Abstract
Rh immune globulin (WinRho SDF; Cangene, Mississauga, ON, Canada) is an effective treatment for autoimmune thrombocytopenic purpura; however, maintaining a sustained supply for its use in autoimmune thrombocytopenic purpura and its primary indication, hemolytic disease of the newborn, makes the development of alternative reagents desirable. We compared Rh immune globulin and 6 human monoclonal anti-D antibodies (MoAnti-D) with differing isotypes and specificities for their ability to opsonize erythrocytes and inhibit platelet phagocytosis in an in vitro assay. Results demonstrated that opsonization of erythrocytes with Rh immune globulin significantly (P < .001) reduced phagocytosis of fluorescently labeled opsonized platelets in an Fc-dependent manner. Of the MoAnti-D that shared specificity but differed in isotype, only IgG3 antibodies could significantly (P < .001) inhibit platelet phagocytosis. In contrast, 2 MoAnti-D shared isotypes and differed in specificity; however, only one could significantly (P < .001) inhibit platelet phagocytosis. The results suggest that MoAnti-D epitope specificity and isotypes are critical requirements for optimal inhibition of opsonized platelet phagocytosis.
Introduction
Autoimmune thrombocytopenic purpura (AITP) is a bleeding disorder in which platelets are opsonized by autoantibodies and destroyed by Fc receptor (R)-mediated phagocytosis.1,2 Treatment options can include corticosteroids or splenectomy, and if these fail to raise platelet counts, more potent compounds may be used.1,2 Rh immune globulin also increases platelet counts in AITP3–5 ; however, the competition for supplies of Rh immune globulin for its primary indication, hemolytic disease of the newborn (HDN), has limited its use in AITP. A potential alternative to this treatment is the use of monoclonal anti-D antibodies (MoAnti-D).
The D antigen is a 32-kD erythrocyte (red blood cell [RBC]) protein encoded by the RHD gene, which has several allelic variants that can generate different epitopes; this allows the production of many MoAnti-D.6–13 Hundreds of MoAnti-D have been produced for phenotypic studies, and many have been characterized for their ability to inhibit physiologic processes in models of HDN.9–13 With respect to AITP, however, only one study attempted to treat patients with AITP with MoAnti-D, but platelet counts were not increased.14 It was suggested that the failure was the result of the monoclonal preparation missing factors present in polyclonal anti-D.15 Subsequently, few studies attempted to understand why MoAnti-D preparations are not effective in AITP. This study examined the ability of MoAnti-D to inhibit opsonized platelet phagocytosis in vitro, and results demonstrate that both epitope specificity and isotype need to be considered for production of a MoAnti-D preparation that mimics the effects of polyclonal Rh immune globulin.
Materials and methods
Antibodies
Serum prepared from a patient with AITP, who was previously screened for high-titered IgG antiplatelet autoantibodies, was obtained (J.F.) with informed consent according to the Declaration of Helsinki. Murine IgG2a monoclonal antihuman major histocompatibility complex (W6/32) was produced from hybridoma HB-95 (ATCC#HB-95). WinRho SDF was obtained from Cangene (Mississauga, ON, Canada). Human MoAnti-D BRAD3 (IgG3; clone 1A3-3) and BRAD5 (IgG1; clone 1A11) that share D specificity (Loop 6/7) were obtained from the International Blood Group Reference Laboratory (Bristol, United Kingdom).10 Human MoAnti-D IgG1r9B8 (IgG1; clone rRh9B8-14-1-M5) and IgG3r9B8 (IgG3; clone rRh9B8-C12-1) share identical V-regions (Loop3/Loop6 M169L/M170R.D350H) but differ in isotypes, whereas IgG1Rh113 (IgG1; clone rRh113) and IgG1Rh178 (IgG1; clone rRh4-29-178) differ in D specificity (Loop6 D350H and Loop 4/6 F223V, D350H, G353/A354N, respectively) but share isotypes and were produced as previously described.11 Because the efficacy of MoAnti-D is proportional to the amount on RBCs,13 the anti-D antibodies were titrated on RBCs and bound with a fluorescein isothiocyanate-labeled goat antihuman IgG (H + L chain-specific, #GTX72737; GeneTex, San Antonio, TX). The anti-D amounts that showed equivalent fluorescence (binding to RBC) were used in the phagocytosis assay; WinRho SDF (1.9 μg), IgG1Rh113 (4.0 μg), IgG1Rh178 (1.1 μg), IgG1r9B8 (3.0 μg), IgG3r9B8 (3.3 μg), BRAD3 (0.7 μg), and BRAD5 (1.1 μg). F(ab′)2 fragments of WinRho SDF were prepared as previously described16 ; purity by high-performance liquid chromatography analysis was greater than 96%.
Platelets and red blood cells
CPDA blood was drawn from laboratory volunteers under a St. Michael's Hospital Research Ethics Board-approved protocol. Informed consent was obtained in accordance with the Declaration of Helsinki. Platelet-rich plasma was prepared, platelets were counted and labeled with 20 μM CellTracker Green CMFDA (CM-G; Invitrogen, Carlsbad, CA), washed, and resuspended in phosphate-buffered saline. When indicated, CM-G–labeled platelets were opsonized with either 5 μg W6/32 or a 1:2 dilution of AITP serum for 30 minutes, washed, and used in the phagocytosis assay. After removal of platelet-rich plasma, the buffy coat was removed and the RBC pellet was washed 6 times in phosphate-buffered saline. RBC were adjusted to 4 × 108 in 100 μL and incubated with the indicated amounts of anti-D for 40 minutes and used in the phagocytosis assay.
Phagocytosis assay
Phagocytosis of platelets was performed by a method previously described.17 Human THP-1 cells (ATCC# TIB-202) were counted and 107 cells/mL were activated with 50 ng/mL phorbol 12-myristate 13-acetate. The reaction was started by incubating 5 × 106 THP-1 cells with 250 × 106 platelets in 0.1-mL duplicate tubes for 2 hours on ice or at 37°C. Extracellular fluorescence was quenched by addition of 0.1% trypan blue. Tubes were centrifuged at 200g for 10 minutes at 4°C, the supernatant discarded, and 200 μL LDS DNA stain (Invitrogen) added. Flow cytometry was performed using a FACSort flow cytometer; cells were acquired through an FL3 gate and intracellular FL1 fluorescence was determined. Phagocytic index was calculated by the formula: median FL1 fluorescence at 37°C/median FL1 fluorescence at 0°C. When indicated, titrations of RBC were added to the assay.
Statistical analysis
An unpaired t test for comparison between means was used.
Results and discussion
When labeled platelets were opsonized with W6/32, a significant increase in THP-1 intracellular fluorescence was observed, as previously described (Figure 1A,B).17 When nonopsonized RBC were added, there was no effect on W6/32-opsonized or autoantibody-opsonized platelet phagocytosis at any RBC:THP-1 ratio (Figure 1C,D). RBC opsonized with WinRho SDF, however, mediated a significant (P < .0001) Fc-dependent inhibition of both W6/32- and autoantibody-mediated platelet phagocytosis (Figure 1C,D). Wiener et al18 determined that anti-D-opsonized RBC phagocytosis was mediated by high-affinity FcγRI on monocytes, whereas Miescher et al19 demonstrated MoAnti-D-mediated RBC clearance was associated with FcγRIIA and FcγRIIIA polymorphisms. Furthermore, Coopamah et al20 reported that anti-D-mediated erythrophagocytosis was associated with a monocytic Fc-dependent oxidative burst. Perhaps anti-D-mediated inhibition of platelet phagocytosis is related to these observations and we are currently studying this.
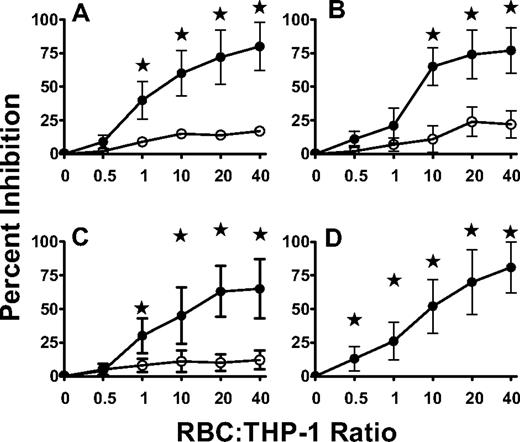
Representative flow cytometric histogram fluorescence of THP-1 phagocytosis of W6/32 platelets. (A) Control THP-1 phagocytosis of W6/32-opsonized platelets at 0°C (······) and 37°C (—) and (B) THP-1 phagocytosis of W6/32-opsonized platelets at 37°C in the presence of nonopsonized red blood cells (RBC; 10:1 RBC:THP-1 ratio, —), Rh immune globulin-opsonized RBC (1:1 RBC:THP-1 ratio, ——), or Rh immune globulin-opsonized RBC (10:1 RBC:THP-1 ratio, ······). The median values of the THP-1 fluorescence for W6/32-opsonized platelets at 0°C was 4.2 (± 1.2) and at 37°C was 79.6 (± 28.4, N = 91). These values were used to calculate the percentage inhibition of (C) W6/32-opsonized platelet phagocytosis by nonopsonized RBC (□), RBC opsonized with Rh immune globulin (●), or RBC bound with Rh immune globulin F(ab′)2 fragments (○); and (D) IgG autoantibody-opsonized platelet phagocytosis by nonopsonized RBC (□), RBC opsonized with Rh immune globulin (●), or RBC bound with Rh immune globulin F(ab′)2 fragments (●). The data in panels C and D are presented as the mean percent inhibition (± SD) at the indicated RBC:THP-1 ratios (N = 20 for panel C and N = 8 for panel D) and were calculated by the formula: (1 − phagocytic index [PI]))×(added RBC)÷PI (platelets alone) × 100. Statistical significance (P values) by an unpaired t test is shown: all solid symbols were compared with the corresponding nonopsonized RBC inhibition, ★; P < .001.
Representative flow cytometric histogram fluorescence of THP-1 phagocytosis of W6/32 platelets. (A) Control THP-1 phagocytosis of W6/32-opsonized platelets at 0°C (······) and 37°C (—) and (B) THP-1 phagocytosis of W6/32-opsonized platelets at 37°C in the presence of nonopsonized red blood cells (RBC; 10:1 RBC:THP-1 ratio, —), Rh immune globulin-opsonized RBC (1:1 RBC:THP-1 ratio, ——), or Rh immune globulin-opsonized RBC (10:1 RBC:THP-1 ratio, ······). The median values of the THP-1 fluorescence for W6/32-opsonized platelets at 0°C was 4.2 (± 1.2) and at 37°C was 79.6 (± 28.4, N = 91). These values were used to calculate the percentage inhibition of (C) W6/32-opsonized platelet phagocytosis by nonopsonized RBC (□), RBC opsonized with Rh immune globulin (●), or RBC bound with Rh immune globulin F(ab′)2 fragments (○); and (D) IgG autoantibody-opsonized platelet phagocytosis by nonopsonized RBC (□), RBC opsonized with Rh immune globulin (●), or RBC bound with Rh immune globulin F(ab′)2 fragments (●). The data in panels C and D are presented as the mean percent inhibition (± SD) at the indicated RBC:THP-1 ratios (N = 20 for panel C and N = 8 for panel D) and were calculated by the formula: (1 − phagocytic index [PI]))×(added RBC)÷PI (platelets alone) × 100. Statistical significance (P values) by an unpaired t test is shown: all solid symbols were compared with the corresponding nonopsonized RBC inhibition, ★; P < .001.
Of the MoAnti-D (BRAD-3/BRAD-5 and IgG1r9B8/IgG3r9B8) that shared epitope specificity but differed in IgG isotype, only IgG3 antibodies significantly (P < .001) inhibited opsonized platelet phagocytosis (Figure 2A,B). These results are consistent with studies demonstrating that some IgG3 isotypes of MoAnti-D are superior to their IgG1 counterparts in mediating responses related to HDN prevention.13,21–23 They may be related to the observation that certain FcγRIIA and IIIA alleles display differential binding to human antibody isotypes.24 Of interest, one IgG3 MoAnti-D (IgG3r9B8) achieved similar levels of platelet phagocytosis inhibition as Rh immune globulin, suggesting even single MoAnti-D products can mimic polyclonal anti-D. Nonetheless, these results may explain why an IgG1 MoAnti-D failed to raise platelet counts in patients with AITP.14
Inhibition of IgG autoantibody-opsonized platelet phagocytosis by red blood cells. RBC opsonized with (A) monoclonal anti-D antibodies (MoAnti-D) IgG3r9B8 (●) or IgG1r9B8 (○), (B) MoAnti-D BRAD3 (●) or BRAD5 (○), (C) MoAnti-D IgG1Rh113 (●) or IgG1Rh178 (○), and (D) RBC opsonized with a mixture of all 6 MoAnti-D (●). The data are presented as the mean percent inhibition (± SD) at the indicated RBC:THP-1 ratios (N = 6) and was calculated as in Figure 1. Statistical significance (P values) by an unpaired t test is shown; all solid symbols were compared with the corresponding nonopsonized RBC inhibition in panel 1B, ★; P < .001.
Inhibition of IgG autoantibody-opsonized platelet phagocytosis by red blood cells. RBC opsonized with (A) monoclonal anti-D antibodies (MoAnti-D) IgG3r9B8 (●) or IgG1r9B8 (○), (B) MoAnti-D BRAD3 (●) or BRAD5 (○), (C) MoAnti-D IgG1Rh113 (●) or IgG1Rh178 (○), and (D) RBC opsonized with a mixture of all 6 MoAnti-D (●). The data are presented as the mean percent inhibition (± SD) at the indicated RBC:THP-1 ratios (N = 6) and was calculated as in Figure 1. Statistical significance (P values) by an unpaired t test is shown; all solid symbols were compared with the corresponding nonopsonized RBC inhibition in panel 1B, ★; P < .001.
When 2 MoAnti-D sharing IgG1 isotypes but differing in specificity (IgG1Rh113/IgG1Rh178) were used in the assay, only one could significantly (P < .01) reduce platelet phagocytosis (Figure 2C). The mechanism of how the MoAnti-D specificity affects opsonized platelet phagocytosis is unknown but may relate to the molecule's orientation on the RBC surface. For example, Christiaansen et al25 demonstrated that orientation of monoclonal antibodies on target cells was critical for determining whether they mediated their biologic effect. Perhaps MoAnti-D with a particular specificity binds D in a manner that does not allow interaction with Fc receptors. In addition, although we equilibrated anti-D binding, some may have been lost from the RBC surface as a result of differing affinities; however, in control experiments with RBC alone, there was no reduction in anti-D fluorescence (not shown). In an attempt to address these issues, we mixed the 6 MoAb expecting that the 3 noninhibitory antibodies would confer at least some of their lack of inhibition; however, the mixture significantly inhibited platelet phagocytosis as well as Rh immune globulin (Figure 2D). This paradoxic finding may be related to studies demonstrating that blending different IgG1 and IgG3 isotypes of MoAnti-D can synergistically inhibit erythrophagocytosis in HDN and respiratory burst in monocytes.21–23
In conclusion, MoAnti-D inhibition of opsonized platelet phagocytosis is dependent on isotype and epitope specificity suggesting that these preparations can be produced to mimic polyclonal anti-D and perhaps be therapeutically effective in AITP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Canadian Blood Services (XT00054), the Danish Physicians Insurance Foundation Codan, Faculty of Health, Aarhus University, the Knud Hoejgaard's Foundation, the Danish Pasteur Society Foundation, and the Reinholdt W. Jorck Foundation.
We thank Drs Greg Denomme (Canadian Blood Services, Toronto, ON, Canada) and Belinda Kumpel (BITS/IBGRL, Bristol, United Kingdom) for their review of this manuscript and Chris Druar and Dave Miller (Cangene, Mississauga, ON, Canada) for their technical assistance.
Authorship
Contribution: M. Kjaersgaard performed the research, analyzed data, and wrote the paper; R.A. designed the research, performed the research, analyzed the data; M. Kim designed and performed the research; E.R.S. designed and performed the research; J.F. designed the research and wrote the paper; D.I.H.S. AND E.J.W. constructed monoclonal antibodies, designed the research, and wrote the paper; and J.W.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: D.I.H.S. and E.J.W. are employed by Cangene, whose product (WinRho SDF) was studied in the present work. All other authors declare no competing financial interests.
Correspondence: John Semple, St. Michael's Hospital, 30 Bond St., Toronto, ON, Canada, M5B 1W8; e-mail: semplej@smh.toronto.on.ca.

![Figure 1. Representative flow cytometric histogram fluorescence of THP-1 phagocytosis of W6/32 platelets. (A) Control THP-1 phagocytosis of W6/32-opsonized platelets at 0°C (······) and 37°C (—) and (B) THP-1 phagocytosis of W6/32-opsonized platelets at 37°C in the presence of nonopsonized red blood cells (RBC; 10:1 RBC:THP-1 ratio, —), Rh immune globulin-opsonized RBC (1:1 RBC:THP-1 ratio, ——), or Rh immune globulin-opsonized RBC (10:1 RBC:THP-1 ratio, ······). The median values of the THP-1 fluorescence for W6/32-opsonized platelets at 0°C was 4.2 (± 1.2) and at 37°C was 79.6 (± 28.4, N = 91). These values were used to calculate the percentage inhibition of (C) W6/32-opsonized platelet phagocytosis by nonopsonized RBC (□), RBC opsonized with Rh immune globulin (●), or RBC bound with Rh immune globulin F(ab′)2 fragments (○); and (D) IgG autoantibody-opsonized platelet phagocytosis by nonopsonized RBC (□), RBC opsonized with Rh immune globulin (●), or RBC bound with Rh immune globulin F(ab′)2 fragments (●). The data in panels C and D are presented as the mean percent inhibition (± SD) at the indicated RBC:THP-1 ratios (N = 20 for panel C and N = 8 for panel D) and were calculated by the formula: (1 − phagocytic index [PI]))×(added RBC)÷PI (platelets alone) × 100. Statistical significance (P values) by an unpaired t test is shown: all solid symbols were compared with the corresponding nonopsonized RBC inhibition, ★; P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2007-03-079848/5/m_zh80150705550001.jpeg?Expires=1767740211&Signature=OQnvLjYGUrthElM3t7fW749XzNuOE-3NHWZ83nltfH4eGg4LocvaOU9RZy2djJSNoLmEXGmWI11L3V~cGCSmDVtmGqQ2pOgnApC5-mcTWOn8DYn0rojk1j1W8ePK111IU69E-zA1r6U1UfFZWshyhbNZAGfNUaCHlJsTYpCkBJhftsZK9-1d3nO1Br3Xh-T-ZbdNLWNqPiyOUO-VtClXi6P81DKlvWE6aJQkTAFEQGeoOZ6Y5ULCBGqX0q6fPPxl6rZQi-2F9Q1UZeDumEcRiMEwcK77TDmYf4RUEuq9k3OIArod-rFOah719tQf0vO1iVPjwF38YfS-F40kR6JOsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal