Abstract
Vγ9/Vδ2 (γδ) T cells play a major role in innate immunity against microbes, stressed, and tumor cells. They represent less than 5% of peripheral blood lymphocytes but can be activated and expanded in vitro by aminobisphosphonates (ABP)–treated monocytes. The aim of this work was to determine whether ABP-treated dendritic cells (DCs) can also activate γδ T cells and regulate immune responses mediated by conventional αβ T cells. Highly purified immature (iDC) and mature DC (mDC) were generated from peripheral blood monocytes of healthy donors and incubated with zoledronic acid (Zol) for 24 hours. Zol-treated iDC and mDC retained their immunostimulatory properties and induced the vigorous expansion of central memory and effector memory γδ T cells. γδ T cells displayed antitumor activity and appropriate cell surface antigens to target secondary lymphoid organs and exert costimulatory activity. Antigen-specific MHC-restricted immune responses, mediated by conventional αβ T cells, were improved by the concurrent γδ T-cell activation. In conclusion, large numbers of γδ T cells with effector and costimulatory activities are rapidly generated by Zol-treated iDC/mDC. This strategy is worthy of further investigation to improve adoptive cell therapy and vaccine interventions against tumors and infections.
Introduction
γδ T cells are unconventional T cells playing a major role in innate immune responses against microbes, stressed cells, and tumor cells. Most of circulating γδ T cells use the same T-cell receptor (TCR) V region pair Vγ9-Vδ2, which enables them to recognize unprocessed nonpeptide compounds, which are referred to as phosphoantigens and produced via the mevalonate or the 1-deoxy-D-xylulose-5-phosphate pathway in mammalian1 and nonmammalian cells.2 γδ T cells also recognize induced self ligands like stress-inducible MHC class I-related MICA/MICB molecules or complexes comprising ATP-synthase subunits, which are induced or upregulated on the surface of stressed cells or some tumor cells.3 This peculiar antigen recognition ability is the basis of the putative role of γδ T cells in tumor immunosurveillance.
Aminobisphosphonates (ABP) are synthetic compounds commonly used to treat bone disease and hypercalcemia in patients with multiple myeloma and breast and prostate cancer.4 Interestingly, ABP have also been shown to activate γδ T cells in vitro and in vivo.5-7 We and others have demonstrated that they induce the activation of γδ T cells via monocytes (Mo).6,8 One interpretation is that ABP share the chemical features of γδ T-cell ligands, but the prevailing view is that they specifically target the mevalonate pathway of Mo and induce the accumulation of phosphorylated metabolites naturally recognized by γδ T cells.6,9,10 Dendritic cells (DCs) are much more efficient than Mo as professional antigen-presenting cells to activate conventional αβ T cells and act as cellular bridges between innate and adaptive immunity.11 A reciprocal and positive influence has recently been described between DC and innate effector cells, including γδ T cells.11 However, whereas a few articles have investigated the effects of activated γδ T cells on DCs,12,13 very limited data are available on the opposite interaction with special regard to the ability of DCs to modulate immune responses mediated by conventional αβ T cells. To this end, we have generated highly purified immature DCs (iDCs) and mature DCs (mDCs) from peripheral blood Mo of healthy donors, and evaluated the effect of short-term incubation with zoledronic acid (Zol) on their phenotypic and functional properties. Our results demonstrate that Zol improves the immunostimulatory ability of iDC and mDC toward unconventional γδ and conventional αβ T cells.
Materials and methods
Blood samples were drawn from healthy donors with informed consent in accordance with the Declaration of Helsinki. The study and the consent form were approved by the Comitato Etico Regionale (Piedmont Ethical Committee).
iDC and mDC generation
iDCs were generated from positively isolated CD14+ cells in 18 healthy donors, as previously reported.14 Briefly, CD14+ cells were purified using CD14 MicroBeads and LS columns, according to the manufacturer's instructions (Miltenyi Biotec, Bologna, Italy; purity > 90%). The standard culture medium was RPMI 1640 (Euroclone, Milano, Italy), containing 10% fetal calf serum (Euroclone), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. CD14+ cells were cultured at 2 × 106/mL in standard medium supplemented with 550 IU/mL recombinant human (rh) GM-CSF (Mielogen 300 Schering-Plough) and 500 IU/mL rh IL-4 (PeproTech EC Ltd, London, United Kingdom) in flat-bottomed microtiter plates (Costar, Cambridge, MA) for 6 days. Fresh cytokines were added on day 3. On day 6, the resulting cells were harvested and confirmed as iDC by morphology and immunophenotype. Contaminating CD3+ and γδ T cells in iDC preparations were less than 2% and 0.5% respectively, as determined by flow cytometry. iDCs were harvested on day 6 and incubated for 24 hours in the presence (iDCZol+) or absence of Zol (iDCZol−), kindly provided by Novartis Pharma, Origgio, Italy.
mDCs were generated by 24-hour stimulation of iDCZol− with IL-1β (100 ng/mL; Alexis Biochemicals, Vinci, Italy) and TNFα (50 ng/mL; PeproTech, Rocky Hill, United Kingdom) in the presence (mDCZol+) or absence of Zol (mDCZol−). In some experiments, iDCs or mDCs were incubated for 24 hours in the presence of Zol and 25 μM mevastatin (Mev) (Sigma-Aldrich, Milan, Italy).
iDCZol+, iDCZol−, mDCZol+, and mDCZol− were scored for cell number and viability by trypan blue staining. Supernatants were collected and stored at −80°C until cytokine contents were assessed.
Cytofluorimetric analyses
Immunophenotyping of DCs and cell subsets was performed with the following monoclonal antibodies: anti-CD3, anti-CD8, anti-CD54, anti-CD62L, anti-CXCR4 (Caltag Laboratories, Burlingame, CA), anti-pan-TCR-γδ (Endogen, Woburn, MA); anti-TCR-αβ, anti-CD56, anti-CD80, anti-HLA-DR (Becton Dickinson, San Jose, CA); anti-CD3, anti-CD14 (Dako SpA, Milano, Italy); anti-Vγ9TCR, anti-CD83 (BD Pharmingen International, San Diego, CA), anti-CD86 (Chemicon, Chandlers Ford, Hampshire, United Kingdom), anti-HLA-A2 (Proimmune, Oxford, United Kingdom), anti-CD1a (Valter Occhiena, Torino, Italy), anti-CD11a (Diaclone, Besancòn, France), anti-CD27 (Ancell, Byport, MN), anti-CCR7 (R&D Systems, Minneapolis, MN), and anti-DC-LAMP (Immunotech, Marseille, France). Appropriate combinations of fluorescein isothiocyanate (FITC), r-phycoerythrin–conjugated, Tri color-conjugated, or allophycocyanin-conjugated antibodies were used.
Quantification of apoptotic and necrotic cells was performed by annexin V and propidium iodide staining with the MEBCYTO-Apoptosis Kit (MBL Medical and Biological Laboratories, Naka-ku Nagoya, Japan).
The internalization capability of iDCZol+ and iDCZol− was tested using FITC-conjugated dextran (FITC-dextran; 10 Kda; Sigma-Aldrich), as previously reported.14
Flow cytometry was conducted with a FACScan cell sorter and CELLQuest software (Becton Dickinson, Mountain View, CA). Total counts of specific cell subsets per well were determined by multiplying total counts of viable cells per well by the percentage of cells of interest, the latter being identified by 2- or 3-color cytofluorimetric analysis and appropriate gating.
Quantification of cytokine production
Quantification IL-4, IL-6, IL-10, and IL-12p70 in the supernatants of iDCZol+, iDCZol−, mDCZol+, and mDCZol− was performed with a multiparametric cytometric bead immunoassay using the Basic FlowCytomix and the Simplex kits (Bender MedSystems GmbH, Vienna, Austria).
Allostimulatory capacity of iDCZol+ and mDCZol+
iDCZol+ and mDCZol+ were incubated in triplicate with allogeneic peripheral blood lymphocytes (allo-PBL) at a ratio of 1:5. On day 5, proliferation was evaluated by pulsing cells with 1 μCi of methyl-[3H]thymidine ([3H]TdR) (47 Ci/mmol specific activity; Amersham, Milano, Italy) per well and harvesting 4 hours later with an automated sample harvester (Packard Instrument) using UNIfilter plates (Perkin Elmer, Wellesley, MA). [3H]TdR uptake was measured with a TopCount microplates scintillation counter (Perkin Elmer).
γδ T-cell stimulatory activity of iDCZol+, mDCZol+, and MoZol+
Autologous PBL were added to iDC/mDC subsets at the DC:PBL ratio of 1:5, and cultured at 1 × 106/mL, in standard medium supplemented with IL-2 (10 U/mL; Eurocetus, Milan, Italy). After 7 days, percentages and total counts of αβ and γδ T cells were determined by flow cytometry. Side-by-side experiments were also performed to compare Zol-treated Mo (MoZol+) with iDCZol+ and mDCZol+. To this end, CD14+ were magnetically purified from cryopreserved peripheral blood mononuclear cells (PBMCs) of the individuals from whom iDC or mDC were generated, and incubated for 24 hours with Zol before mixing with autologous PBL as described.
In 3 experiments, PBL were labeled with 5 μM carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR), according to the manufacturer's instructions. CFSE-labeled PBL were washed once and incubated with iDCZol+ or mDCZol+ as reported. On day 7, cells were surface-stained with anti-TCRγδ mAb and proliferation was determined by evaluating the logarithmic decrease of CFSE fluorescence intensity after gating on γδ T cells.
Modulation of adaptive immune responses
iDCs were generated from CD14+ cells of HLA-A*0201+ healthy donors and pulsed with the HLA-A*0201+ restricted influenza matrix protein-derived peptide GILGFVFTL (MP; Proimmune, Oxford, United Kingdom) at 10 μM final concentration for 2 hours at room temperature in serum-free medium (iDCMP+).15 After washing, aliquots of iDCMP+ or unpulsed iDC (iDCMP−) were induced to fully mature by incubation for 18 hours with TNFα + IL-1β in the presence or absence of 5 μM Zol. Thus, 4 subsets of mDC were available on day 7: mDCMP−Zol−, mDCMP+Zol−, mDCMP−Zol+, and mDCMP+Zol+. On the same day, CD3+ cells were isolated from autologous cryopreserved PBMCs (Miltenyi Pan T Cell Isolation Kit), according to the manufacturer's instructions. Purity of CD3+ cells (always > 90%) and percentages of γδ T cells were determined by flow cytometry. mDC subsets and autologous T cells were incubated for 10 days at the DC:T ratio of 1:10 in 96-well round-bottom plates, in the presence of 10 IU/mL IL-2, which was replenished every 3 days. On day 10, T cells were restimulated for additional 10 days with a second batch of freshly generated appropriate mDC subset.
On days 10 and 20, the frequency of MP-specific CD8+ T cells was determined by flow cytometry with the r-phycoerythrin–labeled A*0201/GILGFVFTL (MP) Pentamer (Proimmune, Oxford, United Kingdom), according to manufacturers' instructions.
On day 20, the cytotoxic activity of T cells generated by 2 rounds of stimulation with mDCMP−Zol−, mDCMP+Zol−, mDCMP−Zol+, or mDCMP+Zol+ was tested against the TAP-deficient HLA-A2+ T2 cell line. This cell line is susceptible to the cytotoxic activity mediated by γδ T cells, as shown by preliminary experiments using PBMCs stimulated for 7 days with Zol plus IL-2.6 Both MP-loaded (T2MP+) and unloaded (T2MP−) T2 cells were used as target cells. The former served as target cells for MP-specific, MHC-restricted, conventional cytotoxic αβ T cells, whereas both T2MP+ and T2MP− cells served as target cells for cytotoxic γδ T cells, which are neither MP-specific nor MHC-restricted. The cytotoxic activity of γδ and αβ T cells was evaluated by flow cytometry based on CFSE staining of T2MP+ and T2MP− target cells and identification of dead cells by propidium iodide staining of CFSE-labeled target cells as previously reported.6,16 This method works as a single-platform assay and does not require cell counting to determine the percentage of killed cells in each well.16
In 2 experiments, T2MP+ cells were incubated with anti-MHC class I A*0201 antibody for 30 minutes at 4°C before washing and mixing with effector cells as reported above.
Statistical analysis
Results are expressed as mean ± SEM. Differences between sample groups were evaluated with the 2-tailed nonparametric Mann-Whitney U test for paired samples or with Fisher exact test for unpaired data. P < .05 was the significance cut-off.
Results
Viability, phenotype, and function of iDCZol+ and mDCZol+ are preserved
The first series of experiments were aimed at determining any toxic effect of short-term incubation with Zol on viability, phenotype, and basic immunostimulatory functions of iDC and mDC.
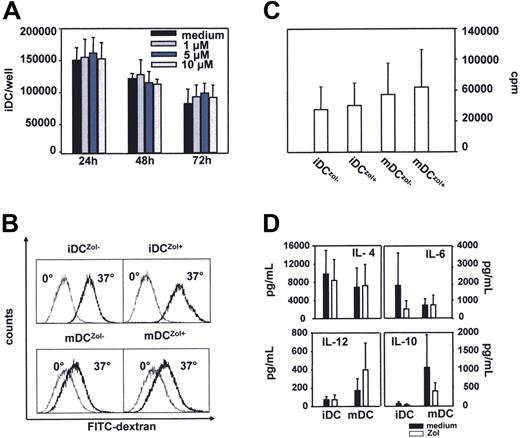
Exposure to Zol up to 10 μM for 72 hours did not affect the viability and total cell counts of iDC (Figure 1A). Based on these and previous results,6 5 μM Zol was selected as the standard treatment concentration. Zol alone did not induce any significant change in the immunophenotype of iDC (Table 1). Rather, a significant increase in the expression of HLA-DR and CD80 was observed in mDC matured with TNF-α plus IL-1β and Zol (mDCZol+) versus TNF-α plus IL-1β alone (mDCZol−) (Table 1).
The immunostimulatory phenotype and functions of iDCZol+ and mDCZol+ are preserved. (A) Dose-response and time course analyses of Zol activity on iDC viability and cell counts. Total counts of viable iDC per well were determined by annexin-V and propidium iodide staining. Bars represent the mean ± SEM of 3 experiments. Results indicate that iDC exposure up to 10 μM Zol for 72 hours does not exert any direct cytotoxic activity. (B) Zol treatment does not affect (1) the capability of iDC to internalize FITC-dextran (iDCZol− 49 ± 17% versus iDCZol+ 53 ± 11%; n = 3, P > .05); and (2) the downregulation of FITC-dextran uptake of mDC (mDCZol− 17 ± 6% versus mDCZol+ 15 ± 3%, n = 3, P > .05). iDCZol−, iDCZol+, mDCZol−, and mDCZol+ were incubated with FITC-dextran for 2 hours at 4°C (dotted light line histograms) or 37°C (solid bold line histograms). mDCZol− and mDCZol+ were matured with TNF-α and IL-1β. Results are 1 of 3 (mDC) to 6 (iDC) experiments. (C) Zol treatment does not affect the allostimulatory activity of iDCZol+ and mDC Zol+. iDC and mDC subsets were incubated for 5 days with allogeneic PBL cells (allo-PBL). Proliferation was determined on day 5 by 3HTdR incorporation. Bars represent the mean (± SEM) of 3 head-to-head experiments. The proliferation rates of allo-PBL stimulated with iDCZol+ and mDCZol+ were slightly higher than those induced by iDCZol− and mDCZol−, but differences are not statistically signficant (iDCZol− 48 600 ± 25 700 cpm/well versus iDCZol+ 52 900 ± 25 800; mDCZol− 65 300 ± 35 800 cpm/well versus mDCZol+ 73 500 ± 42 800; P > .05). (D) Release of IL-4, IL-6, IL-10, and IL-12 in the supernatants of iDCZol−, iDCZol+, mDCZol−, and mDCZol+. Cytokines were measured with a multiparametric cytometric bead immunoassay and results are expressed as pg/mL. Bars represent the mean (± SEM) of 4 experiments. Closed bars represent iDCZol− and mDCZol−, open bars represent iDCZol+ and mDCZol+. mDCZol− and mDCZol+ were matured with TNF-α and IL-1β. IL-10 production was decreased in iDCZol+, whereas IL-12 was increased and IL-6 was decreased in mDCZol+. None of these differences reached a statistical significance.
The immunostimulatory phenotype and functions of iDCZol+ and mDCZol+ are preserved. (A) Dose-response and time course analyses of Zol activity on iDC viability and cell counts. Total counts of viable iDC per well were determined by annexin-V and propidium iodide staining. Bars represent the mean ± SEM of 3 experiments. Results indicate that iDC exposure up to 10 μM Zol for 72 hours does not exert any direct cytotoxic activity. (B) Zol treatment does not affect (1) the capability of iDC to internalize FITC-dextran (iDCZol− 49 ± 17% versus iDCZol+ 53 ± 11%; n = 3, P > .05); and (2) the downregulation of FITC-dextran uptake of mDC (mDCZol− 17 ± 6% versus mDCZol+ 15 ± 3%, n = 3, P > .05). iDCZol−, iDCZol+, mDCZol−, and mDCZol+ were incubated with FITC-dextran for 2 hours at 4°C (dotted light line histograms) or 37°C (solid bold line histograms). mDCZol− and mDCZol+ were matured with TNF-α and IL-1β. Results are 1 of 3 (mDC) to 6 (iDC) experiments. (C) Zol treatment does not affect the allostimulatory activity of iDCZol+ and mDC Zol+. iDC and mDC subsets were incubated for 5 days with allogeneic PBL cells (allo-PBL). Proliferation was determined on day 5 by 3HTdR incorporation. Bars represent the mean (± SEM) of 3 head-to-head experiments. The proliferation rates of allo-PBL stimulated with iDCZol+ and mDCZol+ were slightly higher than those induced by iDCZol− and mDCZol−, but differences are not statistically signficant (iDCZol− 48 600 ± 25 700 cpm/well versus iDCZol+ 52 900 ± 25 800; mDCZol− 65 300 ± 35 800 cpm/well versus mDCZol+ 73 500 ± 42 800; P > .05). (D) Release of IL-4, IL-6, IL-10, and IL-12 in the supernatants of iDCZol−, iDCZol+, mDCZol−, and mDCZol+. Cytokines were measured with a multiparametric cytometric bead immunoassay and results are expressed as pg/mL. Bars represent the mean (± SEM) of 4 experiments. Closed bars represent iDCZol− and mDCZol−, open bars represent iDCZol+ and mDCZol+. mDCZol− and mDCZol+ were matured with TNF-α and IL-1β. IL-10 production was decreased in iDCZol+, whereas IL-12 was increased and IL-6 was decreased in mDCZol+. None of these differences reached a statistical significance.
Immunophenotype of iDCZol+ and mDCZol+
| . | iDCZol−* . | iDCZol+ . | mDCZol−† . | mDCZol+ . |
|---|---|---|---|---|
| HLA-DR | 680 ± 283 | 622 ± 249 | 1.287 ± 586 | 1.698 ± 910‡ |
| CD86 | 78 ± 25 | 142 ± 55 | 113 ± 30 | 121 ± 30 |
| CD80 | 235 ± 57 | 246 ± 55 | 263 ± 57 | 349 ± 85‡ |
| CD54 | 1.617 ± 341 | 1.685 ± 487 | 2.219 ± 780 | 2.168 ± 520 |
| CD83 | 82 ± 23 | 59 ± 15 | 139 ± 69 | 121 ± 46 |
| CD11a | 116 ± 58 | 113 ± 55 | 121 ± 48 | 619 ± 517 |
| CCR7 | 367 ± 35 | 368 ± 13 | 356 ± 36 | 317 ± 25 |
| CXCR4 | 132 ± 58 | 128 ± 57 | 136 ± 66 | 136 ± 72 |
| DC-LAMP | 58 ± 2 | 54 ± 5 | 78 ± 10 | 88 ± 2 |
| . | iDCZol−* . | iDCZol+ . | mDCZol−† . | mDCZol+ . |
|---|---|---|---|---|
| HLA-DR | 680 ± 283 | 622 ± 249 | 1.287 ± 586 | 1.698 ± 910‡ |
| CD86 | 78 ± 25 | 142 ± 55 | 113 ± 30 | 121 ± 30 |
| CD80 | 235 ± 57 | 246 ± 55 | 263 ± 57 | 349 ± 85‡ |
| CD54 | 1.617 ± 341 | 1.685 ± 487 | 2.219 ± 780 | 2.168 ± 520 |
| CD83 | 82 ± 23 | 59 ± 15 | 139 ± 69 | 121 ± 46 |
| CD11a | 116 ± 58 | 113 ± 55 | 121 ± 48 | 619 ± 517 |
| CCR7 | 367 ± 35 | 368 ± 13 | 356 ± 36 | 317 ± 25 |
| CXCR4 | 132 ± 58 | 128 ± 57 | 136 ± 66 | 136 ± 72 |
| DC-LAMP | 58 ± 2 | 54 ± 5 | 78 ± 10 | 88 ± 2 |
Results are mean fluorescence intensity (MFI) plus or minus the SEM from 10 (HLA-DR) to 3 (DC-LAMP) experiments.
iDCs were generated from peripheral monocytes by incubation for 6 days with IL-4 and granulocyte-macrophage colony-stimulating factor as reported in “Materials and methods.” On day 6, iDCs were harvested and incubated for 24 hours in the presence (iDCZol+) or absence of 5 μM Zol (iDCZol−).
mDCs were generated by incubation of iDCs with TNF-α and IL-1β for 24 hours in the presence (mDCZol+) or absence (mDCZol−) of 5 μM Zol. DC-LAMP expression is determined after 48 hours of incubation.
Difference statistically significant between mDCZol− and mDCZol+ (both P < .05).
The ability of iDC to internalize FITC-dextran was not affected, confirming that Zol alone is unable to induce the maturation of iDC (Figure 1B). As expected, mDC downregulated their ability to internalize FITC-dextran, and this downregulation was unaffected by Zol (Figure 1B), indicating that mDC can fully mature and maintain this status in the presence of Zol.
The allostimulatory capacity of iDCZol+ and mDCZol+ was slightly increased compared with iDCZol− and mDCZol−, even if the differences were not statistically different (Figure 1C).
The release of IL-6, IL-10, IL-4, and IL-12 in the supernatants by iDCZol+ and mDCZol+ is shown in Figure 1D. IL-10 production was decreased in iDCZol+, whereas IL-12 was increased, and IL-6 decreased, in mDCZol+. None of these differences was statistically significant. More importantly, these changes did not invalidate the intrinsic ability of iDCs and mDCs to drive Th1 immune responses, as shown by the results of pentamer and cytotoxic experiments reported (Figure 5A-C).
Altogether, these data indicate that Zol has no toxic effect and does not hamper the basic phenotype and immunostimulatory functions of iDC and mDC.
iDCZol+ and mDCZol+ are strong inducers of autologous γδ T-cell proliferation
Next, we evaluated the ability of iDCZol+ and mDCZol+ to activate autologous γδ T cells. Neither iDCZol− nor mDCZol− induced the expansion of autologous γδ T cells, whereas both iDCZol+ and mDCZol+ were strong inducers (Figure 2A). MoZol+ also induced the proliferation of autologous γδ T cells as previously reported6 (Figure 2A). Total counts of viable γδ T cells per well were significantly increased in iDCZol+ versus iDCZol−, mDCZol+ versus mDCZol− and MoZol+ versus MoZol− (Figure 2B). When the comparison was restricted to side-by-side experiments (n = 4), iDCZol+ and mDCZol+ were confirmed to act as better inducers than MoZol+, although the differences were not statistically significant.
Proliferative expansion of γδ T cells by iDCZol+, mDCZol+, and MoZol+. (A) Flow cytometry of representative γδ T-cell expansions after 7 days stimulation with iDCZol+ (upper quadrants), mDCZol+ (middle quadrants), and MoZol+ (lower quadrants). mDCZol+ were matured with TNF-α and IL-1β. Results are from 1 representative out of 11 (iDC) to 4 (Mo) experiments. (B) Total counts of viable cells, γδ T cells, and αβ T cells per well after 7 days of stimulation with different iDCs, mDCs, and Mo subsets. Bars represent the mean (± SEM) of 11 (iDCs) to 4 (mDCs) experiments. Side-by-side experiments (n = 4) and unpaired experiments are pooled together. Total counts of γδ T cells are significantly higher in iDCZol+ versus iDCZol− (133 000 ± 44 000 versus 14 000 ± 4200; n = 11, P < .001), mDCZol+ versus mDCZol− (124 000 ± 41 000 versus 21 700 ± 6000; n = 6, P < .005), and MoZol+ versus MoZol− (104 000 ± 40 000 versus 21 000 ± 9000; n = 4, P < .05). When the comparison is restricted to side by side experiments, differences are not statistically different between iDCZol+ (285 000 ± 96 000 γδ T cells/well), mDCZol+ (250 000 ± 67 000 γδ T cells/well) and MoZol+ (103 000 ± 40 000 γδ T cells/well). Differences among total counts of αβ T cells are also not statistically different (iDCZol−: 208 000 ± 36 000 αβ T cells/well; iDCZol+: 188 000 ± 33 000 αβ T cells/well; mDCZol−: 189 000 ± 43 000 αβ T cells/well, mDCZol+: 177 000 ± 33 000 αβ T cells/well; MoZol−: 152 000 ± 54 000 αβ T cells/well, MoZol+: 146 000 ± 39 000 αβ T cells/well). P > .05).
Proliferative expansion of γδ T cells by iDCZol+, mDCZol+, and MoZol+. (A) Flow cytometry of representative γδ T-cell expansions after 7 days stimulation with iDCZol+ (upper quadrants), mDCZol+ (middle quadrants), and MoZol+ (lower quadrants). mDCZol+ were matured with TNF-α and IL-1β. Results are from 1 representative out of 11 (iDC) to 4 (Mo) experiments. (B) Total counts of viable cells, γδ T cells, and αβ T cells per well after 7 days of stimulation with different iDCs, mDCs, and Mo subsets. Bars represent the mean (± SEM) of 11 (iDCs) to 4 (mDCs) experiments. Side-by-side experiments (n = 4) and unpaired experiments are pooled together. Total counts of γδ T cells are significantly higher in iDCZol+ versus iDCZol− (133 000 ± 44 000 versus 14 000 ± 4200; n = 11, P < .001), mDCZol+ versus mDCZol− (124 000 ± 41 000 versus 21 700 ± 6000; n = 6, P < .005), and MoZol+ versus MoZol− (104 000 ± 40 000 versus 21 000 ± 9000; n = 4, P < .05). When the comparison is restricted to side by side experiments, differences are not statistically different between iDCZol+ (285 000 ± 96 000 γδ T cells/well), mDCZol+ (250 000 ± 67 000 γδ T cells/well) and MoZol+ (103 000 ± 40 000 γδ T cells/well). Differences among total counts of αβ T cells are also not statistically different (iDCZol−: 208 000 ± 36 000 αβ T cells/well; iDCZol+: 188 000 ± 33 000 αβ T cells/well; mDCZol−: 189 000 ± 43 000 αβ T cells/well, mDCZol+: 177 000 ± 33 000 αβ T cells/well; MoZol−: 152 000 ± 54 000 αβ T cells/well, MoZol+: 146 000 ± 39 000 αβ T cells/well). P > .05).
Total counts of viable αβ T cells were unaffected by the proliferative expansion of γδ T cells (Figure 2B), indicating that neither a shortage of nutrients nor the production of cytokines, such as IFN-γ, or the expansion of γδ T cells with effector activity had a detrimental effect on conventional αβ T cells. Total γδ and αβ T-cell counts also indicate that the former only accounted for the increased overall cellularity observed after stimulation with iDCZol+, mDCZol+, and MoZol+ (Figure 2B), further confirming that active proliferation, and not a relative enrichment, was the main cause of the selective γδ T-cell expansion. Experiments with CFSE-labeled T cells confirmed the selective proliferation of γδ T cells induced by iDCZol+ and mDCZol+ (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
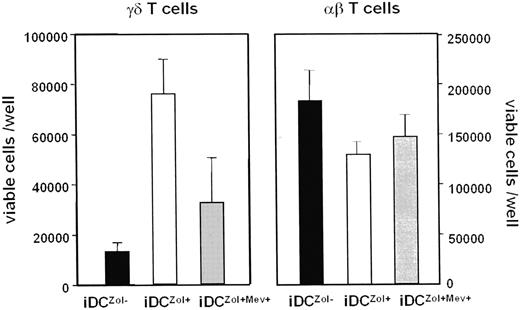
To further demonstrate the ability of Zol to specifically recruit γδ T cells, iDC were incubated with 25 μM Mev immediately before treatment with Zol. iDCZol+Mev+ were then incubated with autologous T cells and total counts of γδ and αβ T cells determined on day 7. The increase of γδ T cells induced by iDCZol+ was blunted by Mev treatment (Figure 3). Mev treatment did not affect αβ T-cell counts. These data further confirm that the immunostimulatory ability of iDCZol+ and mDCZol+ is specifically directed toward γδ T cells and highly dependent on the mevalonate pathway.
The proliferative expansion of γδ T cells induced by iDCZol+ is dependent on the mevalonate pathway. Total counts of viable γδ and αβ T cells after stimulation with iDCZol−, iDCZol+, and iDCZol+Mev+. The former only are significantly increased by iDCZol+ stimulation (see also Figure 2B). This increase is blunted by blocking the mevalonate pathway with Mev (iDCZol+ 76 000 ± 14 000; iDCZol + Mev+ 33 000 ± 18 000; P < .02). Mev treatment does not affect αβ T-cell counts. Error bars represent mean ± SEM.
The proliferative expansion of γδ T cells induced by iDCZol+ is dependent on the mevalonate pathway. Total counts of viable γδ and αβ T cells after stimulation with iDCZol−, iDCZol+, and iDCZol+Mev+. The former only are significantly increased by iDCZol+ stimulation (see also Figure 2B). This increase is blunted by blocking the mevalonate pathway with Mev (iDCZol+ 76 000 ± 14 000; iDCZol + Mev+ 33 000 ± 18 000; P < .02). Mev treatment does not affect αβ T-cell counts. Error bars represent mean ± SEM.
Zol-treated iDCs and mDCs preferentially induce the expansion of central memory and effector memory γδ T cells with enhanced expression of specific homing and costimulatory receptors
These experiments were performed to determine γδ T-cell subset distribution after stimulation with iDCZol+ and mDCZol+. γδ T cells can be divided into 4 subsets according to their phenotype, proliferative capacity and effector functions. Naive (N: CD45RA+, CD27+) and central memory (CM: CD45RA−, CD27+) γδ T cells display high proliferative capacity, but low effector functions, whereas effector memory (EM: CD45RA−, CD27−) and terminally differentiated late effector (TEMRA: CD45RA+, CD27−) γδ T cells display the opposite pattern.17 Immunophenotyping on day 7 showed that total counts of viable CM and EM γδ T cells per well were significantly increased after stimulation with iDCZol+ compared with iDCZol− (Figure 4A,B).
iDCZol+ induce the expansion of CM and EM γδ T cells with specific homing and costimulatory receptors. (A) Representative dot plots of γδ T-cell subset distribution after 7 days stimulation with iDCZol− and iDCZol+. Four subsets are identified according to the expression of cell surface CD45RA and CD27 antigens after backgating on γδ T cells: naive (N: CD45RA+, CD27+), central memory (CM: CD45RA−, CD27+), effector memory (EM: CD45RA−, CD27−), and terminally differentiated late effector cells (TEMRA: CD45RA+, CD27−). (B) Total counts of γδ T-cell subsets after 7 days stimulation with iDCZol− and iDCZol+. Bars represent the mean ± SEM of 5 experiments. Differences between CM and EM γδ T cells are statistically significant (CM γδ T cells: iDCZol− 3600 ± 1400 versus iDCZol+ 54 000 ± 20 000; P < .03; EM γδ T cells: iDCZol− 4700 ± 2100 versus iDCZol+ 28 000 ± 11 000; P < .04). (C) Expression of homing receptors (CD62L) and costimulatory molecules (HLA-DR, CD80) on the surface of γδ T cells after stimulation with iDCZol+. Results are from 1 of 5 experiments. (D) Total counts of viable CD62L+, CD80+, and HLA-DR+ γδ T cells after stimulation with iDCZol+. Bars represent the mean (± SEM) of 5 experiments. Total counts of viable CD62L+ γδ T cells per well are significantly increased (CD62L+ γδ T cells: iDCZol− 13 500 ± 5300 versus iDCZol+ 62 000 ± 9000; P < .005; HLA-DR+ γδ T cells: iDCZol− 12 000 ± 4000 versus iDCZol+ 78 400 ± 21 000; P > .05; CD80+ γδ T cells: iDCZol− 4000 ± 1200 versus iDCZol+ 36 000 ± 16 000; P > .05).
iDCZol+ induce the expansion of CM and EM γδ T cells with specific homing and costimulatory receptors. (A) Representative dot plots of γδ T-cell subset distribution after 7 days stimulation with iDCZol− and iDCZol+. Four subsets are identified according to the expression of cell surface CD45RA and CD27 antigens after backgating on γδ T cells: naive (N: CD45RA+, CD27+), central memory (CM: CD45RA−, CD27+), effector memory (EM: CD45RA−, CD27−), and terminally differentiated late effector cells (TEMRA: CD45RA+, CD27−). (B) Total counts of γδ T-cell subsets after 7 days stimulation with iDCZol− and iDCZol+. Bars represent the mean ± SEM of 5 experiments. Differences between CM and EM γδ T cells are statistically significant (CM γδ T cells: iDCZol− 3600 ± 1400 versus iDCZol+ 54 000 ± 20 000; P < .03; EM γδ T cells: iDCZol− 4700 ± 2100 versus iDCZol+ 28 000 ± 11 000; P < .04). (C) Expression of homing receptors (CD62L) and costimulatory molecules (HLA-DR, CD80) on the surface of γδ T cells after stimulation with iDCZol+. Results are from 1 of 5 experiments. (D) Total counts of viable CD62L+, CD80+, and HLA-DR+ γδ T cells after stimulation with iDCZol+. Bars represent the mean (± SEM) of 5 experiments. Total counts of viable CD62L+ γδ T cells per well are significantly increased (CD62L+ γδ T cells: iDCZol− 13 500 ± 5300 versus iDCZol+ 62 000 ± 9000; P < .005; HLA-DR+ γδ T cells: iDCZol− 12 000 ± 4000 versus iDCZol+ 78 400 ± 21 000; P > .05; CD80+ γδ T cells: iDCZol− 4000 ± 1200 versus iDCZol+ 36 000 ± 16 000; P > .05).
Ag-specific immune responses mediated by αβ T lymphocytes are improved by the concurrent activation of γδ T cells
The last series of experiments was carried out to determine whether Zol treatment was beneficial or detrimental to the ability of mDC to generate Ag-specific immune responses mediated by conventional αβ T cells.
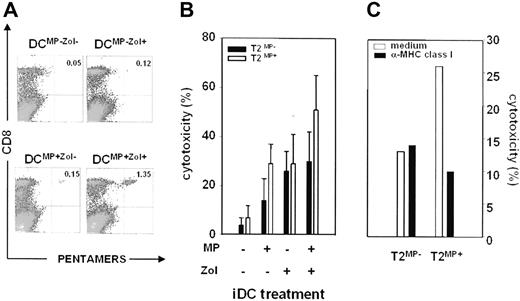
First, we evaluated the frequency of A*0201-restricted MP-specific CD8+ αβ T cells after stimulation with mDCMP−Zol−, mDCMP+Zol−, mDCMP−Zol+, and mDCMP+Zol+. The highest frequency was observed after stimulation with mDCMP+Zol+, both after the first (day 10; Figure 5A) and the second round (day 20) of stimulation (one additional experiment is shown in Figure S2).
Modulation of antigen-specific immune responses by mDCZol+. (A) Detection of MP-specific CD8+ cells by pentamer staining after 10 days of stimulation of autologous T cells with different mDC subsets (mDCMP−Zol−, mDCMP+Zol−, mDCMP−Zol+, and mDCMP+Zol+). MP-specific CD8+ cells are identified by APC-CD8 and pentamer–r-phycoerythrin staining after backgating on viable T cells. The highest frequency is observed after stimulation with mDCMP+Zol+. Representative dot plots are from 1 of 2 experiments. Values in the top right quadrants represent the frequency of MP-specific CD8+ cells in the T lymphocyte population. (B) Cytotoxic activity exerted by T cells against T2MP− (■) and T2MP+ cells (□) after 2 rounds of stimulation with autologous mDCMP−Zol−, mDCMP+Zol−, mDCMP−Zol+, and mDCMP+Zol+. Bars represent the mean (± SEM) of 3 experiments and refer to an effector:target ratio of 1, 3:1. The cytotoxic activity of T cells stimulated with mDCMP+Zol− is higher than after stimulation with mDCMP−Zol−, indicating the generation of MP-specific cytotoxic CD8+ T cells. Cytotoxicity after stimulation with mDCMP−Zol+ is not antigen-specific, because it is similar against T2MP− and T2MP+ cells. Cytotoxicity against T2MP+ cells after stimulation with mDCMP+Zol+ is significantly higher than after stimulation with mDCMP+Zol− (P = .01), indicating that further MP-specific cytotoxicity is generated under these conditions. Cytotoxicity against T2MP− cells, which is similar after stimulation with mDCMP−Zol+ and mDCMP+Zol+, represents the nonantigen-specific background mediated by γδ T cells. (C) Cytotoxic activity detected after stimulation with mDCMP+Zol+ is partly abrogated by anti-MHC class I antibodies. T2MP− and T2MP+ cells were incubated with anti-MHC class I antibodies for 30 minutes before mixing with effector cells. Results are from 1 of 2 experiments. Bars represent cytotoxic values at an effector:target ratio of 1, 3:1.
Modulation of antigen-specific immune responses by mDCZol+. (A) Detection of MP-specific CD8+ cells by pentamer staining after 10 days of stimulation of autologous T cells with different mDC subsets (mDCMP−Zol−, mDCMP+Zol−, mDCMP−Zol+, and mDCMP+Zol+). MP-specific CD8+ cells are identified by APC-CD8 and pentamer–r-phycoerythrin staining after backgating on viable T cells. The highest frequency is observed after stimulation with mDCMP+Zol+. Representative dot plots are from 1 of 2 experiments. Values in the top right quadrants represent the frequency of MP-specific CD8+ cells in the T lymphocyte population. (B) Cytotoxic activity exerted by T cells against T2MP− (■) and T2MP+ cells (□) after 2 rounds of stimulation with autologous mDCMP−Zol−, mDCMP+Zol−, mDCMP−Zol+, and mDCMP+Zol+. Bars represent the mean (± SEM) of 3 experiments and refer to an effector:target ratio of 1, 3:1. The cytotoxic activity of T cells stimulated with mDCMP+Zol− is higher than after stimulation with mDCMP−Zol−, indicating the generation of MP-specific cytotoxic CD8+ T cells. Cytotoxicity after stimulation with mDCMP−Zol+ is not antigen-specific, because it is similar against T2MP− and T2MP+ cells. Cytotoxicity against T2MP+ cells after stimulation with mDCMP+Zol+ is significantly higher than after stimulation with mDCMP+Zol− (P = .01), indicating that further MP-specific cytotoxicity is generated under these conditions. Cytotoxicity against T2MP− cells, which is similar after stimulation with mDCMP−Zol+ and mDCMP+Zol+, represents the nonantigen-specific background mediated by γδ T cells. (C) Cytotoxic activity detected after stimulation with mDCMP+Zol+ is partly abrogated by anti-MHC class I antibodies. T2MP− and T2MP+ cells were incubated with anti-MHC class I antibodies for 30 minutes before mixing with effector cells. Results are from 1 of 2 experiments. Bars represent cytotoxic values at an effector:target ratio of 1, 3:1.
Next, we tested the ability of effector T cells generated by 2 rounds of stimulation to exert cytotoxic activity against T2MP+ or T2MP− cells. Results are shown in Figure 5B. mDCMP−Zol− did not induce any cytotoxic activity against T2MP+ or T2MP− cells. As expected, mDCMP+Zol− generated MP-specific cytotoxic responses against T2MP+ cells only, mediated by conventional CD8+ αβ T cells, but did not induce the expansion of γδ T cells. mDCMP−Zol+ induced the expansion of γδ T cells and generated comparable responses against both T2MP+ and T2MP− cells, indicating lack of MHC restriction and MP specificity. Interestingly, the cytotoxic activity mediated by CD8+ αβ T cells against T2MP+ cells was significantly increased after stimulation with DCMP+Zol+ compared with that observed in the absence of γδ T-cell expansion (ie, after activation by mDCMP + Zol−; effector target [E/T] ratio 1,3:1; 51 ± 13% versus 29 ± 12%; P = .01). On the contrary, cytotoxicity against T2MP− cells was similar after stimulation with mDCMP+Zol+ and mDCMP−Zol+, representing the nonantigen specific background mediated by γδ T cells.
Finally, 2 experiments were performed in which T2MP+ cells were incubated with anti-MHC class I antibody before mixing with effector cells stimulated with mDCMP + Zol+. One representative experiment is shown in Figure 5C, showing that the cytotoxicity against T2MP+ cells was partly abrogated by anti-MHC class I antibody. Thus, MP-specific CD8+ αβ T cells are generated under these conditions and contribute to the cytotoxic response against T2MP+ cells.
In conclusion, iDC can be concurrently loaded with antigens specific for αβ and γδ T cells and effectively induce the activation of both subsets. The largely predominant activation of γδ T cells does not overwhelm, but rather it has beneficial effects on the generation of antigen-specific cytotoxic responses mediated by αβ T cells.
Discussion
The aim of this work was to determine whether short-term incubation with Zol had any impact on the immunostimulatory properties of iDC, with special regard to their ability to activate autologous γδ T cells and modulate antigen-specific immune responses mediated by αβ T cells. Cell count and viability of iDC as well as their subsequent differentiation into mDC, induced by TNFα and IL-1β, were not affected by Zol exposure up to 10 μM for 72 hours. Similar results have been reported by Wolf et al.19 On the contrary, Zol did affect the generation of iDC, as previously shown by von Lilienfeld-Toal et al20 and Wolf et al.19 However, these experiments were performed with Mo incubated with Zol for 3 days during their differentiation process into iDC, and not with freshly generated iDC as in our case.
Zol alone was unable to induce iDC maturation, as shown by the very similar immunophenotype and ability of iDCZol− and iDCZol+ to internalize FITC-dextran. The immunostimulatory phenotype of mDCZol+ was slightly improved, as shown by the increased cell surface HLA-DR and CD80 expression, whereas both mDCZol− and mDCZol+ equally downregulated their ability to internalize FITC-dextran, according to their full maturation status. Curiously, the increased HLA-DR and CD80 expression of mDCZol+ did not translate into an increased allostimulatory activity, as already reported by Wolf et al.19
Last, the pattern of cytokine production by iDCZol+ and mDCZol+ was not statistically different from that of iDCZol− and mDCZol−, even if a trend toward a more favorable cytokine production to drive Th1 immune responses could be envisaged in mDCZol+ (eg, higher and lower production of IL-12 and IL-6, respectively). Altogether, these data indicate that Zol has no toxic effect and does not hamper the basic phenotype and immunostimulatory functions of iDCs and mDCs.
Previous reports have shown that exogenous phosphoantigens, like isopentenyl pyrophosphate and bromohydrin pyrophosphate, mimic the natural ligands of γδ T cells and do not induce any phenotypic change or cytokine modulation in iDCs.12,20,21 However, phosphoantigens and ABP act by very different mechanisms. The former mimic the natural ligands of γδ T cells and are not internalized by iDCs or mDCs, whereas ABP target the intracellular mevalonate pathway by blocking the farnesyl pyrophosphate synthase. Among ABP, Zol is approximately 100-fold more potent than pamidronate in blocking the farnesyl pyrophosphate synthase.22 Thus, it is not surprising that ABP induce a deeper immunomodulation than exogenously added phosphoantigens, nor that Zol is more active than pamidronate.
Next, we have evaluated the ability of iDCZol+ and mDCZol+ to activate autologous γδ T cells. So far, very few studies have investigated the ability of highly purified iDCs and mDCs to activate such cells after treatment with phosphoantigens or ABP. We used resting autologous PBL or purified T cells as a source of γδ T cells rather than γδ T-cell clones or purified γδ T cells, because we wished to investigate both the reciprocal interaction between DCs and γδ T cells, and its effect on the ability of DCs to induce antigen-specific immune responses mediated by conventional αβ T cells. iDCZol+ and mDCZol+ very efficiently induced the proliferative expansion of autologous γδ T cells. Zol was the main cause of γδ T-cell activation because: (1) iDCZol− and mDCZol− were totally ineffective; (2) iDC and mDC subsets were extensively washed before adding autologous PBL or purified T cells; (3) both PBL and T cells were devoid of CD14+ cells potentially targetable by residual Zol, if any; and (4) the immunostimulatory ability of iDCZol+ and mDCZol+ was abrogated by Mev, indicating that targeting the mevalonate pathway of iDC and mDC is an essential prerequisite to recruit γδ T cells, as previously reported in Mo and tumor cells.6,9,10
So far, only von Lilienfeld-Toal et al20 have investigated and failed to show that ABP-treated iDCs induce the proliferation of γδ T cells. However, these experiments were performed with positively selected γδ T cells, fully activated by anti-CD3 mAb and high doses of IL-2 and IL-1β, and proliferation was evaluated 3 days only after coculturing with iDC. Given the strong γδ T-cell baseline activation status and the short incubation time, it is not surprising that ABP-treatment of iDC did not significantly affect the proliferation of γδ T cells. Devilder et al13 did not observe any proliferation after incubation of γδ T-cell clones with iDC or mDC in the presence of bromohydrin pyrophosphate. These results further confirm the inferiority of phosphoantigens to ABP in the ability to specifically prime DCs to induce γδ T-cell activation.
iDCZol+ were slightly better than mDCZol+, and both subsets were better than MoZol+ in inducing the expansion of γδ T cells. This is the first time that these subsets are side-by-side compared. Previous experiments were performed with PBMCs, rather than purified Mo, and cultures were never washed free of Zol.6,10 Devilder et al13 have shown that pamidronate-treated iDCs are superior to mDCs in inducing the expression of intracellular TNFα in clonal or polyclonal γδ T cells. To the best of our knowledge, this is the only other demonstration that ABP specifically prime iDCs and mDCs to activate γδ T cells.
The vigorous γδ T-cell expansion induced by iDCZol+ and mDCZol+ indicates that this strategy is worthy of further investigation as a platform for adoptive cell therapy. It has recently become clear that the successful outcome of adoptive cell therapy very much depends on the differentiation status of the cells infused. CM T cells have more effective antitumor activity in vivo than EM or TEMRA T cells, even though the latter are more potent in vitro.23,24 These data have been validated in vivo with CD8+ cells only, but recent findings indicate that γδ T cells, too, can be subdivided in the same subsets (N, CM, EM, TEMRA) with similar phenotypic and functional properties.6,17 Indeed, iDCZol+ preferentially induced the expansion of CM and EM γδ T cells, which are well-fitted to exert antitumor activity, as shown by their cytotoxic activity against the T2 cell line and other tumor cell lines, as previously reported.6
iDCZol+ and mDCZol+ induced the expansion of γδ T cells expressing homing receptors for secondary lymphoid organs (CD62L+) and costimulatory molecules (HLA-DR+, CD80+). The former are especially expressed at the stage of CM cells25 and enable γδ T cells to migrate to lymph nodes where they interact with DC and other cells to improve the outcome of adaptive immune responses. The expression of costimulatory molecules has been related to the capacity of γδ T cells to act as APC themselves,18 even if these data are controversial and we were not able to confirm the endocytic function of activated γδ T cells. Whether γδ T cells can act as antigen-presenting cells, the phenotype expressed by activated γδ T cells, the well-preserved immunostimulatory functions of iDCZol+ and mDCZol+, and the preserved numbers of conventional αβ T cells prompted us to investigate whether iDC could be primed with the appropriate antigens to simultaneously activate both αβ and γδ T cells. To gain insights into this issue, we pulsed iDCs with MP in the presence or absence of Zol and then induced their differentiation into mDC with TNF-α + IL-1β. Then, we determined the frequency of MP-specific CD8+ T cells and cytotoxic responses against T2MP+ and T2MP− cells. Notably, the highest frequency of MP-specific CD8+ αβ T cells and the highest cytotoxicity against T2MP+ cells were observed after stimulation with mDCMP+Zol+. These results indicate that iDC can be concurrently loaded with antigens specific for αβ and γδ T cells and effectively induce the activation of both subsets. The largely predominant activation of γδ T cells has beneficial effects on the generation of MP-specific CD8+ αβ T cells as shown by the higher frequency of MP-specific CD8+ αβ T cells and higher cytotoxic activity against T2MP+ cells detected after stimulation with mDCMP+Zol+ compared with mDCMP+Zol−. Blocking experiments with anti-MHC class I antibody also indicate that: (1) MP-specific CD8+ αβ T cells are generated after stimulation with mDCMP+Zol+; (2) these cells are not overwhelmed by the much more abundant activated γδ T cells; and (3) they contribute to the bulk cytotoxic activity detected against T2MP+ cells.
The generation of MHC-restricted antigen-specific cytotoxic αβ T cells seemed to draw more benefit from the concurrent γδ T-cell activation than vice versa, as shown by the similar cytotoxic activity against T2MP− cells induced by mDCMP+Zol+ and mDCMP−Zol+. Given the very different frequencies, it is not surprising that the much more abundant activated γδ T cells improve the efficiency of the much less represented MHC-restricted antigen-specific αβ T cells. This is the first report in humans showing that iDCs can be simultaneously primed to activate both γδ and αβ T cells, and that the former act as cellular adjuvants for the development of adaptive immune responses. These data further support the notion that γδ T cells are endowed with more sophisticated functions than simply providing a first-line defense against pathogens or self-induced stress antigens in the peripheral tissues. Mouse models have confirmed that γδ T cells do provide help to adaptive immune responses by promoting the formation of germinal centers, the somatic hypermutation of B cells,26 and by positively regulating superantigen-specific immune responses mediated by αβ T cells.27
In conclusion, our results indicate that it is possible to quickly generate large numbers of activated γδ T cells with effector and costimulatory activities by short-term incubation with iDCs/mDCs pulsed with the appropriate antigens. This strategy is worthy of further investigation to improve adoptive cell therapy and vaccine interventions against tumors and infections.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Lorena Passoni from the Oncogenic Fusion Genes and Proteins Unit, Istituto Nazionale per lo Studio e la Cura dei Tumori, Milan, Italy, for the T2 cell line, and to the Blood Bank of Azienda Ospedaliera San Giovanni Battista, Turin, Italy for providing normal buffy coats.
This work was supported by Ministero dell'Università e della Ricerca (MIUR; cofinanziamento [COFIN], Roma, Italy), Compagnia San Paolo di Torino (Torino, Italy), Regione Piemonte (Italy), Fondazione Neoplasie Sangue (Torino, Italy), and by Novartis Farma (Origgio, Italy). F.F. is a fellowship recipient of Fondazione Cassa di Risparmio di Torino (CRT)–Comitato Gigi Ghirotti. B.C. is a fellowship recipient of Fondazione Amici di Jose' Carreras, Torino, Italy.
Authorship
Contribution: M.M. and F.F. designed the study, analyzed the data, and wrote the paper. F.F., B.C., S.M., F.P., and M.F. performed the research and analyzed the data. B.N. assisted in experimental analyses. R.B., B.B., and M.B. helped to design the study and provided critical suggestions.
F.F. and B.C. contributed equally to this work.
Conflict-of-interest disclosure: R.B. is employed by a company whose product (zoledronic acid) was studied in the present work. The other authors declare no competing financial interests.
Correspondence: Massimo Massaia, MD, Divisione di Ematologia dell'Università di Torino, Via Genova 3, 10126 Torino, Italy; e-mail: massimo.massaia@unito.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal