In this issue of Blood, the report by Camaschella and colleagues of the first patient with a defective iron-sulfur cluster (ISC) assembly and microcytic sideroblastic anemia is a vital piece of information in the rapidly accumulating evidence that there is cross-talk between ISC assembly and the heme biosynthetic pathway, especially in erythroid cells.
Congenital or acquired sideroblastic anemias (SAs) are a heterogeneous group of disorders characterized by the presence of ring sideroblasts (erythroblasts with abnormal mitochondrial iron deposits) in the bone marrow. X-linked SA (XLSA) is due to mutations in the ALAS2 gene encoding the erythroid-specific isoform of the first enzyme of the heme biosynthetic pathway. Some rare forms of SA associated with ataxia in early childhood result from a mutation in ABCB7, a mitochondrial membrane protein thought to export components of ISC out of the mitochondria to participate in the maturation of cytosolic Fe-S proteins. The identification of the molecular defect in these rare patients was the first hint that ISC assembly plays an important role in the control of heme formation in erythroid cells. Further demonstration of this fact occurred with the discovery of a large deletion in the glutaredoxin 5 (glrx5) gene in the shiraz zebrafish mutant,1 displaying hypochromic microcytic anemia at birth, and again with Camaschella and colleagues' identification of a patient with GLRX5 deficiency, microcytic sideroblastic anemia, and iron overload.
In eukaryotes, GLRX5 is part of a complex and highly conserved machinery necessary for assembly and export of ISC and for the maturation of mitochondrial, cytosolic, or nuclear Fe-S containing proteins.2 In yeast, mutants in components of Fe-S biogenesis present abnormal accumulation of iron in the mitochondria, resulting from the transcriptional activation of the so-called iron regulon, encompassing genes important for iron acquisition and intracellular distribution. These regulations are mediated by the Aftp transcription factors that act as sensors of cellular Fe-S status.3
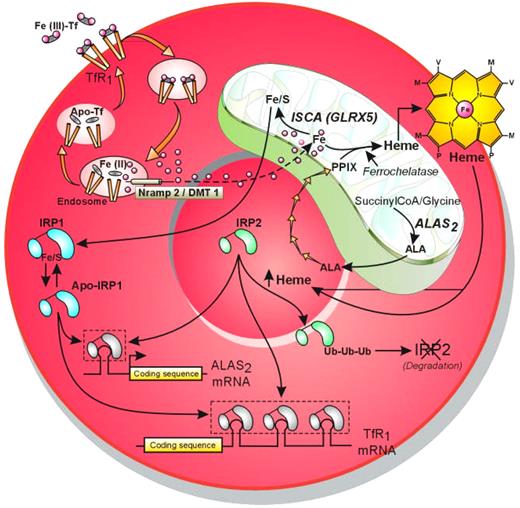
In developing erythroblasts, high iron demand for heme and hemoglobin production is met by a high density of transferrin receptors (TfRs) at the cell surface. Following endocytosis of the iron-transferrin-TfR1 complex, iron is released from transferrin and principally targeted to the mitochondria for incorporation into protoporphyrin IX (PPIX) to form heme, a step catalyzed by ferrochelatase, or for ISC assembly. Cellular iron uptake and PPIX formation are coordinated by posttranscriptional regulations mediated by 2 iron sensors called IRP1 and IRP2 (see the figure). A 4Fe-4S cluster is required for IRP1 iron sensing, and when ISC assembly is defective, as in the patient with GLRX5 mutation or in the shiraz zebrafish mutant, IRP1 remains in the apo form with high RNA binding affinity. As a consequence, translation of the IRE-containing mRNA coding for ALAS2 is repressed, preventing PPIX and thereby heme formation. Molecular defects in the ALAS2 gene, as found in patients with XLSA, also block PPIX formation. In both cases, there is an activation of TfR1expression through IRPs-mediated stabilization of its mRNA, increased influx of iron into the mitochondria despite the lack of heme precursor, and formation of abnormal deposits of iron bound to mitochondrial ferritin, contributing to the formation of ring sideroblasts. It is not clear whether both IRP1 and IRP2 contribute to stabilization of TfR1 mRNA because IRP2 has been proposed to be the main iron sensor in erythroid cells.4,5 IRP2 is stabilized by heme deficiency, whereas accumulation of free heme induces its ubiquitination and degradation by the proteasome.6
Coordination of the cellular iron-acquisition pathway with mitochondrial PPIX formation and ISC assembly through modulation of IRP1 and IRP2 RNA binding affinities. Illustration by Jean-Pierre Laigneau.
Coordination of the cellular iron-acquisition pathway with mitochondrial PPIX formation and ISC assembly through modulation of IRP1 and IRP2 RNA binding affinities. Illustration by Jean-Pierre Laigneau.
Interestingly, anemia of the patient with the GLRX5 mutation improved with iron-chelation therapy, suggesting that these mitochondrial iron deposits are deleterious for developing erythroblasts.
In view of the complexity of the ISC assembly, it is likely that mutations in genes encoding other enzymes of this pathway underlie other forms of non–X-linked congenital sideroblastic anemia, although one can expect more severe phenotypes and/or the presence of additional symptoms such as ataxia or metabolic disorders.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal