Abstract
Until recently, IgE-activated mast cells have been regarded merely as effector cells of adaptive immune responses, involved in allergic reactions and mucosal immunity to parasites. Herein, we report that murine dermal mast cells, activated by local administration of a cream containing the synthetic TLR7 ligand imiquimod, are essential to initiate an early inflammatory reaction. The mast-cell–derived cytokines TNF-α and IL-1β play an important role in this process. Furthermore, TLR7-activated mast cells are also able to promote the emigration of Langerhans cells, which partly depends on the expression of mast-cell–derived IL-1β. We have previously shown that TLR7 ligation enhances transcutaneous immunization evoked by topical application of vaccine antigens to the skin, a procedure that directly targets skin-resident antigen-presenting cells. Consequently, we now demonstrate here that the capacity to mount a peptide-specific cytotoxic T-lymphocyte response following transcutaneous immunization using imiquimod as adjuvant is severely impaired in mast-cell–deficient mice. Thus, these findings demonstrate the potent versability of alternatively activated mast cells at the interface of innate and adaptive immunity.
Introduction
IgE-dependent reactions including dysregulated allergic responses to environmental antigens and mucosal immunity to some extracellular parasites were believed to be the hallmarks of mast-cell immunology for several decades
We know by now that mast cells, predominantly localized at the interface between host and environment (ie, skin and mucosal surfaces), are in addition able to perceive a variety of allergens and invading pathogens. Based on 2 groundbreaking publications using murine models of acute bacterial infection, mast cells were found to be critical effectors of innate immunity. In these studies, mast-cell–deficient mice were highly susceptible to induced septic peritonitis1 and Klebsiella pneumoniae instillation.2 In these settings, the unique ability of mast cells to secrete preformed TNF-α within minutes following IgE-independent stimulation enables the host to mount an early and protective neutrophil response to bacterial challenge. Evidence further accumulates that mast cells are implicated in host defense against a still increasing range of clinically relevant bacterial infections.3-5 Furthermore, mast cells are also thought to participate in response to viruses, but this is less understood and deserves further research.5,6 With respect to IgE-independent activation, both rodent and human mast cells were found to express a variety of Toll-like receptors, some of which are expressed only on certain mast cell subsets.5-7 However, several murine in vivo models clearly showed the importance of TLR-mediated mast cell activation, for example, for the clearance of enterobacterial infection by triggering TLR48 and for the recruitment of CD8+ T cells into the peritoneal cavity following ligation of TLR3 with poly(I:C), which mimics viral dsRNA.9
It is also increasingly being appreciated that mast cells are able to finely control the magnitude of the secretory response following activation.10 Thus, IgE-independent activation in combination with the aptitude to flexibly respond to a variety of stimuli allow these cells to participate in both adaptive and innate immune responses.11
It was also suggested that mast cells can directly promote the development of adaptive immunity, as it was shown that they can trigger the migration of antigen-presenting cells in several murine models.12-14
Besides their potential influence on antigen-presenting cells, mast cells were also shown to directly promote T-cell proliferation and cytokine secretion. This requires IgE-mediated mast-cell activation, TNF-α produced by mast cells, and T-cell and mast-cell expression of OX40L, triggering the costimulatory molecule OX40 on T cells.15,16 This ability of mast cells seems suitable to enhance the recall reaction of primed T cells locally upon re-encountering the antigen.
However, heretofore it was not yet clear whether mast cells are associated with the development or magnitude of an adaptive T-cell response.
We previously reported that the simple epicutaneous application of a cream containing a cytotoxic T lymphocyte (CTL) epitope in combination with the synthetic TLR7 ligand imiquimod leads to the transport of applied peptide to local lymph nodes (LNs). Consequently, this method is highly effective to mount a full-blown immune response against the target peptide.17 Local epicutaneous treatment with imiquimod (Aldara) as an adjuvant induces both an inflammatory response as well as the migration of Langerhans cells (LCs) out of the epidermis.18 LCs, the dendritic cells (DCs) of the epidermis, are able to cross-present exogenous antigen on major histocompatibility class (MHC) class I molecules to CD8+ cytotoxic T cells in skin-draining lymph nodes.19-21
Herein, we show that both early cutaneous inflammation and migration of LCs are promoted by dermal mast cells activated through TLR7, and we identify mast-cell–derived TNF-α and IL-1 as essential mediators. Most importantly, dermal mast cells are crucial to initiate a CTL response in this model, definitively positioning mast cells at the interface of innate and adaptive immunity.
Materials and methods
Mice
Genetically mast-cell–deficient KitW-sh/W-sh mice and congenic Kit+/+ wild-type littermates were obtained by intercrossing KitW-sh/+ mice kindly provided by Marcus Maurer (Department of Dermatology, Charite, Berlin, Germany). Mice deficient for TNF-α, IL-1 α/β, and TLR7 were obtained from Kerstin Steinbrink (Department of Dermatology, University of Mainz, Germany), Yoichiro Iwakura (Center for Experimental Medicine, University of Tokyo, Japan), and Stefan Bauer (Institute for Immunology, University of Marburg, Germany), respectively. All these mice were on a C57Bl/6 background. Mice homozygous for a bicistronic IL-4 GFP reporter (4get mice) on a BALB/c genetic background were kindly provided by André Gessner (Institute of Clinical Microbiology, Immunology, and Hygiene, University of Erlangen-Nurnberg, Germany). Mice were used at the age of 7 to 8 weeks. Animal procedures were conducted in accordance with the institutional guidelines.
Generation of bone marrow–derived mast cells and reconstitution of mast cell–deficient mice
Bone marrow–derived mast cells (BM–CMCs) from Kit+/+ mice and TNF-α–, TLR7-, and IL-1α/β–deficient mice were generated according to standard procedures.22 KitW-sh/W-sh mice were locally reconstituted by injecting 5 × 105 BMMCs intracutaneously into the ear pinna 6 weeks before the experiments were conducted. To assess reconstitution efficiencies, 5-μm sections of paraffin-embedded ears previously fixed with formaldehyde were used for fluorescence staining of tissue mast cells with avidin-Alexa-488 (Molecular Probes, Leiden, the Netherlands). Avidin is a heparin-binding protein that specifically binds to mast-cell granules.23,24 Mast-cell numbers were counted over the entire ear and expressed as mast cells/mm ear cartilage.
Application of imiquimod, measurement of ear thickness, preparation of epidermal sheets, and lymph node cell suspensions
To induce local inflammation and migration of LCs, one ear of each animal was treated daily with approximately 40 mg Aldara for up to 7 days, and the contralateral ear was treated with vehicle (both from 3M Pharmaceuticals, Neuss, Germany) or was left untreated. Ear swelling following local application of Aldara was measured as ear thickness with a micrometer (Mitutoyo, Neuss, Germany). Aldara contains 5% imiquimod (1-(2-methylpropyl)-1H-imidazo (4,5-C) quinolin-4-amine) in vehicle: isooctadecanoic acid (20%-30%), ethoxylated sorbitan monostearate (1%-5%), octadecan-1-ol (1%-5%), petrolatum (1%-5%), hexadecan-1-ol (1%-5%), glycerol (1%-5%), benzyl alcohol (1%-5%), and water (40%-60%). In vitro, BMMCs were treated with 10 μg/mL imiquimod (Invitrogen, Karlsruhe, Germany) for 16 hours prior to RNA isolation. To prepare epidermal sheets,25 ears were divided into dorsal and ventral halves. The dorsal halves were incubated in 0.5% dispase solution for 20 minutes (Dispase II; Roche, Mannheim, Germany). After incubation, epidermis was peeled off and fixed in acetone. LCs were stained with a mAb against MHC class II (clone M5.114.15.2) and an Alexa594-labeled secondary goat antirat antibody (Molecular Probes). All washing steps were done in PBS/0.5% BSA. LC numbers were determined by fluorescence microscopy using CellR imaging software (Olympus, Hamburg, Germany) by counting at least 3 independent areas of 0.3 × 0.4 mm2 each.
Alternatively, LCs in epidermal sheets were stained with anti-Langerin antibody. In agreement with a recent report, stainings with either anti-MHCII or anti-Langerin were identical (data not shown), as all MHCII-positive cells in murine epidermis were shown to coexpress Langerin.26
LN cell suspensions were generated by collagenase digestion as described previously27 and immunostained using APC-conjugated anti-MHCII, PE-conjugated anti–PD-L1, clone MIH5 (both from eBioscience, San Diego, CA) and Alexa488-conjugated anti-Langerin (clone 923F3 from AbCys).
Isolation of dermal mast cells
It was recently shown that dermal and serosal mast cells in naive mice expressing a bicistronic IL-4/GFP gene (4get mice) are uniformly GFP positive.28 Single cell suspensions from ear skin of 4get mice were obtained as described.29 In brief, ears were divided into dorsal and ventral halves and both halves were incubated (dermis down) in MEM containing 0.5 mg/mL Liberase CI (Roche) and 0.5 mg/mL DnaseI (Sigma, Taufkirchen, Germany) for 90 minutes at 37°C. Single cells were released from pretreated ear tissue by a 7-minute treatment in a Medimachine with 50 μm Medicons (BD Biosciences, Heidelberg, Germany). Cell suspensions were stained for c-Kit using PE-conjugated mAb ACK2 (eBioscience), and c-Kit/GFP double-positive mast cells were separated using a cell sorter (FACS Vantage SE and CELLQuest Pro; BD Biosciences). Approximately 2.5% of dermal cells were c-Kit+GFP+ and reanalyzes of sorted cells revealed a purity of more than 97% (not shown).
PCR
Mouse ears were frozen in liquid nitrogen and total RNA was purified using the RNeasy kit (Qiagen, Hilden, Germany). RNA from BMMCs and fluorescence-activated cell sorting (FACS)–sorted dermal mast cells was isolated with TRI REAGENT (Molecular Research Center, Cincinnati, OH). In addition, RNA from dermal mast cells was treated with heparinase I (Sigma) as described.30 RNA was reverse transcribed into cDNA and analyzed by polymerase chain reaction (PCR) using Advantage 2 Polymerase Mix (Clontech, Palo Alto, CA) and the following primers: HGPRT.forward: 5′-GTT GGA TAC AGG CCA GAC TTT GTT G-3′; HGPRT.reverse: 5′-GAG GGT AGG CTG GCC TAT AGG CT-3′; mTNF-α.forward: 5′-TCT ACT GAA CTT CGG GGT GAT CGG TCC-3′; mTNF-α.reverse: 5′-AGA TAG CAA ATC GGC TGA CGG TGT GGG-3′; mIL-1β.forward: 5′-CAA CCA ACA AGT GAT ATT CTC CAT G-3′; mIL-1β.reverse: 5′-GAT CCA CAC TCT CCA GCT GCA-3′; TLR7.forward: 5′-CCA CCA GAC CTC TTG ATT CC-3′; TLR7.reverse: 5′-TCC AGA TGG TTC AGC CTA CG-3′; MMCP-4.forward: 5′-ACC ACT GAG AGA GGG TTC ACA GC-3′; MMCP-4.reverse: 5′-GAA GAC TCT GAT GCA CGC AGG TC-3′. The PCR products were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining. The number of PCR cycles were as follows: 30 for HGPRT, and 35 for TNF-α, IL-1β, TLR7, and MMCP-4.
Mouse DC generation and activation
Immature dendritic cells (DCs) from mast-cell–deficient KitW-sh/W-sh mice or from Kit+/+ mice were generated from bone marrow according to standard protocols.31,32 In brief, DCs were differentiated with GM-CSF for 6 days with medium replacements on days 2 and 4. On day 6, the immature DCs were activated with poly-(I:C) (Amersham, Freiburg, Germany) for 18 hours.
Immunization with peptide-pulsed DCs was done according to Warger et al.32 Briefly, activated DCs were loaded with SIINFEKL peptide, and 106 DCs were injected intraperitoneally into KitW-sh/W-sh or the Kit+/+ control mice.
Transcutaneous immunization
Transcutaneous immunization (TCI) was according to the protocol established by our group.17 In brief, TCI was performed on 2 consecutive days by mixing of 100 μg SIINFEKL peptide with 60 mg preformulated imiquimodcream per mouse per application. TCI was applied onto the back of anesthetized mice (∼ 10 cm2), which had been shaved at least 2 days prior to immunization. Two days after shaving and prior to immunization, the mice did not have skin irritation on their shaved back.
Flow cytometric analysis and in vivo cytotoxicity assay
Six days after immunization, blood samples were collected after tail vein incision and incubated on ice with specific mAbs as indicated after a hypotonic lysis step. The following mAbs were used for analyses by flow cytometry: APC-Cy7–conjugated anti-CD8, PE-Cy7–conjugated anti-CD25, APC-conjugated anti-CD62L (BD Pharmingen, Heidelberg, Germany), FITC-conjugated anti-CD44 (Immunotools, Friesoythe, Germany). PI was used to exclude nonviable cells from analysis. For detection of peptide-specific CD8+ T cells, PE-labeled SIINFEKL-H2-Kb tetramer was used (Orpegen, Heidelberg, Germany). Functional analyses of CTL responses induced by immunizations were performed by an in vivo cytotoxicity assay (20 hours) with differentially 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled syngeneic target cells 6 days after DC immunization (4 μM and 0.4 μM CFSE; Molecular Probes). The CFSElow population was pulsed with 1 μM SIINFEKL, while the CFSEhigh population was left without peptide. Target cells (2 × 107) were adoptively transferred into immunized mice at a 1:1 ratio (CFSElow/CFSEhigh). Twenty hours after intravenous injection of the target cells, mice were killed and spleens processed to single cell suspensions. Specific in vivo lysis of target cells was evaluated as follows: specific lysis [%] = (number of cells non peptide pulsed − number of cells pulsed with peptide)/number of cells non–peptide pulsed. To assess intracellular IFN-γ production, splenocytes were restimulated in vitro for 4 hours with or without SIINFEKL peptide (1 μM) in the presence of brefeldin A (1 μg/mL; Sigma). After surface staining, cells were treated with Cytofix/Cytoperm (BD Pharmingen) according to the manufacture's protocol and finally an APC-conjugated anti–IFN-γ antibody (BD Pharmingen) was added. All washes after the permeabilization step and the intracellular staining itself were performed in PBS containing 0.1% BSA, 0.05% saponin (Sigma). All analyses were performed with a FACSCanto flow cytometer and FACSDiva software (BD Pharmingen).
Statistical analysis
Data are expressed as mean (± SD). Student t test was used to compare mean values between 2 experimental groups where appropriate.
P values less than .05 were considered significant.
Results
Mast cells initiate inflammation and promote emigration of LCs following local treatment with imiquimod
The expression of TLR7 mRNA in murine fetal skin–derived cultured mast cells (FSMCs) was recently described, and we therefore hypothesized that dermal mast cells may contribute to the initiation of an inflammatory response following local treatment with imiquimod.7 Due to the lack of appropriate antibodies, we were unable to stain TLR7-positive mast cells—or any other TLR7-expressing cell type—in tissues. Therefore, we used reverse-transcription (RT)–PCR to investigate TLR7 mRNA expression in BMMCs and dermal mast cells, the latter isolated as c-Kit+GFP+ cells from the skin of 4get mice.28 As depicted in Figure 1A, BMMCs constitutively express low but detectable levels of TLR7 mRNA, which is in accordance with published data.7 In BMMCs, TLR7 mRNA expression increases upon incubation with the TLR7 ligand imiquimod in vitro. Dermal mast cells freshly isolated from untreated mice also express TLR7 mRNA. In dermal mast cells isolated from mice that were topically treated once daily with imiquimod on 2 consecutive days, TLR7 mRNA expression level remains unaffected (Figure 1A).
Dermal mast cells activated via TLR7 are critical for the early onset of inflammation and promote emigration of LCs. (A) Total RNA from BMMCs and c-Kit+GFP+ dermal mast cells isolated from 4get mice was analyzed for the expression of the indicated mRNA species. BMMCs were left untreated or incubated in the presence of 10 μg/mL imiquimod for 16 hours. c-Kit+GFP+ cells were isolated from naive mice or following daily topical treatment with imiquimod on 2 consecutive days. HGPRT served as housekeeping gene transcript and mouse mast-cell protease 4 as positive control expressed by connective tissue–type mast cells.33 Representatives of 3 independent experiments with equivalent results. (B) Ears of wild-type (Kit+/+), mast-cell–deficient (KitW-sh/W-sh), and mast-cell–deficient mice that had previously been reconstituted (indicated by an arrow) with BMMCs from either wild-type or TLR7-deficient mice were treated daily with imiquimod (starting on day 0). The contralateral ear of each animal was treated with vehicle. Ear swelling was measured and the indicated values refer to the ear thickness at the beginning of the experiment. (C) At the end of the experiment shown in panel B, epidermal sheets were prepared and stained for MHC class II+ LCs. The values shown for LC migration refer to the reduction in LC numbers compared with the untreated contralateral ears. (D) Epidermal sheets from Kit+/+ and KitW-sh/W-sh mice were stained for LCs on day 7 after daily treatment with imiquimod. Shown are the means (± SD) from 4 to 5 mice per condition. *P < .01 versus Kit+/+.
Dermal mast cells activated via TLR7 are critical for the early onset of inflammation and promote emigration of LCs. (A) Total RNA from BMMCs and c-Kit+GFP+ dermal mast cells isolated from 4get mice was analyzed for the expression of the indicated mRNA species. BMMCs were left untreated or incubated in the presence of 10 μg/mL imiquimod for 16 hours. c-Kit+GFP+ cells were isolated from naive mice or following daily topical treatment with imiquimod on 2 consecutive days. HGPRT served as housekeeping gene transcript and mouse mast-cell protease 4 as positive control expressed by connective tissue–type mast cells.33 Representatives of 3 independent experiments with equivalent results. (B) Ears of wild-type (Kit+/+), mast-cell–deficient (KitW-sh/W-sh), and mast-cell–deficient mice that had previously been reconstituted (indicated by an arrow) with BMMCs from either wild-type or TLR7-deficient mice were treated daily with imiquimod (starting on day 0). The contralateral ear of each animal was treated with vehicle. Ear swelling was measured and the indicated values refer to the ear thickness at the beginning of the experiment. (C) At the end of the experiment shown in panel B, epidermal sheets were prepared and stained for MHC class II+ LCs. The values shown for LC migration refer to the reduction in LC numbers compared with the untreated contralateral ears. (D) Epidermal sheets from Kit+/+ and KitW-sh/W-sh mice were stained for LCs on day 7 after daily treatment with imiquimod. Shown are the means (± SD) from 4 to 5 mice per condition. *P < .01 versus Kit+/+.
Using both mast-cell–deficient KitW-sh/W-sh mice and their congenic Kit+/+ wild-type littermates, we measured the ear swelling response following topical daily treatment with this adjuvant. As shown in Figure 1B, ear swelling of wild-type animals can easily be detected 24 hours after the first application of imiquimod. In contrast, the onset of an inflammatory reaction in mast-cell–deficient KitW-sh/W-sh mice is significantly delayed, reaching only on day 3 an extent comparable with that seen in wild-type mice on day 1. In general, between days 3 and 4 both mast-cell–deficient and wild-type mice reach a comparable maximum of ear swelling (Figure 2B,C).
To further investigate the role of mast-cell TLR7 signaling, KitW-sh/W-sh mice were locally reconstituted with BMMCs derived from either wild-type or TLR7-deficient mice (Figure 1B). Inflammation is fully recovered only upon reconstitution with wild-type BMMCs. Thus, mast cells are critical for the fast inflammatory response to TLR7 ligation. Reconstitution with TLR7-deficient BMMCs leads to an inflammatory reaction with delayed kinetics, even slower to the one observed in mast-cell–deficient mice.
It was also recently reported that treatment with imiquimod induces the migration of LCs, although murine LCs themselves lack expression of TLR7.18,34 Therefore, we assumed that dermal mast cells, activated via TLR7, might promote the emigration of LCs. In order to investigate this idea, epidermal sheets from animals, previously treated over 4 days once daily with imiquimod or vehicle, were stained for MHC class II–positive LCs (Figure 1C). In untreated skin, we observed no significant differences in LC numbers between mast-cell–deficient and wild-type mice, which is in accordance with previous reports12-14 (and data not shown). Following treatment with imiquimod, the migration of LCs in KitW-sh/W-sh mice is reduced by 50% compared with wild-type mice. Migration could be efficiently restored in mast-cell–deficient mice upon transfer of BMMCs derived from wild-type but not from TLR7-deficient animals. Hence, dermal mast cells are able to act as a sensor for TLR7 ligand, thereby boosting both early local inflammation and migration of LCs. It should be noted that in contrast to the ear swelling response, the numbers of emigrating LCs under the specific conditions shown in Figure 1C did not approximate to each other over time, even after daily treatment with imiquimod for up to 7 days (Figure 1D).
Mast-cell–derived cytokines contribute to inflammation and migration of LCs
Activated mast cells are a rich source of proinflammatory cytokines, which may promote both inflammation (ie, ear swelling) and emigration of LCs in imiquimod-treated skin. In order to pursue this hypothesis further, we analyzed the mRNA expression pattern of important proinflammatory mediators in the ears of both mast-cell–deficient and wild-type mice following treatment with imiquimod. As displayed in Figure 2A, expression of mRNAs encoding TNF-α and IL-1β is increased in the ears of wild-type mice 2 hours after application of the cream, whereas no augmentation of mRNA production is detectable in either vehicle-treated controls or in KitW-sh/W-sh mice. We followed expression of IL-1β and TNF-α mRNAs for up to 8 hours after treatment with imiquimod. In wild-type mice, sustained expression of both mRNAs was observed. In mast-cell–deficient mice, no increase in TNF-α mRNA production was measurable, but IL-1β mRNA expression started 4 hours after application of imiquimod (data not shown).
Mast-cell–derived cytokines boost inflammation and emigration of LCs. (A) Two hours after application of either imiquimod or vehicle, RNA was prepared from the ears and analyzed using RT-PCR. Representative of 3 experiments with equivalent results. (B-D) These experiments were performed as described in Figure 1 to examine the role of mast-cell–derived TNF-α (B) and IL-1 (C) on inflammation and emigration of LCs (D). Shown are the means (± SD) from 4 to 5 mice per condition. *P < .01 and **P = .015 versus Kit+/+.
Mast-cell–derived cytokines boost inflammation and emigration of LCs. (A) Two hours after application of either imiquimod or vehicle, RNA was prepared from the ears and analyzed using RT-PCR. Representative of 3 experiments with equivalent results. (B-D) These experiments were performed as described in Figure 1 to examine the role of mast-cell–derived TNF-α (B) and IL-1 (C) on inflammation and emigration of LCs (D). Shown are the means (± SD) from 4 to 5 mice per condition. *P < .01 and **P = .015 versus Kit+/+.
To further dissect the possible role(s) of mast-cell–derived cytokines, we locally reconstituted ears from KitW-sh/W-sh mice with BMMCs from mice deficient for either TNF-α or IL-1α/β or from their congenic littermates. As summarized in Figure 2B-C, ear swelling of mast-cell–deficient mice can be completely restored following reconstitution with wild-type BMMCs only. Compared with these results, the ear swelling response after transfer of either TNF-α– (Figure 2B) or IL-1α/β–deficient mast cells (Figure 2C) is delayed by one day. This indicates that both mast-cell–derived cytokines play a role in the fast onset of inflammation. However, only mast-cell–derived IL-1, but not TNF-α, contributes significantly to the emigration of LCs (Figure 2D). In order to rule out that these results are due to differences in engraftment efficiencies between mast-cell–deficient mice reconstituted with BMMCs derived from different knock-out strains, we assessed mast-cell numbers in the ears 6 weeks after selective engraftment with mast cells in the ear pinnae. As shown in Table 1, local engraftment of KitW-sh/W-sh mice with BMMCs derived from either wild-type, TLR7, or cytokine-deficient animals results in mast-cell numbers that are statistically indistinguishable from those seen in wild-type mice.
Engraftment of mast-cell–deficient mice with BMMCs derived from different donors
| . | Mast cells/mm ear cartilage . |
|---|---|
| KitW-sh/W-sh | ND |
| Kit+/+ | 30.6 (± 2.4) |
| TLR7−/− → KitW-sh/W-sh | 28.7 (± 4.2) |
| TNF-α−/− → KitW-sh/W-sh | 25.7 (± 4.1) |
| IL1α/β−/− → KitW-sh/W-sh | 29.7 (± 4.5) |
| Kit+/+ → KitW-sh/W-sh | 26.9 (± 4.5) |
| . | Mast cells/mm ear cartilage . |
|---|---|
| KitW-sh/W-sh | ND |
| Kit+/+ | 30.6 (± 2.4) |
| TLR7−/− → KitW-sh/W-sh | 28.7 (± 4.2) |
| TNF-α−/− → KitW-sh/W-sh | 25.7 (± 4.1) |
| IL1α/β−/− → KitW-sh/W-sh | 29.7 (± 4.5) |
| Kit+/+ → KitW-sh/W-sh | 26.9 (± 4.5) |
Mice were reconstituted as described in “Materials and methods.” After 6 weeks, ear sections were stained with avidin-fluorophor conjugate and mast-cell numbers counted. Shown are the means (± SD) from 4 animals per group.
ND indicates not detectable.
LNs are critical structures for the initiation of adaptive immune responses as they provide the platform for the interaction of antigen-loaded antigen-presenting cells with naive T cells. Following treatment with imiquimod, the increase in total cell numbers is severely impaired in LNs derived from KitW-sh/W-sh mice compared with wild-type animals (Figure 3A). LN hypertrophy in KitW-sh/W-sh mice is recovered only upon transfer of mast cells from wild-type or IL-1–deficient animals, but not upon reconstitution with TLR7- or TNF-α–deficient mast cells (Figure 3A). This indicates that mast cells and mast-cell–derived TNF-α are critical initiators of LN hypertrophy, which has previously been reported for LN hypertrophy in response to infection with E coli.35
Mast cells are critical for LN hypertrophy and immigration of LCs. (A) Imiquimod was applied daily to the ears of mice and total cell numbers in draining auricular LN were assessed on day 3. (B,C) LN cell suspensions from the experiments shown in panel A were stained for the expression of MHCII, Langerin, and PD-L1 using FACS analyses. Given are the percentages of Langerin-positive LCs out of MHCII-positive cells (B) and PD-L1–positive cells within the LC population (C). Shown are the means (± SD) from 6 to 8 mice per condition. *P < .05 versus KitW-sh/W-sh treated with imiquimod.
Mast cells are critical for LN hypertrophy and immigration of LCs. (A) Imiquimod was applied daily to the ears of mice and total cell numbers in draining auricular LN were assessed on day 3. (B,C) LN cell suspensions from the experiments shown in panel A were stained for the expression of MHCII, Langerin, and PD-L1 using FACS analyses. Given are the percentages of Langerin-positive LCs out of MHCII-positive cells (B) and PD-L1–positive cells within the LC population (C). Shown are the means (± SD) from 6 to 8 mice per condition. *P < .05 versus KitW-sh/W-sh treated with imiquimod.
To follow the immigration of LCs to the LN, we stained Langerin-positive cells (ie, LCs) in auricular LN cell suspensions following application of imiquimod to the ears. As depicted in Figure 3B, numbers of Langerin-positive cells in LN increase more than 3-fold in wild-type mice on day 3 following daily treatment with imiquimod, whereas the influx is significantly reduced in KitW-sh/W-sh mice. In pilot experiments, we found that numbers of LCs in LN increased between day 2 and day 3 after daily treatment with imiquimod and then rapidly declined to baseline levels around day 7 (data not shown). In accordance with our data on the emigration of LCs out of the epidermis (Figures 1C and 2D), their immigration to the draining LN can be restored in KitW-sh/W-sh mice engrafted with mast cells derived from either wild-type or TNF-α–deficient mice. Consequently, reconstitution with IL-1– or TLR7-deficient BMMCs does not significantly restore immigration; LC numbers do not exceed those observed in KitW-sh/W-sh mice (Figure 3B). However, although the number of LCs immigrating into the draining LN is severely reduced in mast-cell–deficient animals, LCs are phenotypically mature with respect to the expression of PD-L1/B7-H1/CD274, as shown in Figure 3C.27
Priming of CTLs by transcutaneous peptide immunization with imiquimod is mast-cell dependent
The finding that mast cells promote the migration of LCs prompted us to investigate whether the induction of a CTL response to the model epitope SIINFEKL from chicken ovalbumin (OVA257-264), administered in combination with imiquimod, is impaired in KitW-sh/W-sh mice. To this end, we first compared the ability of mast-cell–deficient and wild-type mice to mount a specific CTL response after immunization with peptide-loaded DCs, generated from bone marrow derived from mast-cell–deficient mice or congenic wild-type littermates. On day 7 after immunization with SIINFEKL-loaded DCs, cytolytic activity was monitored in vivo after injecting CFSElow-labeled syngeneic splenocytes loaded with peptide in combination with CFSEhigh-labeled splenocytes without the target peptide in a ratio of 1:1. As expected and shown in Figure 4, nonimmunized KitW-sh/W-sh mice and their congenic littermates are unable to lyse the injected target cells. In contrast, both mouse strains develop a full-blown cytolytic response upon vaccination with peptide-loaded DCs, irrespective of the source of the DCs. Hence, the ability to present the target antigen and to mount a specific CTL response is unimpaired in mast-cell–deficient mice immunized with mature and antigen-loaded antigen-presenting cells.
Immunization with peptide-loaded DCs elicits a potent CTL response in mast-cell–deficient mice.KitW-sh/KitW-sh or Kit+/Kit+ mice were immunized intraperitoneally with 1 × 106 DCs that had been activated with the TLR3 ligand poly(I:C) overnight, and peptide-loaded with the immunodominant H2-Kb peptide SIINFEKL. DCs were derived from either mast-cell–deficient or wild-type mice. At day 6, the blood was monitored for the presence of SIINFEKL-tetramer+ CD8+ T cells (data not shown). Seven days after immunization, cytolytic activity against SIINFEKL-loaded CFSElow or non–peptide-loaded CFSEhigh syngeneic splenocytes was evaluated after 20 hours; target cells had been injected intravenously at day 6. Negative controls show no lysis, while the other groups of mice lysed the target cells 94% to 98%. One representative graph for each group is shown; the experiment was performed 2 times independently, using 3 mice per group.
Immunization with peptide-loaded DCs elicits a potent CTL response in mast-cell–deficient mice.KitW-sh/KitW-sh or Kit+/Kit+ mice were immunized intraperitoneally with 1 × 106 DCs that had been activated with the TLR3 ligand poly(I:C) overnight, and peptide-loaded with the immunodominant H2-Kb peptide SIINFEKL. DCs were derived from either mast-cell–deficient or wild-type mice. At day 6, the blood was monitored for the presence of SIINFEKL-tetramer+ CD8+ T cells (data not shown). Seven days after immunization, cytolytic activity against SIINFEKL-loaded CFSElow or non–peptide-loaded CFSEhigh syngeneic splenocytes was evaluated after 20 hours; target cells had been injected intravenously at day 6. Negative controls show no lysis, while the other groups of mice lysed the target cells 94% to 98%. One representative graph for each group is shown; the experiment was performed 2 times independently, using 3 mice per group.
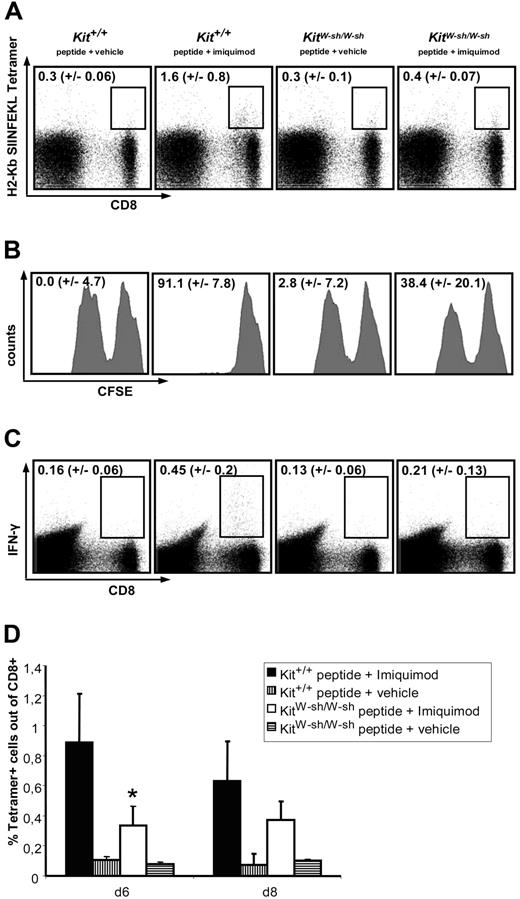
However, KitW-sh/W-sh and Kit+/+ mice show dramatic differences with respect to CTL responses after transcutaneous peptide immunization (TCI) with imiquimod. In Figure 5A, peptide-specific CTLs are detected in peripheral blood using tetramer staining on day 6, after animals were creamed twice on days 0 and 1 of the experiment. In wild-type mice immunized using peptide in combination with imiquimod, a significant number of peptide-specific CTLs can be detected, whereas these cells are scarce in immunized mast-cell–deficient mice (Figure 5A,D). Before day 6, the observed values do not rise above background staining (ie, controls that did not receive peptide; not shown). Furthermore, as depicted in Figure 5B, the appearance of tetramer-positive CTLs in immunized wild-type mice is paralleled by a strong cytolytic activity in vivo, which is severely impaired in mast-cell–deficient mice. In addition, the production of IFN-γ was analyzed by intracellular FACS staining of CD8+ cells after restimulation with peptide in vitro (Figure 5C). According to the expectations, the number of CTLs expressing IFN-γ is also reduced by approximately 50% in KitW-sh/W-sh mice compared with wild-type littermates.
Mast-cell–deficient mice display an impaired CTL response after transcutaneous peptide immunization with imiquimod (TCI). Mice were treated with peptide + imiquimod or peptide + vehicle once daily on 2 consecutive days. (A) After 6 days, peripheral blood was stained for peptide-specific CD8+ T cells using a SIINFEKL-specific tetramer. The depicted numbers represent the percentages of tetramer-positive cells within the CD8+ populations. (B) Seven days after the first immunization, cytolytic activity against SIINFEKL-loaded CFSElow or non–peptide-loaded CFSEhigh syngeneic splenocytes was evaluated after 20 hours of in vivo killing; target cells had been injected intravenously at day 6. The depicted numbers correspond to the percentages of peptide-specific lysis. (C) Splenocytes were restimulated for 4 hours in the presence or absence (data not shown) of SIINFEKL and brefeldin. The production of IFN-γ ex vivo was then analyzed by intracellular FACS staining. The numbers shown indicate the percentages of cells expressing IFN-γ within the CD8+ population. (D) On days 6 and 8, peripheral blood was stained for SIINFEKL-specific CTL using tetramer. All depicted results are representative of 2 independent experiments using 5 mice per group. *P < .01 versus Kit+/+ immunized with peptide + imiquimod.
Mast-cell–deficient mice display an impaired CTL response after transcutaneous peptide immunization with imiquimod (TCI). Mice were treated with peptide + imiquimod or peptide + vehicle once daily on 2 consecutive days. (A) After 6 days, peripheral blood was stained for peptide-specific CD8+ T cells using a SIINFEKL-specific tetramer. The depicted numbers represent the percentages of tetramer-positive cells within the CD8+ populations. (B) Seven days after the first immunization, cytolytic activity against SIINFEKL-loaded CFSElow or non–peptide-loaded CFSEhigh syngeneic splenocytes was evaluated after 20 hours of in vivo killing; target cells had been injected intravenously at day 6. The depicted numbers correspond to the percentages of peptide-specific lysis. (C) Splenocytes were restimulated for 4 hours in the presence or absence (data not shown) of SIINFEKL and brefeldin. The production of IFN-γ ex vivo was then analyzed by intracellular FACS staining. The numbers shown indicate the percentages of cells expressing IFN-γ within the CD8+ population. (D) On days 6 and 8, peripheral blood was stained for SIINFEKL-specific CTL using tetramer. All depicted results are representative of 2 independent experiments using 5 mice per group. *P < .01 versus Kit+/+ immunized with peptide + imiquimod.
Discussion
The development of successful adaptive immune responses critically depends on the fast onset of innate (ie, inflammatory) reactions of appropriate magnitude. Based on the work by Echtenacher et al1 and Malaviya et al,2 it is now widely accepted that mast cells are able to act as a first line of defense in murine models of acute bacterial inflammation, which is due to their ability to induce inflammatory reactions within minutes.1,2 These findings ultimately led to the development of a novel concept of mast-cell immunology, mainly based on alternative (ie, IgE-independent) stimulation of mast cells. Activation of mast cells via TLR ligation was shown to be critically involved in several inflammatory models, including chronic inflammation and innate immune responses.8,9,36,37
Our work presented herein describes an important role for TLR7-activated dermal mast cells with respect to the early onset of inflammatory skin reaction, emigration of LCs, and the magnitude of CTL response, the latter most likely by promoting the migration of peptide-loaded LCs to the local lymph nodes.17 Hence, mast cells together with DCs represent the hinge between innate and adaptive immunity, able to boost the development of a specific immune reaction.
Impaired migration of epidermal LCs and attenuated contact hypersensitivity following topical hapten exposure was also shown in mast-cell–deficient mice and animals lacking either IgE or FcϵRI.12 Furthermore, in a model for passive cutaneous anaphylaxis, it was reported that local IgE/antigen-mediated mast-cell activation provides a strong signal for the migration of LCs to the draining lymph nodes. This was partly dependent on histamine acting on H2 receptors, but it was also noted that local administration of histamine alone did not induce LC migration, indicating that other mast-cell–derived products must play a role in this process.13 Very recently, it was shown that efficient LC migration induced by topical application of the contact allergen FITC depends on both mast cells and mast-cell–derived TNF-α.14 In contrast to our findings, this study also revealed that the levels of LC migration to skin-draining LN in TNF-α–deficient and mast-cell–deficient mice caught up with—and even slightly exceeded—those observed in wild-type animals at later time points (ie, 48-72 hours after hapten application). More importantly, 6 days after hapten application, identical levels of T-cell proliferation induced by restimulation with hapten ex vivo was measured in wild-type, mast-cell–deficient, or TNF-α–deficient mice. Thus, in the study by Suto et al,14 mast cells obviously accelerate the migration of LCs to the draining LN but are not required to promote an adaptive T-cell response. This is in contrast to our present study where the migration of LCs following application of imiquimod is not only delayed but also remains reduced at later time points, which correlates with a severely impaired CTL response in mast-cell–deficient mice.
A recent study examined the mechanisms of LC migration and LN hypertrophy in response to Gram-positive Staphylococcus aureus peptidoglycan (PGN).38 In agreement with our data, PGN-induced LC migration and LN hypertrophy were both profoundly mast-cell dependent. However, IgE/antigen-induced LC migration was completely TNF-α dependent while PGN-induced LC migration was partially independent of TNF-α. Furthermore, this study also revealed that LN hypertrophy following IgE cross-linking requires TNF-α, while PGN-induced LN hypertrophy is TNF-α independent. As a conclusion, we hypothesize that the mechanisms of mast-cell–dependent phenomena (eg, role of TNF-α in LC migration) are deeply influenced by the mode of mast-cell activation. This hypothesis is corroborated by the finding that mediator production by mast cells differs in response to various stimuli (eg, IgE cross-link versus stimulation with PGN)38 or even among different TLR activators.7
In addition, it should be noted that different stimuli can trigger different cell types in situ. In our model, obviously other cell types in the skin are also able to recognize TLR7 ligands and to induce an inflammatory reaction, albeit with slower kinetics (Figure 1B). Likely candidates for this phenomenon are dermal DCs and a recently described population of plasmacytoid DC-like cells.39,40 The latter accumulate in the skin several days after daily treatment with imiquimod and are believed to promote inflammation by producing type I interferons.40 However, our results show that mast cells are necessary to induce an early inflammatory skin response.
At present, transcutaneous immunization protocols appear promising, as they are noninvasive, easy to perform, and cost effective.41,42 An understanding of the underlying mechanisms may help to develop such strategies more specifically in the future.
In addition to the use of imiquimod as an adjuvant, antiviral and antitumor effects of this drug have also been described.40,43-45 Therefore, it is tempting to speculate that mast cells might also be critically involved in these reactions, which is currently under investigation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft, project STA 984/1–1 and Sonderforschungsbereich 548, projects A10 (M.S.) and A11 (C.T.), and Sonderforschungsbereich 432, project B10 (H.S.).
We cordially thank our colleagues Marcus Maurer, Andre Gessner, Kerstin Steinbrink, Yoichiro Iwakura, Stefan Bauer, and Esther von Stebut for providing us with mouse strains and bone marrow. We are indebted to Nikolaus Romani and Patrizia Stoitzner (Department of Dermatology, Innsbruck Medical University, Austria) for their help and advice. We also thank Steffen Schmitt, Sandra Gerecht, and Alex Hobel for expert technical assistance. The continuous support by Jürgen Knop, Head of the Department of Dermatology, University of Mainz, is greatly appreciated.
Authorship
Contribution: V.H., M.B., M.K., and C.T. performed research, data collection, and analysis; T.W., G.R., T.B., H.S., and C.T. contributed new analytical tools, data collection, and analysis; E.S. and M.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Stassen, Institute for Immunology, Johannes Gutenberg University, Hochhaus am Augustusplatz, 55131 Mainz, Germany; e-mail: stassenm@uni-mainz.de.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal