Abstract
The pathogenesis of type 1 diabetes (T1D) involves the immune-mediated destruction of insulin-producing β cells in the pancreatic islets of Langerhans. Genetic analysis of families with a high incidence of T1D and nonobese diabetic (NOD) mice, a prototypical model of the disorder, uncovered multiple susceptibility loci, although most of the underlying immune defects remain to be delineated. Here we report that aged mice doubly deficient in granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3) manifest insulitis, destruction of insulin-producing β cells, and compromised glucose homeostasis. Macrophages from mutant mice produce increased levels of p40 after LPS stimulation, whereas concurrent ablation of interferon-γ (IFN-γ) ameliorates the disease. The administration of antibodies that block cytotoxic T lymphocyte associated antigen-4 (CTLA-4) to young mutant mice precipitates the onset of insulitis and hyperglycemia. These results, together with previous reports of impaired hematopoietic responses to GM-CSF and IL-3 in patients with T1D and in NOD mice, indicate that functional deficiencies of these cytokines contribute to diabetes.
Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disease in which a loss of tolerance to insulin-producing β cells in the pancreatic islets results in impaired glucose homeostasis.1 T1D clusters in families and is frequently associated with other autoimmune disorders, suggesting that an underlying genetic susceptibility compromises tolerance to multiple normal tissues. Nonobese diabetic (NOD) mice are widely used as a model for T1D because they display many similar aspects of disease pathogenesis and harbor a general predisposition to autoimmunity, which is modulated by genetic background.2 In NOD mice, the development of diabetes proceeds from an initial phase of insulitis, characterized by T and B cell infiltrates in the absence of β-cell damage, to an aggressive stage in which β cells are destroyed and glucose homeostasis is disrupted.
Extensive linkage analysis of families with T1D and NOD mice yielded more than 20 genetic susceptibility loci.3 Among these, the major histocompatibility (MHC) class II locus exerts the most potent influence on disease development. Several non-MHC-related genes have also been implicated, including insulin, CTLA-4, IL-2, CD25, the protein tyrosine phosphatase PTPN22, and the membrane transporter NRAMP-1. Nonetheless, multiple additional loci remain to be identified, although characterization of these gene products has been hampered by the large number of immune defects associated with disease and a limited understanding of the key pathogenic mechanisms.
Antigen-presenting cells are thought to play an important role in the development of diabetes.4 Dendritic cells and macrophages contribute to the maintenance of tolerance through central deletion of autoreactive thymocytes and the induction of recessive and dominant modes of suppression in the periphery.5 Among the phenotypic abnormalities observed in patients with T1D and NOD mice are the impaired responses of hematopoietic cells to granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3).6-19 Alterations in the number and/or function of dendritic cells, macrophages, and granulocytes derived from cultures of hematopoietic precursors in GM-CSF and IL-3 have been described, but the contribution of these cytokine defects to altered antigen presenting cell function in vivo and the pathogenesis of diabetes remains unclear.
We previously established roles for GM-CSF and IL-3 in the maintenance of immune homeostasis through promoting the efficient phagocytosis of apoptotic cells by macrophages.20 Consistent with other strains of mice that display an impaired uptake of dying cells,21 aged GM-CSF and, to a greater extent, GM-CSF/IL-3 doubly deficient mice developed a systemic lupus erythematous (SLE)-like disorder characterized by anti-double-stranded DNA antibodies and immune complex-mediated glomerulonephritis. Here we report that aged GM-CSF/IL-3–deficient mice also develop insulitis, destruction of insulin-producing β cells, and compromised glucose homeostasis. Similar to patients with T1D and NOD mice, disease pathogenesis in this model involves p40 and CTLA-4, suggesting that functional defects in GM-CSF and IL-3 contribute to autoimmune diabetes.
Methods
Mice
Mice deficient in GM-CSF,22 IL-3,23 GM-CSF/IL-3,24 interferon-γ (IFN-γ),25 and GM-CSF/IL-3/IFN-γ20 were backcrossed at least 9 generations onto the C57Bl/6 strain and housed under specific pathogen-free conditions. Genotypes were confirmed by polymerase chain reaction (PCR), as described previously.20 All mouse experiments were conducted under a protocol approved by the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Dana-Farber Cancer Institute Institutional Animal Care and Use Committee (IACUC).
Pathology
Pancreases were fixed in 10% buffered formalin, embedded in paraffin, cut in 5-μm sections, and stained with hematoxylin and eosin. Islets were examined in 7 to 11 fields per specimen at magnification ×100. Inflammation was evaluated as peri-insulitis and insulitis. Peri-insulitis was noted when an aggregate of lymphocytes surrounded the islet. The inflammatory infiltrates were graded as 1-3+; 1+ represented an infiltrate of less than 10 cells, 2+ represented an infiltrate of 10-50 cells, and 3+ represented an infiltrate greater than 50 mononuclear cells. Insulitis was noted when lymphocytes were present within the islets. Each islet was evaluated for necrosis as evidenced by marked nuclear pallor and loss of nuclear content with vacuolization of the cytoplasm and ghost-like remnants of cells or marked nuclear pyknosis surrounded by shrunken, intensely staining cytoplasmic masses.
Immunohistochemical staining for CD3, CD4, CD8, and B220 proteins was performed by an automated method on the Ventana ES immunohistochemistry instrument (Ventana Medical Systems, Inc., Tucson, AZ) using an indirect biotin avidin diaminobenzidine (DAB) detection system on contiguous formalin-fixed, paraffin-embedded 4-μm sections from a representative block in each case. Monoclonal antibodies were from BD Biosciences (San Jose, CA).
To stain for islet cell hormone production, we treated 5-μm paraffin sections with 3% H2O2 overnight to quench endogenous peroxide and then added goat serum for blocking. An anti-insulin monoclonal antibody (mAb) (HB125, Biogenex) was incubated overnight at 4°C followed by a horseradish peroxidase (HRP)–labeled goat anti-mouse secondary antibody (sc-2055; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature. The staining was developed with the DAB detection system as above, followed by hematoxylin counterstaining. Somatostatin and glucagon immunohistochemical stainings were performed similarly using rabbit polyclonal anti-somatostatin (N1551; Dako North America, Inc, Carpinteria, CA), rabbit polyclonal anti-glucagon (18-0064; Zymed, South San Francisco, CA), and the HRP-labeled goat anti-rabbit secondary Ig (sc-2054; Santa Cruz Biotechnology). For insulin and somatostatin double-stainings, somatostatin was detected using rabbit polyclonal anti-somatostatin Ig (N1551; Dako North America), while insulin was detected with the anti-insulin monoclonal antibody (clone HB125; Biogenex). Detection of both primary antibodies was performed with the combined HRP/alkaline phosphatase Envision Doublestain Kit (K1395; Dako North America) with somatostatin labeled using the DAB chromagen (SK-4100; Vector Laboratories, Burlingame, CA) and insulin labeled using fast red (K0597; Dako North America). Appropriate positive and negative control sections were included with each immunohistochemical staining reaction. The histologic samples were analyzed at room temperature using a Zeiss Axiophot microscope (Carl Zeiss Microimaging, Thornwood, NY) that contained the following lenses: Plan-NEOFLUAR 6x/0,15; Plan-APOCHROMAT 10x/0,32 (44 06 30); Plan-APOCHROMAT 20x/0,60 (44 06 40); EC Plan-NEOFLUAR 40x/0,75 (440350-9903); and Eyepiece: P1 10x/25 (44 40 34). The microscopic images were taken in air with a SPOT RT color digital camera and SPOT software version 4.6 (Diagnostic Instruments, Sterling Heights, MI) and then cropped using Adobe Photoshop, version 7 (Adobe Systems, San Jose, CA).
Blood sugar measurements
Age- and sex-matched mice were studied after 6 hours of fasting. Blood sugar levels were measured with the ExacTech RSG testing system (Abbott Diabetes Care, Alameda, CA) using samples obtained from tail veins. For glucose tolerance tests, mice were injected intraperitoneally with a glucose solution (3 g/kg body weight) in a volume of 50 μL 0.9% NaCl solution per 10 g body weight. Glucose measurements from tail blood were performed at 0, 15, 30, 60, and 120 minutes.
Anti-CTLA4 antibody experiments
Supernatants from the 9H10 hybridoma were collected and purified on a HiTrap protein A Sepharose column (GE Healthcare, Chalfont St Giles, Buckinghamshire, United Kingdom). The anti-CTLA-4 monoclonal antibodies were eluted with 0.1 M citric acid, pH 3.0, and flowthrough was collected in 1-mL aliquots and rapidly neutralized with 100 μl 1 M Tris-Cl, pH 8.0. Protein concentrations were measured by Bradford analysis (Bio-Rad Laboratories, Hercules, CA). Aliquots with the highest protein concentrations were pooled, and antibody concentrations were determined by coating a MaxiSorp enzyme-linked immunosorbent assay (ELISA) plate (Nalge Nunc International, Rochester, NY) with 1:50 diluted pooled aliquots after an incubation at 4°C overnight. Hamster IgG (BD Pharmingen, San Diego, CA) was used for standardization. After washing, the plates were incubated with an alkaline phosphatase-conjugated goat anti-hamster Ig (Southern Biotechnology Associates, Birmingham, AL) and developed with p-nitrophenyl phosphate (Bio-Rad Laboratories). The absorbances at 405 nm were measured. One hundred micrograms of purified anti-CTLA4 or hamster IgG monoclonal antibodies were injected into the peritoneal cavities of young female mice at the age of 12, 15, and 18 days in a volume of 200 μL 0.9% NaCl. Blood sugar measurements from tail vein blood started at day 10 after the first injection.
Cytokine measurements
Peritoneal macrophages (5 × 105 or 1 × 106) were cultured in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine and 10% fetal calf serum and stimulated with 100 ng of lipopolysaccharide (LPS) (Escherichia coli serotype B5:055; Sigma) for 24 hours. Supernatants were harvested for ELISA, and pellets were used for RNA isolation (QIAGEN method; QIAGEN, Valencia, CA). For real-time PCR, reverse transcriptions were performed with 0.5 μg total RNA using the SuperScript First Strand Synthesis System (Invitrogen, Carlsbad, CA). cDNA was amplified with specific primers, and output was monitored by SYBR Green (Roche, Indianapolis, IN) and the ABI Prism 7700 System (Applied Biosystems, Foster City, CA). The results were normalized to the level of cyclophilin mRNA. The primer sequences were: p40: forward, atccagcgcaagaaagaaaa; reverse, ggaacgcacctttctggtta; p35: forward, atgaagctctgcatcctgct; reverse, cagatagcccatcaccctgt; p19: forward, ccagcgggacatatgaatct; reverse, tggatacggggcacattatt; and TNF-α: forward, acagaaagcatgatccgcg; reverse, gccccccatcttttggg; and cyclophilin: forward, atggtcaaccccaccgtgt; reverse, ttcttgctgtctttgttggaactttgtc. Absolute data were normalized to cyclophilin for each reaction according to the formula: 2 [CT(Cyclophilin) − CT(Sample)]. For ELISAs, culture supernatants were concentrated with Centricon 25 columns (Millipore, Billerica, MA) and then analyzed with the mouse IL-12 ELISA kit (BD Biosciences) according to the manufacturer's instructions.
Results
GM-CSF/IL-3–deficient mice developed insulitis and β-cell destruction
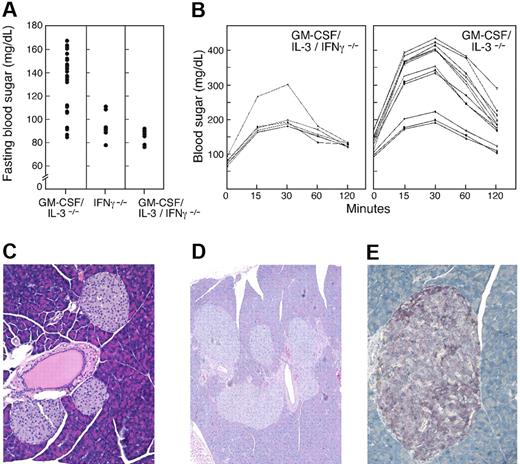
Previous studies characterized the development of pulmonary alveolar proteinosis and a SLE-like disorder in mice deficient in GM-CSF and IL-3.20,22 Further analysis of aged mutant mice (8+ months) on the C57Bl/6 background revealed an inflammatory pathologic condition in the pancreas (Figure 1 and Table 1). Although wild-type controls demonstrated minimal abnormalities, GM-CSF singly deficient animals manifested large lymphoid aggregates that circumscribed a significant proportion of the islets; these aggregates rarely penetrated the islets and were not associated with necrosis. Approximately 60% of the infiltrates were graded as 3+, with more than 50 mononuclear cells per reaction. IL-3 singly deficient mice23 also displayed peri-insulitis, albeit of reduced intensity.
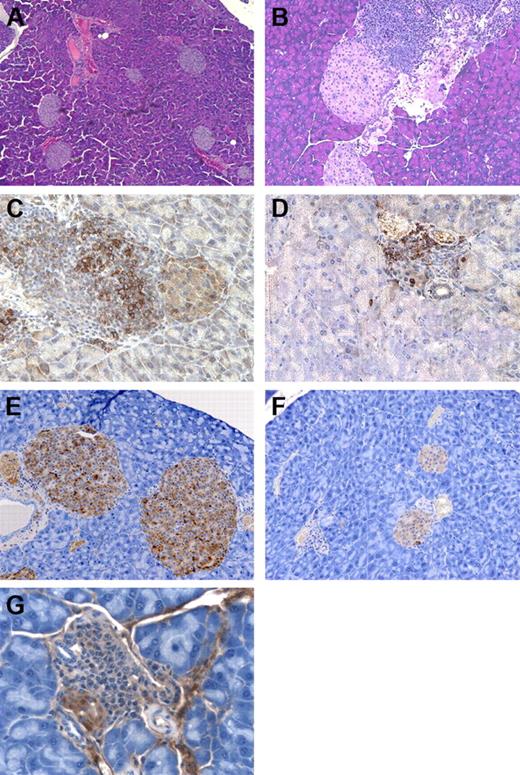
GM-CSF/IL-3–deficient mice develop insulitis. (A) Wild-type mouse with normal islets, original magnification × 100. (B) Insulitis and peri-insulitis in GM-CSF/IL-3–deficient mouse, original magnification × 200. (C) B220+ B cells in the islets of GM-CSF/IL-3–deficient mouse, original magnification × 400. (D) CD3+ T cells in the islets of GM-CSF/IL-3–deficient mouse, original magnification × 400. (E) Insulin-producing β cells in wild-type mouse, original magnification × 200. (F) Decreased insulin-producing β cells in GM-CSF/IL-3–deficient mouse, original magnification × 200. (G) Insulitis with β-cell destruction in GM-CSF/IL-3–deficient mouse, original magnification × 400.
GM-CSF/IL-3–deficient mice develop insulitis. (A) Wild-type mouse with normal islets, original magnification × 100. (B) Insulitis and peri-insulitis in GM-CSF/IL-3–deficient mouse, original magnification × 200. (C) B220+ B cells in the islets of GM-CSF/IL-3–deficient mouse, original magnification × 400. (D) CD3+ T cells in the islets of GM-CSF/IL-3–deficient mouse, original magnification × 400. (E) Insulin-producing β cells in wild-type mouse, original magnification × 200. (F) Decreased insulin-producing β cells in GM-CSF/IL-3–deficient mouse, original magnification × 200. (G) Insulitis with β-cell destruction in GM-CSF/IL-3–deficient mouse, original magnification × 400.
Pathologic analysis of insulitis in GM-CSF– and IL-3–deficient mice
| Genotype and islets scored . | Peri-insulitis . | Insulitis . | Necrosis . |
|---|---|---|---|
| Wild-type | |||
| 11 | 0 | 0 | 0 |
| 14 | 1 | 0 | 0 |
| 5 | 1 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| GM-CSF−/− | |||
| 9 | 2 | 0 | 0 |
| 25 | 13 | 0 | 0 |
| 16 | 14 | 0 | 0 |
| 14 | 4 | 0 | 0 |
| 22 | 7 | 0 | 0 |
| 19 | 3 | 1 | 0 |
| 22 | 9 | 1 | 0 |
| IL-3−/− | |||
| 15 | 3 | 0 | 0 |
| 19 | 5 | 0 | 0 |
| 19 | 4 | 0 | 0 |
| 20 | 9 | 0 | 0 |
| GM-CSF/IL-3−/− | |||
| 19 | 0 | 8 | 0 |
| 17 | 4 | 4 | 0 |
| 5 | 1 | 4 | 0 |
| 11 | 3 | 4 | 0 |
| 11 | 4 | 7 | 1 |
| 18 | 2 | 13 | 1 |
| 6 | 2 | 4 | 2 |
| 17 | 7 | 8 | 6 |
| 14 | 7 | 7 | 6 |
| Genotype and islets scored . | Peri-insulitis . | Insulitis . | Necrosis . |
|---|---|---|---|
| Wild-type | |||
| 11 | 0 | 0 | 0 |
| 14 | 1 | 0 | 0 |
| 5 | 1 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| GM-CSF−/− | |||
| 9 | 2 | 0 | 0 |
| 25 | 13 | 0 | 0 |
| 16 | 14 | 0 | 0 |
| 14 | 4 | 0 | 0 |
| 22 | 7 | 0 | 0 |
| 19 | 3 | 1 | 0 |
| 22 | 9 | 1 | 0 |
| IL-3−/− | |||
| 15 | 3 | 0 | 0 |
| 19 | 5 | 0 | 0 |
| 19 | 4 | 0 | 0 |
| 20 | 9 | 0 | 0 |
| GM-CSF/IL-3−/− | |||
| 19 | 0 | 8 | 0 |
| 17 | 4 | 4 | 0 |
| 5 | 1 | 4 | 0 |
| 11 | 3 | 4 | 0 |
| 11 | 4 | 7 | 1 |
| 18 | 2 | 13 | 1 |
| 6 | 2 | 4 | 2 |
| 17 | 7 | 8 | 6 |
| 14 | 7 | 7 | 6 |
GM-CSF indicates granulocyte-macrophage colony-stimulating factor; IL-3, interleukin 3.
Mice doubly deficient in GM-CSF and IL-3 showed more severe disease, with lymphocytic infiltrates extending into the islets, which in some cases evidenced necrosis of scattered single cells. Immunohistochemistry revealed the presence of both CD3+ T cells and B220+ B cells in these reactions. To determine whether this insulitis resulted in a loss of β cells, we performed immunostaining for insulin. Compared with the wild-type control mice, GM-CSF/IL-3 doubly deficient mice showed a marked reduction in the size of many islets, and the total number of insulin-secreting β cells was greatly reduced. Moreover, immune-mediated destruction was evidenced by the lymphocyte satellitosis of β cells, which was accompanied by islet cell cytoplasmic swelling and nuclear degeneration. Mega-islets were not observed.
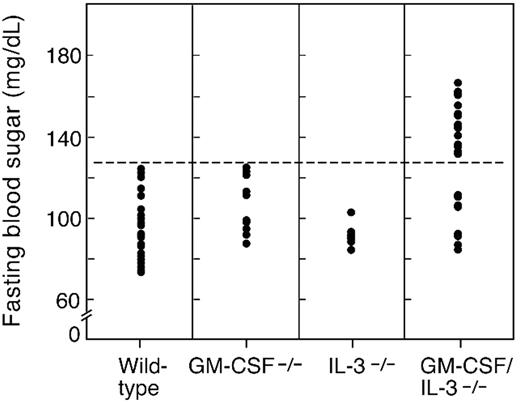
To determine whether these pathologic features were associated with abnormalities in glucose homeostasis, we measured fasting blood sugar levels in age-matched mice (8 months). Analysis of 30 wild-type animals yielded a mean fasting glucose of 96 mg/dL, with a standard deviation of 14.3 (Figure 2). The fasting sugar levels for IL-3–deficient mice (n = 8) were comparable with those of wild-type control mice (mean, 91.5 mg/dL; SD 5.4; P = .21), whereas GM-CSF–deficient animals (n = 10) showed a modest elevation of borderline statistical significance (mean, 107 mg/dL; SD, 13.9; P = .04). In contrast, 22 of 30 GM-CSF/IL-3 doubly deficient mice demonstrated fasting hyperglycemia (mean, 147 mg/dL; SD 10.4; P < .001), whereas the other 8 were comparable with controls (mean, 99.1 mg/dL; SD 11; P = .26). Young GM-CSF/IL-3–deficient mice (1 or 2 months) did not show fasting hyperglycemia (data not shown), although additional studies are required to define the precise time frame of disease onset.
Aged GM-CSF/IL-3–deficient mice develop fasting hyperglycemia. Blood sugar levels were determined for wild-type (n = 30), GM-CSF−/− (n = 10), IL-3−/− (n = 8), and GM-CSF/IL-3−/− (n = 30) mice. Of 30 GM-CSF/IL-3 doubly deficient mice, 22 showed fasting hyperglycemia compared with control mice (P < .001, Wilcoxon rank sum test).
Aged GM-CSF/IL-3–deficient mice develop fasting hyperglycemia. Blood sugar levels were determined for wild-type (n = 30), GM-CSF−/− (n = 10), IL-3−/− (n = 8), and GM-CSF/IL-3−/− (n = 30) mice. Of 30 GM-CSF/IL-3 doubly deficient mice, 22 showed fasting hyperglycemia compared with control mice (P < .001, Wilcoxon rank sum test).
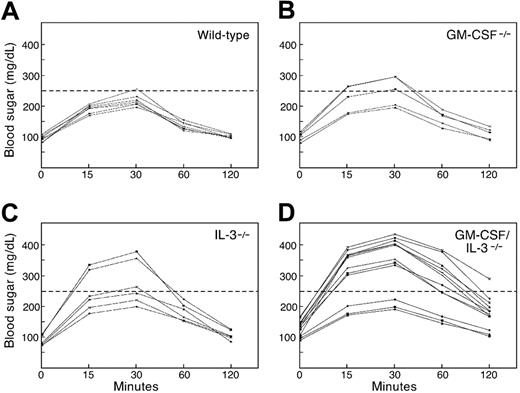
To further characterize glucose homeostasis, we administered a glucose challenge intraperitoneally into 8-month-old mice and followed blood sugar levels serially (Figure 3). The maximum glucose levels achieved at 30 minutes after infusion were comparable among wild-type (n = 8; mean, 213 mg/dL), GM-CSF–deficient (n = 5;, mean, 256 mg/dL; P = .59), and IL-3–deficient (n = 6; mean, 252 mg/dL; P = .13) animals. However, 9 of 12 GM-CSF/IL-3–deficient mice showed significantly greater peak blood sugar levels (mean, 402 mg/dL; P < .001) that remained elevated at the end of the test, whereas levels in the remaining 3 mice were comparable with those of control mice. All of the double-deficient mice with a diabetic glucose tolerance profile demonstrated an elevated fasting blood sugar. Taken together, these results indicate that a high proportion of aged GM-CSF/IL-3–deficient mice develop autoimmune diabetes. However, further work will be necessary to understand the mechanisms underlying the heterogeneity in disease development in these animals.
GM-CSF/IL-3–deficient mice show abnormal glucose tolerance tests. Wild-type (n = 8), GM-CSF–deficient (n = 5), IL-3–deficient (n = 6), and GM-CSF/IL-3–deficient (n = 12) mice were injected intraperitoneally with a glucose challenge, and blood (taken from the mice's tails) were serially analyzed. Nine GM-CSF/IL-3–deficient mice showed significantly greater peak blood sugar levels that remained elevated at the end of the test compared with all other groups (P < .001, Wilcoxon rank sum test).
GM-CSF/IL-3–deficient mice show abnormal glucose tolerance tests. Wild-type (n = 8), GM-CSF–deficient (n = 5), IL-3–deficient (n = 6), and GM-CSF/IL-3–deficient (n = 12) mice were injected intraperitoneally with a glucose challenge, and blood (taken from the mice's tails) were serially analyzed. Nine GM-CSF/IL-3–deficient mice showed significantly greater peak blood sugar levels that remained elevated at the end of the test compared with all other groups (P < .001, Wilcoxon rank sum test).
GM-CSF/IL-3–deficient mice show altered macrophage cytokine production
Our previous analysis of the mechanisms underlying the development of SLE in GM-CSF/IL-3–deficient mice uncovered a marked impairment in macrophage phagocytosis of apoptotic cells.20 A similar defect has been reported in NOD mice,26 raising the possibility that some macrophage abnormalities in diabetes might reflect alterations in GM-CSF and IL-3 function. In this context, several studies have shown increased macrophage production of p40 in response to LPS, and this cytokine has been mapped to the Idd4 (mouse) or IDDM18 (human) susceptibility loci.27,28 Persistently elevated NF-κB activity and compromised phagocytosis of apoptotic cells might contribute to the enhanced p40 production.29,30
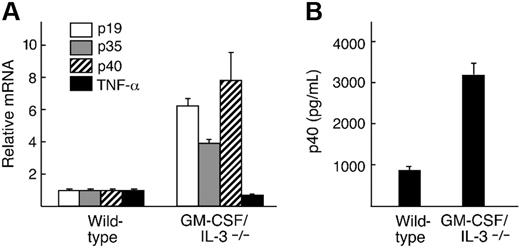
To examine whether inflammatory cytokine profiles were altered in GM-CSF/IL-3–deficient mice, we harvested peritoneal macrophages and stimulated them in vitro with 100 ng of LPS for 24 hours (Figure 4). Real-time PCR disclosed a marked increase in p40 transcripts of the cytokine-deficient macrophages compared with wild-type control mice (P = .001), using cyclophilin as a reference standard. An ELISA performed on culture supernatants confirmed the increased production of p40 protein (P = .001). Because p40 is a subunit of the heterodimeric cytokines IL-12 and IL-23,31 we also examined expression levels of the unique p19 and p35 chains. Real-time PCR demonstrated increases in both transcripts compared with wild-type control mice (P = .05), whereas TNF-α levels were not altered.
GM-CSF/IL-3–deficient macrophages produce increased inflammatory cytokines in response to LPS stimulation. (A). Real-time PCR analysis shows increased levels of p19, p35, and p40 in GM-CSF/IL-3–deficient macrophages compared with wild-type control mice. (B). GM-CSF/IL-3–deficient macrophages secrete elevated levels of p40 protein compared with wild-type macrophages. Shown are 3 animals per group. Similar results were observed in 3 independent experiments. Error bars refer to the standard deviations for the 3 samples in each group.
GM-CSF/IL-3–deficient macrophages produce increased inflammatory cytokines in response to LPS stimulation. (A). Real-time PCR analysis shows increased levels of p19, p35, and p40 in GM-CSF/IL-3–deficient macrophages compared with wild-type control mice. (B). GM-CSF/IL-3–deficient macrophages secrete elevated levels of p40 protein compared with wild-type macrophages. Shown are 3 animals per group. Similar results were observed in 3 independent experiments. Error bars refer to the standard deviations for the 3 samples in each group.
Although recent studies have underscored a central role for IL-23 and Th-17 cells in experimental allergic encephalomyelitis and collagen-induced arthritis,32,33 previous work showed that IL-12 administration could exacerbate diabetes in NOD mice, in part through the stimulation of IFN-γ-secreting Th1 cells that infiltrate the islets.34,35 Although p40 and IFN-γ–deficient NOD mice display minimal alterations in the frequency or kinetics of diabetes development,36-38 the progression to diabetes in several models is associated with increased expression of IFN-γ–induced genes.39-41 Thus, IFN-γ might play an important role, although other pathways may contribute to disease development in the absence of this cytokine.
To determine whether IFN-γ is involved in the diabetes in GM-CSF/IL-3–deficient mice, we introgressed an IFN-γ–null allele and thereby generated triply cytokine-deficient mice on the C57Bl/6 background (Figure 5). GM-CSF/IL-3/IFN-γ–deficient mice (n = 10) showed significantly reduced fasting blood sugar levels compared with GM-CSF/IL-3–deficient mice (P < .001), and these levels were similar to those of IFN-γ–deficient mice and wild-type control mice. Moreover, glucose tolerance tests for GM-CSF/IL-3/IFN-γ–deficient mice (n = 6) were significantly improved compared with GM-CSF/IL-3–deficient animals (n = 12). Mean peak glucose values were 190 mg/dL for triple knockouts compared with 402 mg/dL for double knockouts with diabetes (P < .001). Consistent with these metabolic parameters, pathologic examination of the pancreases of aged GM-CSF/IL-3/IFN-γ–deficient mice failed to disclose peri-insulitis or insulitis. Interestingly, however, 2 of 16 triply deficient mice analyzed showed islet cell adenomas. Immunostaining revealed that these pancreatic lesions were composed primarily of insulin-secreting β cells rather than glucagon- or somatostatin-producing cells, although the insulin staining was more punctate and heterogeneous than in wild-type mice. These findings raise the possibility that insulin metabolism and/or islet cell homeostasis might be altered by the compound cytokine deficiency. In this context, we previously demonstrated that GM-CSF/IL-3/IFN-γ–deficient animals succumb to a high incidence of hematologic and solid malignancies within a background of chronic infection.20 Further studies are required to define the precise impact of chronic infection and cytokine dysfunction on islet cell function.
IFN-γ is required for diabetes in GM-CSF/IL-3–deficient mice. (A) Fasting blood sugar levels were determined for IFN-γ–deficient (n = 8) and GM-CSF/IL-3/IFN-γ–deficient (n = 10) mice and compared with GM-CSF/IL-3–deficient mice shown in Figure 2. GM-CSF/IL-3/IFN-γ–deficient animals showed significantly reduced fasting blood sugar levels compared with GM-CSF/IL-3–deficient mice (P < .001). (B) Glucose tolerance tests were performed on GM-CSF/IL-3/IFN-γ–deficient mice (n = 6). Peak glucose levels were significantly reduced compared with GM-CSF/IL-3–deficient animals shown in Figure 3 (P < .001). (C) Absence of insulitis in a GM-CSF/IL-3/IFN-γ–deficient mouse, original magnification ×200. (D) Islet cell adenomas in GM-CSF/IL-3/IFN-γ–deficient mouse, original magnification ×200. (E). Insulin (red) and somatostatin (brown) staining in adenoma in GM-CSF/IL-3/IFN-γ–deficient mouse, original magnification ×400.
IFN-γ is required for diabetes in GM-CSF/IL-3–deficient mice. (A) Fasting blood sugar levels were determined for IFN-γ–deficient (n = 8) and GM-CSF/IL-3/IFN-γ–deficient (n = 10) mice and compared with GM-CSF/IL-3–deficient mice shown in Figure 2. GM-CSF/IL-3/IFN-γ–deficient animals showed significantly reduced fasting blood sugar levels compared with GM-CSF/IL-3–deficient mice (P < .001). (B) Glucose tolerance tests were performed on GM-CSF/IL-3/IFN-γ–deficient mice (n = 6). Peak glucose levels were significantly reduced compared with GM-CSF/IL-3–deficient animals shown in Figure 3 (P < .001). (C) Absence of insulitis in a GM-CSF/IL-3/IFN-γ–deficient mouse, original magnification ×200. (D) Islet cell adenomas in GM-CSF/IL-3/IFN-γ–deficient mouse, original magnification ×200. (E). Insulin (red) and somatostatin (brown) staining in adenoma in GM-CSF/IL-3/IFN-γ–deficient mouse, original magnification ×400.
CTLA-4 antibody blockade stimulated rapid progression to destructive insulitis in GM-CSF/IL-3–deficient mice
Whereas diabetes development in GM-CSF/IL-3–deficient mice manifests a relatively long latency, the addition of other susceptibility loci might accelerate disease pathogenesis. Indeed, recent investigations have delineated a key role for the negative immune regulator CTLA-4 in diabetes.42 Reduced expression of a soluble isoform of CTLA-4 in patients with T1D or of a ligand-independent CTLA-4 product in NOD mice is associated with increased disease risk. Consistent with these findings, the administration of blocking antibodies to CTLA-4 to young NOD or BDC2.5 TCR transgenic mice precipitated destructive disease, although older animals were refractory to this manipulation.43,44 Moreover, lentivirus-mediated RNA interference knockdown of CTLA-4 similarly evoked insulitis.45
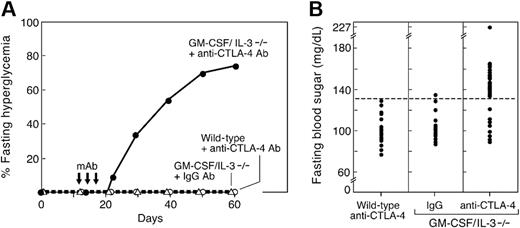
In view of these data, we wondered whether disrupting CTLA-4 function might increase the kinetics of diabetes development in GM-CSF/IL-3–deficient mice, thereby uncovering a potential important interaction between cytokine deficiency and loss of negative immune regulation. We thus administered a blocking anti–CTLA-4 monoclonal antibody to 12-day-old GM-CSF/IL-3–deficient animals, which do not demonstrate islet pathology at this age (Figure 6). Administration of anti–CTLA-4 antibody to age-matched, wild-type mice failed to elicit fasting hyperglycemia, in agreement with earlier reports that CTLA-4 blockade alone was insufficient to provoke diabetes.43,44 Similarly, the injection of isotype control antibody into 12-day-old GM-CSF/IL-3–deficient mice did not evoke disease. In contrast, 22 of 30 young GM-CSF/IL-3–deficient mice treated with anti–CTLA-4 antibody developed fasting hyperglycemia within 3 weeks. Mean fasting sugar levels were 153 mg/dL (SD 19.5) for diabetic cytokine knockouts versus 99.8 mg/dL (SD 13.1) for anti–CTLA-4 antibody-treated wild-type mice (P < .001); the 8 remaining treated GM-CSF/IL-3–deficient animals were comparable with control mice (mean fasting glucose, 102.5 mg/dL; SD, 11). Moreover, the administration of anti–CTLA-4 antibody to 4 weeks old GM-CSF/IL-3–deficient mice failed to provoke disease, consistent with the narrow window of CTLA-4 function in NOD and BDC2.5 transgenic mice.44
CTLA-4 antibody blockade precipitates diabetes in young GM-CSF/IL-3–deficient mice. (A) Time course of disease development. GM-CSF/IL-3–deficient (n = 30) or wild-type mice (n = 25) were injected intraperitoneally with 100 μg of anti-CTLA-4 antibody (clone 9H10) three times as indicated (arrows). A cohort of GM-CSF/IL-3–deficient mice (n = 15) was similarly treated with isotype-matched IgG control antibody. (B) Fasting blood sugar levels were determined 50 days after the initiation of antibody injections. Of 30 treated GM-CSF/IL-3–deficient mice, 22 developed fasting hyperglycemia (P < .001 versus all other groups).
CTLA-4 antibody blockade precipitates diabetes in young GM-CSF/IL-3–deficient mice. (A) Time course of disease development. GM-CSF/IL-3–deficient (n = 30) or wild-type mice (n = 25) were injected intraperitoneally with 100 μg of anti-CTLA-4 antibody (clone 9H10) three times as indicated (arrows). A cohort of GM-CSF/IL-3–deficient mice (n = 15) was similarly treated with isotype-matched IgG control antibody. (B) Fasting blood sugar levels were determined 50 days after the initiation of antibody injections. Of 30 treated GM-CSF/IL-3–deficient mice, 22 developed fasting hyperglycemia (P < .001 versus all other groups).
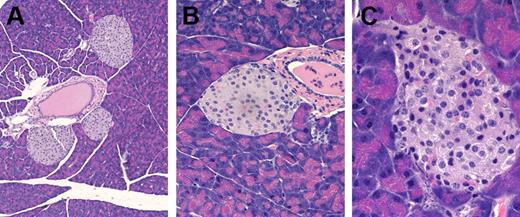
Pathologic examination of anti–CTLA-4 antibody-treated GM-CSF/IL-3–deficient mice that developed diabetes revealed lymphocytes infiltrating the islets (Figure 7). In contrast, wild-type mice that received anti–CTLA-4 antibody and GM-CSF/IL-3–deficient mice that received control antibody did not show islet abnormalities. Together, these results show that defects in CTLA-4 cooperate with deficiencies of GM-CSF and IL-3 in the pathogenesis of diabetes.
Anti–CTLA-4 antibody triggers insulitis in young GM-CSF/IL-3–deficient mice. (A) Normal islets in a wild-type mouse treated with anti–CTLA-4 mAb, original magnification, × 60. (B) Normal islets in a young GM-CSF/IL-3–deficient mouse treated with control Ig, original magnification, × 200. (C) Insulitis in a young GM-CSF/IL-3–deficient mouse treated with anti–CTLA-4 mAb, original magnification, × 400.
Anti–CTLA-4 antibody triggers insulitis in young GM-CSF/IL-3–deficient mice. (A) Normal islets in a wild-type mouse treated with anti–CTLA-4 mAb, original magnification, × 60. (B) Normal islets in a young GM-CSF/IL-3–deficient mouse treated with control Ig, original magnification, × 200. (C) Insulitis in a young GM-CSF/IL-3–deficient mouse treated with anti–CTLA-4 mAb, original magnification, × 400.
Discussion
The ability of GM-CSF to enhance protective immune responses is well established. Vaccination with irradiated tumor cells engineered to secrete GM-CSF stimulates potent, specific, and long-lasting anti-tumor immunity through improved antigen presentation by dendritic cells and macrophages.46 Similarly, the provision of GM-CSF through pharmacologic strategies activates innate immune effectors and increases antigen-specific T- and B-cell responses against diverse pathogens.47
The role of GM-CSF in immune regulation, however, is less clear.48 Transgenic mice in which GM-CSF was expressed in the stomach epithelium developed gastritis,49 whereas GM-CSF expression in the islets either exacerbated or inhibited diabetes, depending on the genetic background.50,51 GM-CSF–deficient mice displayed reduced susceptibility to collagen-induced arthritis and experimental allergic encephalomyelitis, which correlated with the induction of weaker autoreactive T-cell responses, but preserved antibody reactions.52,53 We previously reported that GM-CSF–deficient and, to a greater degree, GM-CSF/IL-3 doubly deficient mice developed an SLE-like disorder with anti–double-stranded DNA antibodies and immune complex deposition in the glomeruli of the kidney.20 The studies presented here extend these findings to demonstrate that GM-CSF/IL-3–deficient mice also manifest insulitis and compromised glucose homeostasis. Consistent with NOD mice, the pathogenesis of diabetes in this model involves p40, IFN-γ, and CTLA-4.
Many studies have documented impaired responses of hematopoietic cells from NOD mice and patients with T1D to GM-CSF and IL-3.6-19 These abnormalities appear to reflect cell-intrinsic defects in myeloid cells, resulting in altered numbers or maturation of dendritic cells, macrophages, and granulocytes. However, the contribution of these defects, if any, to diabetes pathogenesis remained unclear. In this context, our characterization of hematopoiesis in GM-CSF/IL-3 doubly deficient mice uncovered only a mild eosinophilia that was probably due to enhanced IL-5 signaling through the βc subunit shared among GM-CSF, IL-3, and IL-5.24 Contact hypersensitivity reactions were markedly diminished, though, and this involved a reduction in priming hapten-specific responses.
Macrophages from GM-CSF/IL-3–deficient mice are impaired in the phagocytosis of apoptotic cells.20 This defect has been linked to the development of SLE in several systems,21 including NOD mice, which manifest a similar abnormality.26 The importance of efficient clearance of dying cells in diabetes has been highlighted by studies that reveal a developmental window of β-cell death that is linked to dendritic cell infiltration of islets and the transport of islet cell antigens to draining lymph nodes.54 Efficient capture and presentation of islet cell antigens is critical for the maintenance of anergic and regulatory T cells that protect against diabetes.55,56 Furthermore, the cross-talk between dendritic cells and CD1d-restricted NKT cells also contributes to tolerance,57 and it is thus noteworthy that optimal dendritic cell activation stimulated by GM-CSF–secreting tumor cells similarly requires CD1d-restricted NKT cells.58
Defects in the uptake of dying cells by macrophages might contribute to autoimmune disease through the decreased production of immunoregulatory cytokines such as TGF-β and the increased production of pro-inflammatory cytokines such as IL-12.30,59 Indeed, the production of p40 after stimulation of macrophages from GM-CSF/IL-3–deficient and NOD mice was markedly increased, and these findings are consistent with the identification of p40 as a disease susceptibility locus in NOD mice and possibly patients with T1D.27,28 Additional studies are required to determine whether these defects affect thymic selection.
Characterization of cytokine profiles in stimulated GM-CSF/IL-3–deficient macrophages revealed elevated levels of both IL-12 and IL-23. Although recent work has shown that IL-23 plays a major role in the inflammatory pathology of collagen-induced arthritis and experimental allergic encephalomyelitis,32,33 earlier studies demonstrated that the administration of IL-12 to NOD mice exacerbated the disease.34,35 The effects of IL-12 involved the stimulation of IFN-γ producing Th1 cells, although the cytokine also worsened disease in IFN-γ–deficient NOD mice through additional cytotoxic mechanisms that included Fas-ligand. This redundancy of function may help explain why IFN-γ–deficient NOD mice display only minor differences in disease development compared with wild-type mice.37,38 Indeed, IFN-γ target genes are highly up-regulated during the progression from benign to destructive insulitis triggered by cyclophosphamide or CTLA-4 antibody blockade.39-41 Our finding that IFN-γ is required for insulitis and compromised glucose homeostasis in GM-CSF/IL-3–deficient mice supports the idea that IFN-γ–regulated pathways are important in autoimmune pathologic conditions.60 Moreover, the development of islet cell adenomas, but not insulitis, in some GM-CSF/IL-3/IFN-γ–deficient mice raises the possibility that IFN-γ might also participate in islet cell growth control. In this context, the distinction between islet cell adenoma and hyperplasia is based on pathologic criteria of size (greater than 2 mm) and alterations in cell morphology. Additional studies are required to clarify the molecular mechanisms underlying these alterations.
Consistent with the identification of CTLA-4 as a disease susceptibility locus in NOD mice and possibly patients with T1D,42 we found that administration of anti–CTLA-4 blocking antibodies to young GM-CSF/IL-3–deficient mice precipitated diabetes in a high proportion of animals. As in the BDC2.5 transgenic TCR model, CTLA-4 blockade was active only within a narrow time interval.43,44 Studies of the BDC2.5 mice indicated that the CTLA-4 effect was restricted to the initial contact of naive T cells with antigen-loaded dendritic cells in the draining lymph node; once insulitis was established, CTLA-4 blockade was inactive. Further studies are necessary to delineate whether a similar mechanism accounts for the therapeutic window in GM-CSF/IL-3–deficient mice. It will also be of interest to examine the impact of blocking antibodies to PD-1 in this model, particularly because IFN-γ is a potent stimulus for PD-L1, which can be expressed at high levels in the islets.61-63
Taken together, the studies presented here indicate that functional deficiencies of GM-CSF and IL-3 contribute to diabetes. The more severe pathologic condition observed in the doubly deficient mice compared with the single knockouts probably reflects some redundancy in signaling through the shared βc subunit.24 Because macrophages from NOD mice and patients with T1D show increased GM-CSF production,18,19 gene products involved in cytokine receptor signaling or downstream targets are candidates for disease susceptibility loci. Indeed, alterations in STAT5 activity have been reported in patients with T1D and NOD mice.18,19,64 It is tempting to speculate that gene products involved in the defective phagocytosis of apoptotic cells by GM-CSF/IL-3–deficient macrophages may be critical determinants of autoimmunity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Rohit Kulkarni and Jiang Hu (Joslin Clinic) for the islet immunohistochemistry; Esther Brisson, Christine Sheehan, Paula North, Adam Mizeraki, and Paul Googe for help with the histologic specimens; and Stephen Conley for help with photography.
This work was supported by the National Institutes of Health and the Leukemia and Lymphoma Society.
National Institutes of Health
Authorship
Contribution: T.E., S.G., M.D., D.A.O., and M.M. performed research; T.E., S.G., M.D., D.N., D.A.O., M.M., and G.D. analyzed data; J.P.A. contributed a vital reagent; and T.E. and G.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Glenn Dranoff, Dana-Farber Cancer Institute, Dana 520C, 44 Binney St, Boston, MA 02115; e-mail: glenn_dranoff@dfci.harvard.edu.