involvement of these facilitating cells in the enhanced engraftment is unlikely because we observed no significant differences between G-CSF and GROβΔ4-mobilized cells.
Osteoblasts are implicated in HSC maintenance,45-47 and imaging studies show an interaction of primitive HSPCs with endosteal bone,16,46,48 leading to the hypothesis that primitive HSCs are found in the endosteum niche, whereas HPCs and mature cells migrate to the vascular niche and egress marrow. The capacity of chemokines such as GROβ/GROβΔ47,8 and IL-849 to mobilize HSPCs within 15 minutes is kinetically inconsistent with mobilization from the endosteal niche and suggests either that these chemokines do not mobilize long-term repopulating-hematopoietic stem cells (LTR-HSCs), which is clearly not the case, or that a more readily accessible pool of HSPCs can be mobilized. Using expression of SLAM (signaling lymphocytic activation molecule) family receptors that distinguish HSCs and hematopoietic progenitor cells (HPCs), it has been shown that a relatively large pool of HSCs is associated with sinusoidal endothelium.50 This may explain the rapid mobilization of primitive and mature HSCs by GROβΔ4 or IL-8. In addition, synergistic mobilization of large numbers of primitive HSCs by GROβΔ4 plus G-CSF suggests that a relatively large pool of primitive HSCs inhabit the vascular niche or are mobilized to the vascular niche from the osteoblast niche by G-CSF, but not released in response to G-CSF alone.
Numerous studies implicate the SDF-1/CXCR4 axis in HSPC trafficking and chemoattraction to the marrow microenvironment.13,14,19-21 Migration of GROβΔ4-mobilized CFU-GM, KL, and SKL cells to SDF-1 in vitro was significantly lower than G-CSF–mobilized cells, which was not caused by differences in CXCR4 expression. Higher CD26 levels on GROβΔ4-mobilized SKL cells that can inactivate SDF-1 is consistent with reduced migration to SDF-1. In addition, plasma and marrow SDF-1α levels in mice mobilized with GROβΔ4 are significantly higher than in mice mobilized by G-CSF,8 raising the possibility that CXCR4 desensitization on GROβΔ4-mobilized HSPCs could also account for their reduced migration in vitro. Alternatively, administration of GROβΔ4 may inhibit HSPC CXCR4 responsiveness, given that KC, the mouse homologue of GROβ, can desensitize CXCR4 on mouse polymorphonuclear neutrophils (PMNs).51 However, there is no clear evidence that HSPCs express CXCR2.52
Despite reduced migration to SDF-1α in vitro, homing of GROβΔ4-mobilized SKL cells to marrow was significantly enhanced compared with G-CSF–mobilized SKL cells, indicating a lack of correlation between in vivo homing and in vitro migration to SDF-1α. Lack of correlation between in vitro migration to SDF-1α and marrow homing of CD34+ cells mobilized by G-CSF plus AMD3100 has also been reported.53 Pretreatment of nonmobilized SKL cells with the CXCR4 antagonist AMD3100 significantly blocked their homing as described;13 however, although AMD3100 inhibited in vitro migration of SKL cells from control and GROβΔ4-mobilized blood to SDF-1α, it failed to block homing of GROβΔ4-mobilized SKL cells, even at doses 10-fold higher than required to block homing of resident marrow HPCs.13 In addition, the CD26 inhibitor Diprotin A, while enhancing homing of G-CSF–mobilized cells, did not enhance homing of GROβΔ4-mobilized SKL cells. Together, these results suggest that homing of GROβΔ4-mobilized SKL cells is not primarily dependent on the SDF-1α/CXCR4 axis. These differences were not related to differences in CXCR4 expression, although we cannot rule out an effect caused by differential CXCR4 truncation by proteases released during the mobilization procedure.19 Human CD34+CXCR4− cells harbor intracellular CXCR4 that can restore NOD/SCID repopulation potential.44 Although we cannot exclude externalization of intracellular CXCR4 or transcriptional upregulation of CXCR4 on GROβΔ4-mobilized HSCs after transplantation, which would probably be unaffected by AMD3100, GROβΔ4, and G-CSF–mobilized-SKL cells demonstrated equivalent intracellular CXCR4 at harvest, making it unlikely that differential expression of CXCR4 from intracellular pools explains enhanced homing. We observed that SKL cells mobilized by either G-CSF or GROβΔ4 showed superior homing compared with KL cells (Table 2). Surface CXCR4 expression on mobilized KL and SKL cells were equivalent, further supporting that CXCR4 expression does not correlate with enhanced homing. Higher CD26 expression on GROβΔ4-mobilized SKL cells, which inactivates SDF-1 in the stem cell niche may facilitate their release to the peripheral circulation and account for increased mobilization of more primitive HSCs. However, CD26 inhibition by Diprotin A or gene knockout did not affect mobilization by GROβΔ4 in contrast to G-CSF, suggesting that similar to a lack of involvement of CD26 in homing, CD26 is not required for the release of HSPC by GROβΔ4.
Restoration of white blood cells in patients who received G-CSF–mobilized PBSCs occurs faster than for bone marrow cells,2,3 which may be associated with enhanced migration of G-CSF–mobilized CD34+ cells to SDF-1α.54,55 However, other studies demonstrate that migration of G-CSF–mobilized CD34+ cells to SDF-1α in vitro is lower than marrow-derived CD34+ cells,56,57 suggesting that engraftment of mobilized HSPCs does not directly correlate with migration to SDF-1α. Pretreatment of marrow cells with Pertussis toxin, a selective Gαi inhibitor, does not impair hematopoietic engraftment, suggesting that engraftment is independent of Gαi-coupled receptors, which includes CXCR4.23 Furthermore, fetal liver cells from CXCR4−/− mice engraft in lethally irradiated recipients,58 and marrow engraftment of CXCR4−/− and CXCR4+/+ SKL cells is equivalent.24 These findings suggest that the SDF-1α/CXCR4 axis may not be absolutely required for engraftment and homing by all HSC populations. Moreover, homing of HPCs refractory to SDF-1 migration is blocked in the absence of α4-integrins, indicating that homing via SDF-1α/CXCR4 can be fully compensated by a functional α4-integrin/VCAM-1 pathway.25 Adhesion of GROβΔ4-mobilized KL or SKL cells to VCAM-1+ endothelial cells under static condition is elevated compared with G-CSF–mobilized cells, but is not caused by changes in adhesion molecule expression. Because firm adhesion to endothelial cells is a critical step for the homing,42 this suggests that enhanced interaction of α4-integrin on HSPCs with VCAM-1 on endothelial cells may account for the enhanced homing of GROβΔ4-mobilized SKL cells. Alternatively, because the majority of cells transplanted are retained in organs outside bone marrow, increased marrow homing of GROβΔ4-mobilized HSPCs may be attributed to the reduction in their uptake by the other organs.
The CXCR4 antagonist AMD3100 mobilizes HSPCs and synergize with G-CSF and AMD3100 plus G-CSF–mobilized cells showed enhanced engraftment compared with G-CSF.53 However, in contrast to GROβΔ4 or GROβΔ4 plus G-CSF–mobilized PBSCs, AMD3100 plus G-CSF–mobilized cells do not display enhanced homing relative to G-CSF–mobilized PBSCs. HSCs mobilized by AMD3100 alone or with G-CSF demonstrated enhanced competitiveness on secondary transplantation, which is also seen with HSCs mobilized by GROβΔ4 or GROβΔ4 plus G-CSF, but not with G-CSF. The reason for enhanced repopulation in secondary transplantation is not clear but may reflect a property of the HSC population mobilized by chemokines or chemokine receptor antagonists. The further enhanced competitiveness of GROβΔ4-mobilized or GROβΔ4 plus G-CSF–mobilized peripheral blood stem cell transplantation (PBSC) seen in tertiary transplantation further support the mobilization of a more highly competitive HSCs compared with G-CSF.
In summary, our data indicate that the accelerated neutrophil and platelet engraftment observed after transplantation of HSPCs mobilized by GROβΔ4 is associated with rapid mobilization of more primitive hematopoietic cells with enhanced engraftment and repopulation activity. In addition, enhanced homing and engraftment of GROβΔ4-mobilized cells is less dependent on the SDF-1/CXCR4 axis. Rapid chemokine mobilization may be an attractive paradigm for PBST either alone or added to a standard or reduced duration G-CSF regimen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Suzan Rice for cell sorting by FACS.
This work was supported by US Public Health Service grants HL69669 and HL079654 (to L.M.P.) from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: S.F. designed research, performed research, collected data, analyzed data, and wrote the paper. H.B. participated in performance of the research. A.G.K. participated in designing research, performance of the study, and analysis of the data. L.M.P. participated in designing research, analysis of the data, coordination and performance of the study, and wrote the paper.
Conflict-of-interest disclosure: A.G.K. is an employee of GlaxoSmithKline, whose potential product is studied in this work. The other authors declare no competing financial interests.
Correspondence: Louis M. Pelus, PhD, Department of Microbiology and Immunology, Indiana University School of Medicine, 950 West Walnut Street, Indianapolis, IN 46202. e-mail: lpelus@iupui.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal