Abstract
More effective therapeutic strategies are required for patients with poor-prognosis systemic sclerosis (SSc). A phase 2 single-arm study of high-dose immunosuppressive therapy (HDIT) and autologous CD34-selected hematopoietic cell transplantation (HCT) was conducted in 34 patients with diffuse cutaneous SSc. HDIT included total body irradiation (800 cGy) with lung shielding, cyclophosphamide (120 mg/kg), and equine antithymocyte globulin (90 mg/kg). Neutrophil and platelet counts were recovered by 9 (range, 7 to 13) and 11 (range, 7 to 25) days after HCT, respectively. Seventeen of 27 (63%) evaluable patients who survived at least 1 year after HDIT had sustained responses at a median follow-up of 4 (range, 1 to 8) years. There was a major improvement in skin (modified Rodnan skin score, −22.08; P < .001) and overall function (modified Health Assessment Questionnaire Disability Index, −1.03; P < .001) at final evaluation. Importantly, for the first time, biopsies confirmed a statistically significant decrease of dermal fibrosis compared with baseline (P < .001). Lung, heart, and kidney function, in general, remained clinically stable. There were 12 deaths during the study (transplantation-related, 8; SSc-related, 4). The estimated progression-free survival was 64% at 5 years. Sustained responses including a decrease in dermal fibrosis were observed exceeding those previously reported with other therapies. HDIT and autologous HCT for SSc should be evaluated in a randomized clinical trial.
Introduction
Systemic sclerosis (SSc) is a multisystem autoimmune disease with vasculopathy and progressive fibrosis that is highly variable in its clinical manifestations, but patients with diffuse cutaneous SSc and internal organ involvement have reduced life span.1–4 Although cyclophosphamide was recently observed to have a small beneficial effect, more effective therapies for the severe forms of SSc are required to improve outcome.5–9 In a report of the first 19 patients in this pilot study, it was observed that there was marked clinical improvement in the degree of the scleroderma and overall function with a median follow-up of 15 months.10 Now, in 34 patients, we report additional information on safety and show that there was a significant decrease in the degree of the scleroderma and dermal fibrosis, improved overall function and, in general, stability of internal organ function, and these responses were sustained at a median of 4 years after high-dose immunosuppressive therapy (HDIT)
Patients, materials, and methods
Patients
Between July 1997 and March 2005, 34 participants were enrolled in the study at the Fred Hutchinson Cancer Research Center (n = 19), University of Michigan (n = 7), Karmanos Cancer Institute (n = 4), Loma Linda University (n = 3), and Texas Transplant Institute (n = 1). The institutional review board at each center approved the study. Patients were registered for the study only after providing a signed consent to confirm that they had been fully informed, in accordance with the Declaration of Helsinki, of the investigational purposes of the study.
Study design
This pilot study was designed to assess the safety and potential efficacy of HDIT for severe SSc. Patients 65 years of age or less were eligible for study participation if they had early (4 years or less) diffuse scleroderma (modified Rodnan skin score [mRSS] 16 or greater) and significant visceral organ involvement as previously described.10 Patients were also included if there was progressive pulmonary disease with a decrease of at least 15% in forced vital capacity (FVC) or diffusion capacity of the lung for carbon monoxide adjusted for hemoglobin levels (DLCOadj) in the previous 6 months with any skin involvement. The eligibility criteria selected patients with a mortality risk from SSc of approximately 50% at 5 years with conventional treatment, and these have been previously published.9–11 Study end points were safety of mobilization with granulocyte colony-stimulating factor (G-CSF), engraftment after transplantation of autologous CD34-selected hematopoietic cells (HCs), early and late regimen-related toxicity or complications, disease response, and immunologic recovery. Immune recovery of the study participants has been reported previously.10,12
Treatment and supportive care
Peripheral blood stem cells (PBSCs) were mobilized with G-CSF (16 μg/kg/d) subcutaneously with the first apheresis scheduled on day 4. The G-CSF–mobilized PBSC products were CD34-selected using a Isolex 300i device (Baxter, Irvine, CA) and cryopreserved. Unmodified autologous HC grafts were stored for treating engraftment failure or severe immunodeficiency after HDIT. The autologous HC grafts were evaluated for content of CD34+ cells and CD3+ T cells by flow cytometry. The HDIT regimen has been previously reported and included fractionated total body irradiation ([TBI] 800 cGy), cyclophosphamide (120 mg/kg), and equine antithymocyte globulin ([ATG] 90 mg/kg; Pfizer, New York, NY).10 Methylprednisolone (1 mg/kg) was given intravenously with each dose of ATG. TBI without lung shielding was used for the first 8 patients, and subsequently lungs were shielded to a total dose of 200 cGy. Prednisone (0.5 mg/kg/d) was given from the start of conditioning to day 30 after hematopoietic cell transplantation (HCT) and was tapered over 1 month after the fifth patient included in the study. The CD34-selected autologous graft was infused on day 0. G-CSF (5 μg/kg/d) was given intravenously from day 0 until the absolute neutrophil count was greater than 0.5 × 109/L (500/μL) for 3 days. Infection prophylaxis included trimethoprim-sulfamethoxazole, acyclovir, and fluconazole.13 A preemptive strategy was used for the prevention of cytomegalovirus (CMV) disease.14
Evaluation of outcomes
The day of neutrophil engraftment was defined as the first of 3 consecutive days after HCT when an absolute count of greater than 0.5 × 109/L (500/μL) had been achieved. The day of platelet engraftment was defined as the first of 3 consecutive days when a count of 20 × 109/L (20 000/μL) had been sustained or the first of 2 days in which the platelet count increased on the second day without transfusions.15 Regimen-related toxicities were defined according to the Bearman scale.16
Scheduled evaluations of patients were performed before mobilization and then at 3 months and annually after HCT. These evaluations included a medical history, physical examination, complete blood count, serum chemistries, autoantibodies (Scl-70, ANA), thyroid function tests, validated standard measurements of the mRSS, pulmonary function testing for FVC and DLCOadj, high-resolution computed tomography (HRCT) scans of the chest, multigated acquisition (MUGA) scan of the heart or echocardiogram for left ventricular ejection fraction (LVEF), and a modified Health Assessment Questionnaire Disability Index (mHAQ-DI).5,17–21 An ophthalmologic examination was done at the last study evaluation to assess for cataracts. In a subset of consenting patients, skin biopsies were done after HDIT next to the site of the baseline biopsy.
For determining progression-free survival, progression was defined as death, respiratory failure (decrease in FVC of more than 20% or decrease in DLCOadj of more than 30%), renal failure (requiring chronic dialysis for more than 6 months), or heart failure (LVEF less than 30%) after HDIT. Progression could be treatment related or disease related. Disease activation was defined as events developing 6 or more months after HDIT, including worsening pulmonary function (decrease in FVC of more than 10% or DLCOadj of more than 15%), arrhythmias requiring medical treatment for 3 or more months, onset of hypertensive or nonhypertensive renal crisis, increase in mRSS (5 or more for baseline of up to 20, or more than 25% for baseline more than 20), increase in mHAQ (more than 0.4), or the initiation of disease-modifying antirheumatic drugs (DMARDs). Disease response was defined as a decrease in mRSS (5 or more for baseline of up to 20, or more than 25% for baseline more than 20), improving pulmonary function (increase in FVC of more than 10% or DLCOadj of more than 15%), or a decrease in mHAQ (more than 0.4). Only patients who survived at least 1 year were considered evaluable for response because there were no scheduled evaluations performed 3 to 12 months after HDIT, and the duration of follow-up at 3 months was not adequate for evaluating response endpoints of fibrosis and organ function.
Assessment of skin biopsies
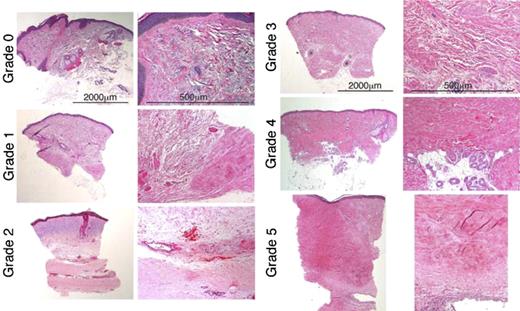
Serial 3- to 4-mm full-thickness punch biopsies of skin were taken from the same location in an individual patient, either the lateral aspect of the forearm or the upper arm in the triceps region, in a subset of patients. Coded slides stained with hematoxylin and eosin (H&E) were prepared from formalin-fixed blocks and were analyzed for changes in the various components of skin, including epidermis, papillary and reticular dermis, adnexae, and subcutis. Deidentification and coding of the slides was done so that a blinded evaluation of the dermal fibrosis could be performed. The histologic sections of the skin were assessed on a Leica DM 3000 microscope (Leica Microsystems, Wetzlar, Germany), relying primarily on the 2.5×nplan/0.07 NA, 5×nplan/0.12 NA, and 10×/hc plan Fluotar/0.30 NA objectives, and also using a 20× HC plan apo/0.70 NA. Images were processed using Adobe Elements 4.0 and Adobe Photoshop CS version 8. Changes in fibrosis were graded on a 0-to-5 semiquantitative scale (Table 1; Figure 1). Because of the local variability in skin, even within a given location, and some qualitative variability between biopsies, a meaningful reduction in overall fibrosis score required changes of at least 2 grades.
Dermal fibrosis grades 0 to 5. Each pair represents a different dermal fibrosis grade. The left-hand column of each pair containing low-power views (original magnification, × 2.5), illustrates the spectrum of change in the overall dermal breadth and distribution of fibrosis. The right-hand column views for each pair (original magnification, × 10), illustrate the qualitative size, shape, distribution, and density in the dermal collagen bundles and surrounding extracellular matrix. All sections are stained with H&E. Grade 0 is thin curlicue collagen bundles with only minimal focal papillary dermal sclerosis. Grade 1 is less than 25% sclerosis with focal dense deep dermal fibrosis. In grade 2, the dense zone of fibrosis within the hypodermis comprises less than 50% of the overall dermal breadth. The remaining reticular dermis is composed of fine collagen bundles with actinic change (blue) in the upper dermis. Higher power shows the distinction between the sclerotic and normal collagen bundles. Grade 3 is extensive sclerosis—more than 50%—throughout the dermis with thickened collagen bundles admixed with thinner bundles and intervening extracellular matrix. Grade 4 is pandermal fibrosis with loss of most extracellular matrix without extension into hypodermis (or entrapment of eccrine coils) or surrounding fat. Grade 5 is markedly fibrotic and thickened—more than 4 mm—dermis replaced with dense waxy collagen with deep extension into hypodermis with fibrous incorporation of a muscular artery. See “Patients and methods; Assessment of skin biopsies” for more information on images.
Dermal fibrosis grades 0 to 5. Each pair represents a different dermal fibrosis grade. The left-hand column of each pair containing low-power views (original magnification, × 2.5), illustrates the spectrum of change in the overall dermal breadth and distribution of fibrosis. The right-hand column views for each pair (original magnification, × 10), illustrate the qualitative size, shape, distribution, and density in the dermal collagen bundles and surrounding extracellular matrix. All sections are stained with H&E. Grade 0 is thin curlicue collagen bundles with only minimal focal papillary dermal sclerosis. Grade 1 is less than 25% sclerosis with focal dense deep dermal fibrosis. In grade 2, the dense zone of fibrosis within the hypodermis comprises less than 50% of the overall dermal breadth. The remaining reticular dermis is composed of fine collagen bundles with actinic change (blue) in the upper dermis. Higher power shows the distinction between the sclerotic and normal collagen bundles. Grade 3 is extensive sclerosis—more than 50%—throughout the dermis with thickened collagen bundles admixed with thinner bundles and intervening extracellular matrix. Grade 4 is pandermal fibrosis with loss of most extracellular matrix without extension into hypodermis (or entrapment of eccrine coils) or surrounding fat. Grade 5 is markedly fibrotic and thickened—more than 4 mm—dermis replaced with dense waxy collagen with deep extension into hypodermis with fibrous incorporation of a muscular artery. See “Patients and methods; Assessment of skin biopsies” for more information on images.
Grading of dermal fibrosis in systemic sclerosis
| Dermal fibrosis grade . | Description . |
|---|---|
| 0 | No homogenization but there may be atrophic, thin straightened collagen bundles with increased amounts of interstitial ground substance |
| 1 | Less than 25% sclerosis with residual foci; some residual straightening or eosinophilic collagen bundles may be present |
| 2 | Focal sclerosis—less than 50% overall |
| 3 | Incomplete homogenization with spaces between the collagen bundles with 50% to 75% sclerotic change |
| 4 | Pandermal sclerosis without obvious expansion of the lower reticular dermis with some sparing of perieccrine adipose tissue |
| 5 | Pandermal sclerosis with homogenization from the papillary to the reticular dermis; includes obvious widening of the reticular dermis below the eccrine coils with extension into the hypodermis and formation of fibrous septa |
| Dermal fibrosis grade . | Description . |
|---|---|
| 0 | No homogenization but there may be atrophic, thin straightened collagen bundles with increased amounts of interstitial ground substance |
| 1 | Less than 25% sclerosis with residual foci; some residual straightening or eosinophilic collagen bundles may be present |
| 2 | Focal sclerosis—less than 50% overall |
| 3 | Incomplete homogenization with spaces between the collagen bundles with 50% to 75% sclerotic change |
| 4 | Pandermal sclerosis without obvious expansion of the lower reticular dermis with some sparing of perieccrine adipose tissue |
| 5 | Pandermal sclerosis with homogenization from the papillary to the reticular dermis; includes obvious widening of the reticular dermis below the eccrine coils with extension into the hypodermis and formation of fibrous septa |
Statistics
For each of the clinical parameters assessed, posttransplantation values were compared with pretransplantation (baseline) values at the last time point in each of several posttransplantation time windows. The 1-sample t test was used to test the null hypothesis that the difference between posttransplantation and baseline values was equal to 0. In addition to comparisons in specific time windows, generalized estimating equations (GEEs) were used to determine if the value of a clinical parameter increased or decreased with time (from baseline to the last measurement available). For these analyses, we tested for linear associations between time and each clinical parameter. All reported P values are 2-sided, and those resulting from regression models were derived from the Wald test.
Results
Patient characteristics
Patient characteristics at baseline are described in Table 2. All patients were considered to have a poor prognosis and had failed a median of 2 (range, 0 to 5) different immune-modifying treatments before being included in the clinical trial. All patients had pulmonary disease, although this may not have been the primary internal organ considered for protocol eligibility. The only patient with a skin score less than 16 (1 subject with an mRSS of 3) had progressive pulmonary disease and a DLCOadj of 48%. One patient had pulmonary arterial hypertension, 1 patient required total parenteral nutrition, 3 patients had a history of significant renal dysfunction or renal crisis, and 5 patients had gastric antral vascular ectasia before HDIT.
Patient characteristics before transplantation (n= 34)
| Clinical parameter . | Value . | Frequency, no.* . |
|---|---|---|
| Median age, y (range) | 41 (23-61) | 34 |
| Sex: M/F | 8/26 | 34 |
| Median skin score: mRSS (range) | 30 (3-48) | 34 |
| Median DLCOadj,† % (range) | 61 (40-83) | 33 |
| Median FVC, % (range) | 71 (27-103) | 34 |
| HRCT of chest | ||
| Abnormal | 27 | 34 |
| Ground-glass | 12 | 34 |
| Interstitial fibrosis | 25 | 34 |
| Normal | 7 | 34 |
| Alveolitis by BAL | 16 | 25 |
| Median ejection fraction, % (range) | 65 (47-80) | 32 |
| Median serum creatinine level, mg/dL (range) | 0.7 (0.4-1.7) | 34 |
| Median mHAQ score (range) | 1.88 (0.25-2.88) | 30 |
| Hypothyroidism | 9 | 34 |
| Organ dysfunction | ||
| Lung‡ | 34 | 34 |
| Kidney§ | 2 | 34 |
| Heart‖ | 9 | 34 |
| Gastrointestinal tract¶ | 23 | 34 |
| Autoantibodies | ||
| Antinuclear antibodies | 25 | 33 |
| Scl-70 | 11 | 34 |
| Anticentromere | 0 | 27 |
| Disease duration, mo (range)# | 21 (4-51) | 34 |
| Immune-based therapy before HDIT | ||
| Corticosteroids | 22 | 34 |
| Cyclophosphamide | 15 | 34 |
| Methotrexate | 13 | 34 |
| D-penicillamine | 10 | 34 |
| Hydroxychloroquine | 6 | 34 |
| Etanercept | 5 | 34 |
| Cyclosporine | 3 | 34 |
| Relaxin | 2 | 34 |
| Imuran | 1 | 34 |
| Other | 4 | 34 |
| Clinical parameter . | Value . | Frequency, no.* . |
|---|---|---|
| Median age, y (range) | 41 (23-61) | 34 |
| Sex: M/F | 8/26 | 34 |
| Median skin score: mRSS (range) | 30 (3-48) | 34 |
| Median DLCOadj,† % (range) | 61 (40-83) | 33 |
| Median FVC, % (range) | 71 (27-103) | 34 |
| HRCT of chest | ||
| Abnormal | 27 | 34 |
| Ground-glass | 12 | 34 |
| Interstitial fibrosis | 25 | 34 |
| Normal | 7 | 34 |
| Alveolitis by BAL | 16 | 25 |
| Median ejection fraction, % (range) | 65 (47-80) | 32 |
| Median serum creatinine level, mg/dL (range) | 0.7 (0.4-1.7) | 34 |
| Median mHAQ score (range) | 1.88 (0.25-2.88) | 30 |
| Hypothyroidism | 9 | 34 |
| Organ dysfunction | ||
| Lung‡ | 34 | 34 |
| Kidney§ | 2 | 34 |
| Heart‖ | 9 | 34 |
| Gastrointestinal tract¶ | 23 | 34 |
| Autoantibodies | ||
| Antinuclear antibodies | 25 | 33 |
| Scl-70 | 11 | 34 |
| Anticentromere | 0 | 27 |
| Disease duration, mo (range)# | 21 (4-51) | 34 |
| Immune-based therapy before HDIT | ||
| Corticosteroids | 22 | 34 |
| Cyclophosphamide | 15 | 34 |
| Methotrexate | 13 | 34 |
| D-penicillamine | 10 | 34 |
| Hydroxychloroquine | 6 | 34 |
| Etanercept | 5 | 34 |
| Cyclosporine | 3 | 34 |
| Relaxin | 2 | 34 |
| Imuran | 1 | 34 |
| Other | 4 | 34 |
BAL indicates bronchoalveolar lavage.
Number of patients with available results for the given parameter.
Adjusted for hemoglobin concentration.
Lung dysfunction was defined as diffusion capacity of the lung for carbon monoxide adjusted for hemoglobin levels below 70% and/or forced vital capacity below 70%.
Renal dysfunction was defined as prior history of renal crisis.
Cardiac dysfunction was defined as ejection fraction below 50%, electrical conduction abnormalities, pulmonary hypertension, or pericardial effusion.
Gastrointestinal dysfunction was defined as esophageal or bowel dysfunction including a history of parenteral nutrition.
Time from onset of first non-Raynaud symptoms.
Autologous hematopoietic cell graft and engraftment
The number of aphereses for collection of the graft, graft composition, and time to engraftment are described in Table 3. Recovery of neutrophil counts occurred in all patients at a median of 9 (range, 7 to 13) days. All patients recovered platelet counts at a median of 11 (range, 7 to 25) days except for 1 patient who developed scleroderma renal crisis. However, engraftment had occurred in this patient based on the presence of marrow megakaryocytes on day 34.
Apheresis, graft composition, and engraftment
| . | Median (range) . |
|---|---|
| Mobilization: no. of apheresis + back-up* | 2 (2-7) |
| CD34-selected grafts | |
| CD34+ cells × 106/kg | 4.02 (2.36-8.6) |
| CD3+ cells × 104/kg; n = 22 | 1.86 (0-25.2) |
| CD34 purity (%) | 91 (55-99) |
| Engraftment after transplantation | |
| Days to neutrophil count above 500/μL† | 9 (7-13) |
| Days to platelet count above 20 000/μL‡ | 11 (7-25) |
| . | Median (range) . |
|---|---|
| Mobilization: no. of apheresis + back-up* | 2 (2-7) |
| CD34-selected grafts | |
| CD34+ cells × 106/kg | 4.02 (2.36-8.6) |
| CD3+ cells × 104/kg; n = 22 | 1.86 (0-25.2) |
| CD34 purity (%) | 91 (55-99) |
| Engraftment after transplantation | |
| Days to neutrophil count above 500/μL† | 9 (7-13) |
| Days to platelet count above 20 000/μL‡ | 11 (7-25) |
Minor complications observed infrequently during mobilization included edema, increased skin tightness, erythema, and arthralgias, but these resolved after G-CSF was stopped. All grafts were collected with a single course of G-CSF except for 1 patient who had discontinued mycophenolate mofetil immediately before starting collection of peripheral blood stem cells.
First of 3 consecutive days with an absolute neutrophil count above 500/μL.
First of 3 consecutive days with a platelet count above 20 000/μL or an increase in platelet count independent of platelet transfusions.
Disease evaluations
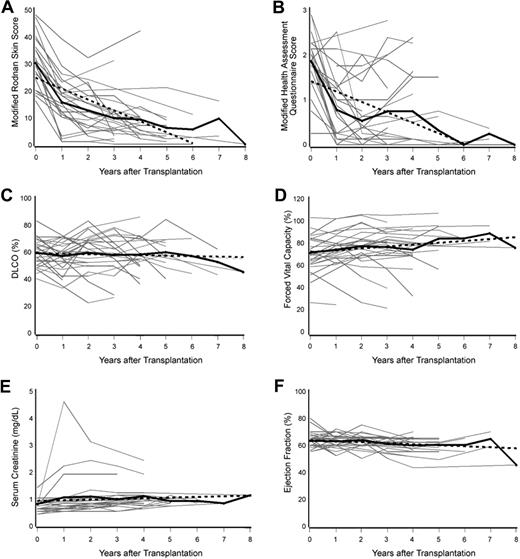
Of the 34 patients enrolled in the study, 27 (79%) survived 1 year and were evaluable for response. Summarized in Table 4 are changes from baseline to the last evaluation within various time windows for each of the clinical parameters that were assessed. The mean decrease in mRSS (baseline, 30.12) and mHAQ score (baseline, 1.85) at final evaluation was 22.08 (−70.3%) and 1.03 (−55%), respectively (both P < .001). There was also a statistically significant linear decrease in mRSS and mHAQ scores over time (both P < .001, GEE; Figure 2A,B). At last follow-up, an mRSS of 0 to 5 was observed in 11 patients. Marked improvement of mRSS was also associated with improvement of the dermal fibrosis in 8 of 10 patients for whom biopsies were available from both before and after HDIT (mean decrease, 19.6; 95% confidence interval [CI], 12.6 to 26.6; P < .001; Figures 3, 4). There was a mean decrease in the dermal fibrosis grade of 3.1 (95% CI, 2.2 to 4.0; P < .001) from a mean baseline grade of 4.3. Using all available skin biopsies (n = 40) including from patients without samples from both before and after HDIT, a strong correlation was noted between the dermal fibrosis grades and the mRSS (r = 0.62, P < .001). The mean increase in FVC between baseline and final evaluation was 2.11% (P = .50), and DLCOadj decreased by an average of 6.04% (P = .05). Considering all values across time, there was a statistically significant increase in FVC (increase of 1.66 per year [95% CI, 0.37 to 2.96], P = .01, GEE; Figure 2C) and DLCOadj values were decreased, but this did not achieve statistical significance (decrease of 0.36 per year [95% CI, 1.42 to -0.69], P = .50; GEE; Figure 2D). The HRCT scans of the chest were centrally reviewed in 21 of the evaluable patients. No significant changes were observed after HDIT except for the 6 patients with reactivation of disease in the lungs. In 6 of 7 patients who survived at least 1 year and were centrally reviewed, “ground-glass” abnormalities at baseline decreased and evolved to interstitial fibrosis, and in the other patient the findings remained unchanged. There was a statistically significant but small increase in serum creatinine and decrease in ejection fraction (Table 4; Figure 2E,F). No patient experienced renal crisis or a significant loss of kidney function after the early treatment-related events. There was no statistically significant change in the ANA titer (P = .26). Of 11 patients who had a positive test for anti-Scl-70, 2 had become negative at last follow-up (6 and 8 years).
Change in clinical parameters after HDIT
| Assessments . | Mean baseline; n = 34 . | Difference from baseline (CI; P)* . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Years 1-2 . | n . | Years 3-4 . | n . | Years 5-8 . | n . | Final evaluation;† . | n . | ||
| mRSS (0-51) | 30.12 | −17.56 (−20.72, −14.40; < .001) | 25 | −21.24 (−25.42, −17.05; < .001) | 21 | −21.82 (−25.88, −17.74; < .001) | 11 | −22.08 (−25.71, −18.45; < .001) | 25 |
| mHAQ (0-3) | 1.85 | −1.26 (−1.60, −0.92; < .001) | 23 | −1.08 (−1.53, −0.64; < .001) | 20 | −1.50 (−1.92, −1.09; < .001) | 11 | −1.03 (−1.40, −0.66; < .001) | 26 |
| DLCOadj, % | 60.09 | −1.37 (−5.64, 4.98; .60) | 27 | −3.70 (−9.86, 4.90; .31) | 23 | −2.27 (−9.69, 5.15; P .51) | 11 | −6.04 (−12.08, 0.002; .05) | 27 |
| FVC, % | 71.53 | 4.48 (0.14, 9.10; .06) | 27 | 2.09 (−5.17, 9.34; .56) | 23 | 10.36 (3.52, 17.20; .007) | 11 | 2.11 (−4.27, 8.49; .50) | 27 |
| Creatinine, mg/dL | 0.78 | 0.26 (0.04, 0.47; .02) | 26 | 0.25 (0.07, 0.44; .009) | 23 | 0.13 (0.04, 0.21; .008) | 11 | 0.25 (0.10, 0.40; .003) | 27 |
| Ejection fraction, % | 63.24 | −0.21 (−3.60, 3.19; .90) | 24 | −2.84 (−5.80, 0.11; .06) | 19 | −3.85 (−7.22, −0.48; .03) | 11 | −2.37 (−4.80, 0.07; .06) | 27 |
| Assessments . | Mean baseline; n = 34 . | Difference from baseline (CI; P)* . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Years 1-2 . | n . | Years 3-4 . | n . | Years 5-8 . | n . | Final evaluation;† . | n . | ||
| mRSS (0-51) | 30.12 | −17.56 (−20.72, −14.40; < .001) | 25 | −21.24 (−25.42, −17.05; < .001) | 21 | −21.82 (−25.88, −17.74; < .001) | 11 | −22.08 (−25.71, −18.45; < .001) | 25 |
| mHAQ (0-3) | 1.85 | −1.26 (−1.60, −0.92; < .001) | 23 | −1.08 (−1.53, −0.64; < .001) | 20 | −1.50 (−1.92, −1.09; < .001) | 11 | −1.03 (−1.40, −0.66; < .001) | 26 |
| DLCOadj, % | 60.09 | −1.37 (−5.64, 4.98; .60) | 27 | −3.70 (−9.86, 4.90; .31) | 23 | −2.27 (−9.69, 5.15; P .51) | 11 | −6.04 (−12.08, 0.002; .05) | 27 |
| FVC, % | 71.53 | 4.48 (0.14, 9.10; .06) | 27 | 2.09 (−5.17, 9.34; .56) | 23 | 10.36 (3.52, 17.20; .007) | 11 | 2.11 (−4.27, 8.49; .50) | 27 |
| Creatinine, mg/dL | 0.78 | 0.26 (0.04, 0.47; .02) | 26 | 0.25 (0.07, 0.44; .009) | 23 | 0.13 (0.04, 0.21; .008) | 11 | 0.25 (0.10, 0.40; .003) | 27 |
| Ejection fraction, % | 63.24 | −0.21 (−3.60, 3.19; .90) | 24 | −2.84 (−5.80, 0.11; .06) | 19 | −3.85 (−7.22, −0.48; .03) | 11 | −2.37 (−4.80, 0.07; .06) | 27 |
Mean difference between baseline and last evaluation for 27 patients who survived more than 1 year in the indicated period. Confidence intervals (95%) are within parentheses. Each patient was compared with his own baseline result.
Mean difference between baseline and final evaluation after HDIT.
Change in modified Rodnan skin score (mRSS), modified Health Assessment Questionnaire (mHAQ) score, and organ function after high-dose immunosuppressive therapy (HDIT) and autologous hematopoietic cell transplantation. A determination was made as to whether a parameter value was statistically significantly increasing or decreasing over time using a generalized estimating equation (GEE) model. The bold black solid line represents the mean value over time for the parameter of interest. The bold black dotted line represents an estimate of the modeled linear relationship between the parameter value and time and summarizes the results of the GEE models. The gray solid lines are parameter values for individual patients. The mean mRSS and mHAQ values statistically significantly decreased with time after HDIT (both P < .001; panels A and B, respectively). The mean values for diffusion capacity of the lung for carbon monoxide (DLCO) adjusted for hemoglobin levels did not statistically significantly change (P = .50, panel C), and forced vital capacity statistically significantly increased with time (P = .01, panel D). The mean values for serum creatinine and ejection fraction statistically significantly increased and decreased with time (P = .01 and P = .04; panels E and F, respectively).
Change in modified Rodnan skin score (mRSS), modified Health Assessment Questionnaire (mHAQ) score, and organ function after high-dose immunosuppressive therapy (HDIT) and autologous hematopoietic cell transplantation. A determination was made as to whether a parameter value was statistically significantly increasing or decreasing over time using a generalized estimating equation (GEE) model. The bold black solid line represents the mean value over time for the parameter of interest. The bold black dotted line represents an estimate of the modeled linear relationship between the parameter value and time and summarizes the results of the GEE models. The gray solid lines are parameter values for individual patients. The mean mRSS and mHAQ values statistically significantly decreased with time after HDIT (both P < .001; panels A and B, respectively). The mean values for diffusion capacity of the lung for carbon monoxide (DLCO) adjusted for hemoglobin levels did not statistically significantly change (P = .50, panel C), and forced vital capacity statistically significantly increased with time (P = .01, panel D). The mean values for serum creatinine and ejection fraction statistically significantly increased and decreased with time (P = .01 and P = .04; panels E and F, respectively).
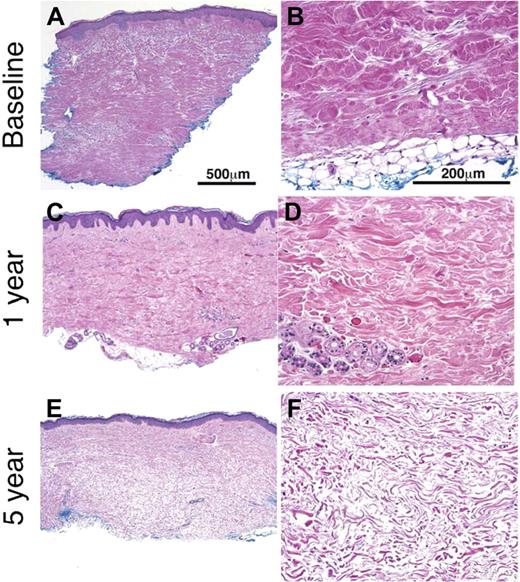
Resolution of dermal fibrosis after HDIT and autologous HCT. Full-thickness skin biopsies from patient no. 11, collected at baseline and then at 1 and 5 years after HDIT, were histologically evaluated (H&E). The skin biopsies performed after HDIT were performed at a site adjacent to the baseline skin biopsy. The biopsies in the left-hand column were taken at original magnification × 5, and those in the right-hand column were taken at original magnification × 20. All sections were stained with H&E. (A) Skin biopsy was obtained before HDIT and autologous HCT. Pandermal sclerosis from the dermal-epidermal border to the hypodermis (subcutaneous fat) was observed. The epidermis is mildly acanthotic (thickened) with loss of rete ridges. The reticular dermis is replaced by dense compact collagen without normal fascicular bundles or dermal appendages. This pretransplantation skin biopsy was determined as grade 5 dermal fibrosis. The thickness of the dermis was measured at more than 2 mm. (B) In the higher-power magnification, the straightened dermal-subcutaneous border demonstrates the abnormal, densely packed, homogenized collagen. (C) The skin biopsy at 1 year after HDIT was determined to be a grade 2 dermal fibrosis and has less fibrosis than at baseline. The low-power magnification view shows crowded collagen fascicles with focal areas of residual thickened bundles. (D) A higher-power view of the 1-year skin biopsy from panel C shows thin and collagen bundles admixed with residual thick straightened hypereosinophilic collagen bundles without dense homogenization at baseline. The residual eccrine unit lacks any surrounding adipose tissue. (E) The skin biopsy at 5 years shows complete resolution of the dermal fibrosis (grade 0) with a reduction in the thickness of the dermis from baseline to 1 mm. The collagen bands in the dermis are thin with a relative increase in the intervening extracellular matrix (space between the collagen bands). The dermal-epidermal border remains straightened with loss of rete ridges. (F) A higher-power view of collagen in the lower reticular dermis demonstrates the change to thin wavy bundles separated by increased ground substance. See “Patients and methods; Assessment of skin biopsies” for more information on images.
Resolution of dermal fibrosis after HDIT and autologous HCT. Full-thickness skin biopsies from patient no. 11, collected at baseline and then at 1 and 5 years after HDIT, were histologically evaluated (H&E). The skin biopsies performed after HDIT were performed at a site adjacent to the baseline skin biopsy. The biopsies in the left-hand column were taken at original magnification × 5, and those in the right-hand column were taken at original magnification × 20. All sections were stained with H&E. (A) Skin biopsy was obtained before HDIT and autologous HCT. Pandermal sclerosis from the dermal-epidermal border to the hypodermis (subcutaneous fat) was observed. The epidermis is mildly acanthotic (thickened) with loss of rete ridges. The reticular dermis is replaced by dense compact collagen without normal fascicular bundles or dermal appendages. This pretransplantation skin biopsy was determined as grade 5 dermal fibrosis. The thickness of the dermis was measured at more than 2 mm. (B) In the higher-power magnification, the straightened dermal-subcutaneous border demonstrates the abnormal, densely packed, homogenized collagen. (C) The skin biopsy at 1 year after HDIT was determined to be a grade 2 dermal fibrosis and has less fibrosis than at baseline. The low-power magnification view shows crowded collagen fascicles with focal areas of residual thickened bundles. (D) A higher-power view of the 1-year skin biopsy from panel C shows thin and collagen bundles admixed with residual thick straightened hypereosinophilic collagen bundles without dense homogenization at baseline. The residual eccrine unit lacks any surrounding adipose tissue. (E) The skin biopsy at 5 years shows complete resolution of the dermal fibrosis (grade 0) with a reduction in the thickness of the dermis from baseline to 1 mm. The collagen bands in the dermis are thin with a relative increase in the intervening extracellular matrix (space between the collagen bands). The dermal-epidermal border remains straightened with loss of rete ridges. (F) A higher-power view of collagen in the lower reticular dermis demonstrates the change to thin wavy bundles separated by increased ground substance. See “Patients and methods; Assessment of skin biopsies” for more information on images.
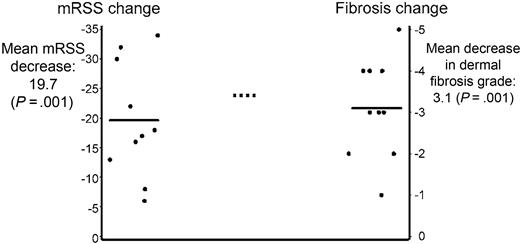
Change in dermal fibrosis grade and mRSS after HDIT and autologous HCT. The change in dermal fibrosis grade and mRSS was evaluated in patients from whom we had skin biopsies from both before and after HDIT and autologous HCT (n = 10). The mean decrease in skin score from baseline after HDIT was 19.7 (P < .001; left horizontal line) and was very comparable to the result for the overall group. The mean decrease in the dermal fibrosis grade from baseline was 3.1 (P = .001; right horizontal line) from a mean baseline grade of 4.3. Seven of the 10 patients had a dermal fibrosis grade of 0 to 1 at the time of last skin biopsy. The median follow-up was 4 (range, 1 to 6) years after HDIT and autologous HCT.
Change in dermal fibrosis grade and mRSS after HDIT and autologous HCT. The change in dermal fibrosis grade and mRSS was evaluated in patients from whom we had skin biopsies from both before and after HDIT and autologous HCT (n = 10). The mean decrease in skin score from baseline after HDIT was 19.7 (P < .001; left horizontal line) and was very comparable to the result for the overall group. The mean decrease in the dermal fibrosis grade from baseline was 3.1 (P = .001; right horizontal line) from a mean baseline grade of 4.3. Seven of the 10 patients had a dermal fibrosis grade of 0 to 1 at the time of last skin biopsy. The median follow-up was 4 (range, 1 to 6) years after HDIT and autologous HCT.
Among patients who survived more than 1 year, overall function as assessed by mHAQ was improved in 19 of 25 and disease responses were observed in skin of 23 of 25 and lung of 8 of 27 patients. All responders in lung had an improved FVC, and 2 patients also had an improved DLCOadj. Disease activation was observed in 10 (37%) patients at a median of 1 (range, 1 to 6) years after HDIT. Disease activation or progression occurred in lung (n = 6), heart (n = 1), or in other systems that required DMARD treatment (n = 3). Four of 6 patients with lung disease activation/progression died at a median of 4 (range, 1 to 5) years after HDIT. A patient with disease activation in the lung also developed a new anti-Smith antibody at more than 3 years associated with new onset of thrombocytopenia. A second patient with disease activation who had the onset of a syndrome resembling rheumatoid arthritis and required DMARD therapy developed a newly positive rheumatoid factor at more than 1 year.
Infections
We previously reported a fatal Epstein-Barr virus (EBV)–associated posttransplantation lymphoproliferative disorder (PTLD) in 1 patient associated with changing from horse to rabbit ATG in the HDIT regimen.10,22 One case of CMV disease (gastroenteritis) occurred that resolved with antiviral treatment. Bacteremic episodes were observed in 11 patients and were usually associated with a central line. The microorganism identified in 6 of these bacteremic episodes was staphylococcus (coagulase negative). Three months after HDIT, infections were infrequent despite persistent lymphopenia. Reactivation of varicella zoster virus (VZV) (shingles) occurred in 6 patients at a median of 8 (range, 3 to 26) months after HDIT in patients not receiving preventive acyclovir (Table 5).
Infections after HDIT for SSc
| Infections . | Frequency, no. . | Site . | |
|---|---|---|---|
| Less than 3 mo . | More than 3 mo . | ||
| Virus | |||
| EBV-associated PTLD | 1 | 0 | Systemic |
| Herpes simplex | 3 | 0 | Perianal, vaginal, oral |
| Cytomegalovirus | |||
| Reactivation* | 6 | 0 | Blood |
| Disease | 1 | 0 | Gastrointestinal tract |
| Varicella zoster† | 1 | 5 | Skin |
| BK virus | 1 | 0 | Hemorrhagic cystitis |
| Bacteria | |||
| Bacteremia | 11 | 0 | |
| Urinary tract infection | 3 | 0 | Bladder |
| Exit site infections | 3 | 0 | Central line, 2; J tube, 1 |
| Osteomyelitis‡ | 1 | 0 | Elbow |
| Cellulitis‡ | 1 | 0 | Thumb |
| Aspiration pneumonia§ | 1 | 3 | Lung |
| Fungus: Aspergillus flavus | 1 | 0 | Isolated skin (1 site) |
| Infections . | Frequency, no. . | Site . | |
|---|---|---|---|
| Less than 3 mo . | More than 3 mo . | ||
| Virus | |||
| EBV-associated PTLD | 1 | 0 | Systemic |
| Herpes simplex | 3 | 0 | Perianal, vaginal, oral |
| Cytomegalovirus | |||
| Reactivation* | 6 | 0 | Blood |
| Disease | 1 | 0 | Gastrointestinal tract |
| Varicella zoster† | 1 | 5 | Skin |
| BK virus | 1 | 0 | Hemorrhagic cystitis |
| Bacteria | |||
| Bacteremia | 11 | 0 | |
| Urinary tract infection | 3 | 0 | Bladder |
| Exit site infections | 3 | 0 | Central line, 2; J tube, 1 |
| Osteomyelitis‡ | 1 | 0 | Elbow |
| Cellulitis‡ | 1 | 0 | Thumb |
| Aspiration pneumonia§ | 1 | 3 | Lung |
| Fungus: Aspergillus flavus | 1 | 0 | Isolated skin (1 site) |
Infectious events were ascertained until 2 years (or longer for VZV infections) after HDIT and autologous HCT.
Cytomeglovirus reactivation was treated preemptively with antiviral therapy.
Varicella zoster reactivation in patients not receiving antiviral prophylaxis.
Secondary to skin ulcer related to SSc.
Late aspiration pneumonia events were attributed to abnormal esophageal function and gastric reflux associated with systemic sclerosis.
Regimen-related complications
Fatal pulmonary toxicities were observed in 2 of the first 8 patients on study and were previously reported.10 No further similar pulmonary events occurred after lung shielding was adopted for TBI. Renal crisis or dysfunction occurred in 6 patients, 3 of whom had a history of renal crisis or renal dysfunction before study treatment. All adverse renal events first occurred less than 2 months after HDIT. Dialysis was required in 2 patients, but other complications developed after prolonged hospitalization and the patients died at day 123 and 191. A third patient required dialysis and died on day 14 after a sepsis syndrome (no microorganism isolated) and multiorgan failure. A fourth patient required dialysis for 20 months, but at 2 and 4 years without dialysis had serum creatinine levels of 212 and 274 μmol/L (3.1 and 2.4 mg/dL), respectively. The final 2 patients had a doubling of serum creatinine but otherwise were clinically stable. Cardiovascular complications were supraventricular arrhythmias (n = 2), heart failure responsive to medication (n = 2), and hypertension. One patient with an abnormal pretreatment ejection fraction of 47% had a fatal arrhythmia at day 22. At autopsy, only SSc-related changes to the heart were observed.
Only 1 case of hypothyroidism developed at 12 months after HDIT among the 17 patients who were evaluable to 4 (range, 1 to 8) years after HDIT. In this single case, antithyroglobulin antibodies had been increased both before and after HDIT. In scheduled evaluations of the eye for 18 patients at last follow-up, only mild posterior subcapsular cataracts were observed in 2 patients. One patient developed neutropenia associated with a dysplastic marrow morphology and multiple cytogenetic abnormalities that were consistent with a diagnosis of myelodysplastic syndrome (MDS). A second patient died from complications related to MDS at 6 years after HDIT. Cytogenetic studies on both of the pretransplantation marrows had been normal. CD34+ cells or marrow that had been cryopreserved before transplantation in these 2 patients were thawed, and fluorescence in situ hybridization (FISH) studies were performed. In the pretransplantation CD34+ sample, 8.3% and 8.0% of the cells were positive for monosomy 5 and 7, respectively, both of which were present when the patient was diagnosed with MDS at 2 years after HDIT. No abnormalities were detected by FISH of the pretransplantation marrow on the other patient.
One patient with a 32-pack-year history of smoking cigarettes and who had lung shielding during TBI had a successful pneumonectomy at 5 years after HDIT for stage I non–small-cell lung carcinoma that had been detected at a routine CT scan of the chest.
Survival
There were 8 (23%) treatment-related and 4 (12%) disease-related deaths (Table 6). SSc-related organ dysfunction contributed to 6 of the 7 treatment-related deaths that occurred in the first year after HCT. There was 1 treatment-related event of renal failure that required dialysis for 20 months. Both the estimated overall and progression-free survival were 64% at 5 years (Figure 5).
Cause of death
| Cause of death . | No. . | Time to event, mo after HDIT . |
|---|---|---|
| Treatment related | ||
| Respiratory failure: idiopathic pneumonitis + SSc | 2 | 2, 2 |
| EBV-PTLD | 1 | 2 |
| Renal crisis/failure | 2 | 4, 6 |
| Cardiac arrhythmia | 1 | 0.5 |
| Multiorgan failure and pulmonary hemorrhage | 1 | 0.5 |
| Myelodysplastic syndrome | 1 | 76 |
| Disease related | ||
| Respiratory failure* | 4 | 12, 44, 50, 59 |
| Cause of death . | No. . | Time to event, mo after HDIT . |
|---|---|---|
| Treatment related | ||
| Respiratory failure: idiopathic pneumonitis + SSc | 2 | 2, 2 |
| EBV-PTLD | 1 | 2 |
| Renal crisis/failure | 2 | 4, 6 |
| Cardiac arrhythmia | 1 | 0.5 |
| Multiorgan failure and pulmonary hemorrhage | 1 | 0.5 |
| Myelodysplastic syndrome | 1 | 76 |
| Disease related | ||
| Respiratory failure* | 4 | 12, 44, 50, 59 |
In 1 patient the terminal event was a gastrointestinal bleed.
Survival and progression-free survival after high-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for systemic sclerosis. The estimated probability of survival and progression-free survival at 5 years was 64%. Tick marks represent censored observations.
Survival and progression-free survival after high-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for systemic sclerosis. The estimated probability of survival and progression-free survival at 5 years was 64%. Tick marks represent censored observations.
Discussion
This report demonstrates a major decrease of the dermal fibrosis by histopathology associated with an improvement of the mRSS after standard treatment regimens or HDIT and autologous HCT for SSc. This suggests that the dermal fibrosis of SSc is in a dynamic state and disease activity is required to maintain it. Improvements of the skin and resolution of dermal fibrosis have also been observed in SSc patients after allogeneic HCT.23,24 No differences in mRSS were observed in a randomized clinical trial of methotrexate.25 At 2 years in another clinical trial, patients with diffuse scleroderma had a mean decrease in mRSS of 4.8 and 6.9 in the low- and high-dose arms of D-penicillamine, respectively.6 A very modest but significant improvement in skin score was observed at 1 and 2 years in a recent randomized clinical trial of oral cyclophosphamide administered for 12 months.5,26 Patients with diffuse scleroderma in this study had a mean baseline mRSS of 21.0 ± 1.0, and at 2 years the mRSS had decreased by 6.2 and 3.8 in the cyclophosphamide and placebo arms, respectively. This compares with a mean decrease of 17.6 at 1 to 2 years after HDIT and autologous HCT as reported in this study. In a recent report, a correlation was observed between the overall skin score and the grade of dermal fibrosis (graded 0 to 3).27 Further in this report, a decrease in the grade of dermal fibrosis was observed in 4 of 5 patients after high-dose cyclophosphamide and autologous HCT (3 patients had a decrease of dermal fibrosis by 1 of 3 possible grades and 1 patient by 2 of 3 possible grades). The sclerosis grades (mild, moderate, and severe) were based on a compilation of the degree of fibrosis in 4 different levels of dermis, with extra emphasis placed on fibrosis in the mid and deep reticular dermis. The dermal fibrosis grading system we developed in this study was based on the overall percentage of dermal sclerosis rather than its character or location. Because the follow-up was shorter, it is difficult to compare those results with our reported experience. However, we observed a mean decrease of 3 grades of a possible 5, and 7 of 10 patients had dermal fibrosis grades of only 0 or 1 at last follow-up after the more intensive HDIT including TBI. More importantly, both grading systems reflect the improvement in the skin score. The skin “remodeling” that has been reported after HDIT and either autologous or allogeneic HCT may be comparable to the “remodeling” of fibrotic bone marrow that was observed after successful allogeneic HCT for myeloproliferative disorders.28 Progression in other organs was observed in some patients even though a skin response occurred.
In this clinical trial of HDIT, there were sustained responses at a median of 4 years consisting of a marked reduction in mRSS, improved overall function and, in general, clinically stable internal organ function. Although overall mortality at 5 years was 36% and treatment-related mortality was 23%, revisions to the treatment plan and better patient selection based on the experience obtained in the pilot study should significantly decrease the risk of treatment-related deaths even further. In the early phase of the experience with HDIT and autologous HCT reported by the European League Against Rheumatism/European Group for Blood and Marrow Transplantation (EULAR/EBMT), treatment-related mortality was 17%.29 In an update of a randomized clinical trial conducted by the same group, there has been no treatment-related mortality or unexpected severe toxicities.30 This was ascribed to better patient selection but also may relate to increased experience in managing patients with SSc in a transplantation program.
Although limited in number, other clinical trials of HDIT have been conducted. In a single center study of high-dose therapy with a single agent (cyclophosphamide or melphalan) and autologous HCT, major or partial responses were observed in 8 of 11 patients, but at a median of 18 months, 8 patients had not responded or had relapsed.31 Four (36%) patients had died by 18 months after HDIT. In a report from the European Group of Blood and Marrow Transplantation on 57 patients in their registry with a median follow-up of 20 months, the cumulative probability of disease progression at 5 years was 48% after treatment with mostly high-dose cyclophosphamide with or without T-cell–specific antibodies.29 The cumulative probability of survival at 5 years was 72%. Skin responses were observed in both studies. The higher response rate that we observed in this study and was sustained for a median of 4 years may have resulted from the increased intensity of the pretransplantation immunosuppressive regimen with cyclophosphamide and ATG in combination with TBI. Increased intensity of the high-dose immunosuppressive therapy regimen has been associated with a lower relapse rate of the autoimmune disease.32
Compared with baseline, there was a statistically but not clinically significant decrease of 7% in DLCOadj at the final evaluation. There was a predictable, transient, early decrease in DLCOadj with recovery by 6 to 12 months after HDIT, which is also observed in patients with normal lungs after HCT for hematologic malignancies.10,33 Six patients had disease activation/progression in the lungs, 4 of whom died. However, the average DLCOadj remained stable over time when evaluated using the general estimating equation, and FVC remained stable or improved. There were small but statistically significant changes in serum creatinine levels and ejection fraction at final evaluation compared with baseline, but these changes were not clinically relevant for most patients. Visceral organ function in general remained stable after HDIT for most patients.
It has been previously reported that there was recovery of CD4+ T-cell counts at 2 years after HDIT. However, sustained responses were observed in 63% of patients at a median of 4 years.10,12 Immune recovery after HDIT is associated with increasing thymic-derived naive CD4+ T cells with a decrease in memory T cells, an increase in regulatory T cells, and broader clonal diversity than was present before HDIT.12,34,35 These late immune changes support the conclusion that sustained responses may have resulted from the immunomodulatory effects of HDIT in addition to the early immunosuppression and depletion of autoreactive T cells. The development of new autoantibodies and clinical changes in the manifestations of the autoimmune disease after HCT might have been the natural history of the disease process. However, the immunomodulatory effects of HDIT and autologous HCT may have also resulted in the development of a second autoimmune disorder.36
In the early phase of the pilot study, risks of HDIT specific to SSc were identified. Shielding of the lungs during TBI reduced the risks of pulmonary toxicity. EBV-associated PTLD developed only after equine ATG was replaced by rabbit ATG, so caution is necessary when adjustments to the intensity of the regimen are made and should be limited.22 Renal crisis/dysfunction was observed after HDIT, and it is not clear if some component of the treatment or supportive care contributed to this. Close monitoring of blood pressure and early intervention may mitigate the risk for development of renal crisis. Late adverse effects previously associated with higher doses of TBI such as hypothyroidism and cataracts were infrequent. MDS was diagnosed in 2 patients, 1 in whom pretransplantation chromosomal abnormalities were observed that were similar to the karyotypic changes of the MDS after HDIT and the other in whom a total dose of cyclophosphamide (more than 27 g) was administered before study treatment.37,38 Although high-dose chemoradiotherapy has been associated with an increased risk of secondary MDS or acute myelocytic leukemia (AML), the intensity of the pretransplantation chemotherapy appears to be an additional risk factor.39,40 A contributing factor to the development of the stage I non–small cell carcinoma of the lung in one of the patients was a history of smoking. In the modified HDIT regimen, lung radiation exposure is only 200 cGy. A higher risk of secondary solid tumors has also been observed after high-dose chemoradiotherapy for treatment of hematologic malignancies. However, TBI at doses below 1000 cGy did not increase the risk of secondary MDS/AML or solid tumors compared with chemotherapy-only regimens.39,41,42 All patients will require continued follow-up after HDIT to monitor stability of the disease and for late effects of treatment. Limiting cytotoxic therapy (eg, cyclophosphamide) before HDIT may help reduce the risk of late secondary malignancies.
In this pilot study with a median follow-up of 4 years, substantial improvement in skin involvement occurred, confirmed by clear histologic improvement or resolution of dermal fibrosis. This is the first description of this grading system for dermal fibrosis, and further studies on a second data set are required for validation if it is to be used as a clinical tool. There was also substantial improvement in overall function, and there was general stability of internal organ involvement overall. Patients included in this study were within 4 years from diagnosis with non-Raynaud symptoms of SSc, so it is unknown if similar results would be observed after HDIT in patients with longer duration of disease. Important clinical issues in the use of HDIT and autologous HCT for SSc were identified and led to successful protocol modifications. Future studies should consider renal shielding during TBI. The observations of sustained disease stabilization and improved function in this study were encouraging; however, we cannot be certain that this outcome is a significant improvement over the natural history of the disease. A National Institutes of Health (NIH)–sponsored randomized clinical trial (Scleroderma: Cyclophosphamide or Transplant [SCOT]) based on the pilot study described in this report is now being conducted in the United States that compares HDIT and autologous HCT to pulse cyclophosphamide.43 In addition, a second randomized clinical trial (Autologous Stem Cell Transplantation International Scleroderma Trial [ASTIS]) is being conducted in Europe in which high-dose cyclophosphamide and autologous HCT are compared with pulse cyclophosphamide.
An lnside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards N01-AI-05419 from the National Institute of Allergy and Infectious Diseases, HL36444 from the National Heart, Lung, and Blood Institute, and CA15704 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD
The authors acknowledge the study coordinators and data technicians who contributed to this study. We thank Helen Crawford, Bonnie Larson, Sue Carbonneau, and Jessica Fleenor for their excellent support in preparing this manuscript. We also thank Amgen for the gift of recombinant human G-CSF used in this study.
National Institutes of Health
Authorship
Contribution: R.A.N., P.A.M., J.L.N., R.S., K.M.S., and D.E.F. were responsible for study concept and design; R.A.N., P.A.M., D.E.F., L.J.C., M.A., C.S.C., J.D.G., L.H., G.H., C.F.L., M.D.M., K.T.M., B.M., J.A.M., F.V., M.H.W., and J.R.S., acquisition of data; R.A.N., P.A.M., L.J.C., M.D.M., and D.E.F., analysis and interpretation; R.A.N., P.A.M., and D.E.F., writing of the manuscript; R.S. and K.M.S., obtaining funding; T.A.G., statistical analysis; R.A.N., P.A.M., D.E.F., L.J.C., C.S.C., M.A., and C.F.L., study supervision at collaborating sites; H.S., analysis of skin biopsies and development of the grading system; and J.D.G., central review of high-resolution computed tomography of the chest.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard A. Nash, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: rnash@fhcrc.org.