Abstract
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome in which the known susceptibility genes (DKC1, TERC, and TERT) belong to the telomere maintenance pathway; patients with DC have very short telomeres. We used multicolor flow fluorescence in situ hybridization analysis of median telomere length in total blood leukocytes, granulocytes, lymphocytes, and several lymphocyte subsets to confirm the diagnosis of DC, distinguish patients with DC from unaffected family members, identify clinically silent DC carriers, and discriminate between patients with DC and those with other bone marrow failure disorders. We defined “very short” telomeres as below the first percentile measured among 400 healthy control subjects over the entire age range. Diagnostic sensitivity and specificity of very short telomeres for DC were more than 90% for total lymphocytes, CD45RA+/CD20− naive T cells, and CD20+ B cells. Granulocyte and total leukocyte assays were not specific; CD45RA− memory T cells and CD57+ NK/NKT were not sensitive. We observed very short telomeres in a clinically normal family member who subsequently developed DC. We propose adding leukocyte subset flow fluorescence in situ hybridization telomere length measurement to the evaluation of patients and families suspected to have DC, because the correct diagnosis will substantially affect patient management.
Introduction
The diagnosis of dyskeratosis congenita (DC) is usually made in individuals who have at least 2 features of the diagnostic triad: dyskeratotic nails, lacy reticular skin pigmentation, and leukoplakia. Because many patients with DC have aplastic anemia (AA), DC is classified under the rubric of inherited bone marrow failure syndromes (IBMFS). Family histories of patients with DC are consistent with X-linked–recessive (MIM [Mendelian inheritance in man]305000), autosomal-dominant (MIM 127550), and autosomal-recessive (MIM 224230) patterns of inheritance and, to date, pathogenic germline mutations have been identified in an X-linked–recessive gene, DKC1, and in 2 autosomal-dominant genes, TERC and TERT.1-4 The protein products of these genes are involved in the intricate telomere maintenance pathway.5 However, mutations have not been identified in approximately 60% of clinically diagnosed DC cases.6
Telomeres consist of TTAGGG nucleotide repeats and associated proteins at the ends of chromosomes. A minimum number of telomere repeats is required to protect chromosome ends from recombination and fusion. In most somatic cells, telomere repeats are lost through several pathways,7 and telomerase partially compensates for such losses.5 Mutations in the genes involved in telomere maintenance are associated with failure to maintain telomere length. When one or more chromosomes contain telomeres that have reached a critically short telomere length, the cell can no longer divide and undergoes apoptosis or becomes senescent. Telomerase is able to maintain telomere length in germline cells, but continuous shortening of telomeres is a consequence of cell division in most somatic cells and thus a feature of aging.8 In patients in families with mutations in telomere maintenance pathway genes, telomeres are shorter with each generation,9 and rapidly dividing hematopoietic cells from such patients provide a sensitive indicator of accelerated telomere shortening. The hematopoietic compartment may develop genetic instability as a result of telomere erosion, resulting in AA and increased risk of myelodysplastic syndrome (MDS) and acute myeloid leukemia.10
Previous reports suggested that average telomeres were shorter in granulocytes or mononuclear cells from some (but not all) patients with acquired AA than healthy control subjects. as well as in granulocytes from patients with paroxysmal nocturnal hemoglobinuria.11-14 Short telomeres were also reported in some patients with inherited bone marrow failure disorders, including Fanconi anemia (FA)11,15 and Shwachman-Diamond syndrome (SDS).16 Correlations with the severity of the cytopenias or the presence of MDS or cytogenetic clones have been inconsistent.
Mitchell et al first observed short telomeres in lymphoblasts of patients with DC as a result of mutations in DKC1.17 Subsequently, short telomeres were reported in patients with DC resulting from mutations in DKC1, TERC, or TERT.3,9,18,19 For those patients in whom these genes are normal, the identification of short telomeres may be a useful surrogate for the diagnosis of DC.6
Most of the early studies used Southern blot analysis of DNA extracted from total leukocytes or mononuclear cells and reported either mean telomere length in patients compared with age-matched control subjects or the difference between the average telomere lengths of patients and controls (“deltaTEL”). The focus of those reports was the average telomere length in groups of patients compared with control subjects rather than on the individuals whose telomeres were abnormally short. More recently, Lansdorp and colleagues developed and improved methods using flow cytometry and fluorescence in situ hybridization (FISH) to analyze telomere length in multiple peripheral blood cell types from the same individual and to compare each result from patients with those from a large group of age-matched normal control subjects.20
We used automated multicolor flow FISH to assay telomere lengths in total leukocytes, granulocytes, total lymphocytes, and lymphocyte subsets. Participants included patients with DC, FA, Diamond-Blackfan anemia (DBA), SDS, and other rare or unclassified bone marrow failure syndromes as well as their first-degree relatives. We focused on identification of very short telomeres in individuals and in specific diagnostic groups. The performance characteristics of the assay were quantified for diagnosing DC as well as for distinguishing patients with DC from their unaffected relatives and from patients with other marrow failure disorders (non-DC patients).
Patients, materials, and methods
Blood samples were obtained from 26 patients with DC, 54 of their first-degree relatives, 17 with FA (one after bone marrow transplantation [BMT], 3 with hematopoietic somatic mosaicism21 ), 14 with DBA, 5 with SDS, 10 with other unclassified but possibly inherited conditions, and 35 clinically healthy family members (ie, normal blood counts and physical examinations with no stigmata of the proband's syndrome) of these non-DC patients. Controls were 400 healthy persons ranging from birth to 100 years of age.3 Participants were enrolled in the Clinical Genetics Branch Inherited Bone Marrow Failure Syndromes protocol (www.marrowfailure.cancer.gov), which was approved by the National Cancer Institute Institutional Review Board; all participants or their guardians provided written informed consent in accordance with the Declaration of Helsinki.
The diagnosis of DC was made if the patient had (1) 2 or 3 components of the diagnostic triad (dyskeratotic nails, lacy reticular skin pigmentation, and leukoplakia); (2) one of the triad plus hypoplastic bone marrow and at least 2 less common physical findings (epiphora, developmental problems, pulmonary disease, short stature, dental caries, esophageal narrowing, hair loss, early gray hair, and others22,23 ); and/or (3) a deleterious germline mutation in a known DC gene.6 Other family members were considered to have DC if they met those criteria or had the same mutant gene as the proband. Patients were classified as the Hoyeraal-Hreidarsson variant (HH) if they had DC features plus intrauterine growth retardation, developmental delay, microcephaly, cerebellar hypoplasia, and immunodeficiency; and as Revesz syndrome if they had exudative retinopathy in addition to the previous findings. Non-DC patients (FA, DBA, SDS, and others) were diagnosed if they met criteria for a specific IBMFS or for none of the major IBMFS. The “other patients” included 2 siblings with unexplained neutropenia, 2 with thrombocytopenia absent radii, 3 patients with early-onset thrombocytopenia, 2 with unexplained AA, and one with skin poikiloderma and macrocytic red blood cells. None of these had at least 2 of the classic DC triad or 2 or more of the minor DC physical findings. Those with thrombocytopenia had wild-type DKC1, TERC, and MPL genes. Non-DC relatives were unaffected parents and siblings of patients with non-DC diagnoses.
Bone marrow failure was defined according to clinical guidelines for the management of FA24 : severe, hemoglobin less than 80 g/L (8 g/dL), absolute neutrophil count less than 0.5 ×109/L (500/μL), platelets less than 30 ×109/L (30 000/μL), or on treatment; moderate, below normal for age but above criteria for severe; or none, normal values for age. For single cytopenias, severe, on treatment; or moderate, below diagnostic values for the relevant lineage (hemoglobin below normal for age for DBA, absolute neutrophil count less than 1.5 ×109/L (1500/μL) for SDS, platelets less than 140 ×109/L (140 000/μL) for thrombocytopenias). Bone marrow data were not included because they were not available for many patients.
Blood was drawn in sodium heparin and shipped at room temperature to the telomere laboratory in Vancouver, British Columbia, Canada; processing was performed within 48 hours of phlebotomy. Details of the technical methods can be found in Baerlocher et al.20 Briefly, preparation of leukocytes involved lysis of the red cells with NH4Cl. Leukocytes were denatured in formamide using 87°C, hybridized with a fluorescein-conjugated (CCCTAA)3 peptide nucleic acid probe, and counterstained with LDS751 DNA dye. Analysis of fluorescence was then performed on a FACSCalibur instrument (BD Biosciences, San Jose, CA). The cell types analyzed included total leukocytes, granulocytes, total lymphocytes, CD45RA-positive/CD20-negative naive T cells (referred to as naive T cells), CD45RA-negative memory T cells (memory T cells), CD20-positive B cells (B cells), and CD57-positive NK/NKT cells (NK/NKT cells). “Very short” telomeres were defined as less than the first percentile for the age-matched normal control range and “short” telomeres were from the first to less than the tenth percentile. The normal curves were derived from best-fit analysis of actual telomere length analysis for each subset in 400 normal individuals; the only standardization was the inclusion of bovine thymocytes with long telomeres in every test as an internal control. The laboratory studies and interpretations were performed on coded samples lacking personal and diagnostic identifiers.
Chromosome breakage studies for FA were done on T-lymphocytes or skin fibroblasts using both diepoxybutane and mitomycin C (Oregon Health and Science University, Portland, OR). Red cell adenosine deaminase for DBA was measured according to standard methods (Stanford University Medical Center, Palo Alto, CA). Mutations in DKC1, TERC, and TERT, as well as genes for FA, DBA, SDS, and MPL were identified by bidirectional sequencing of polymerase chain reaction-amplified fragments (GeneDx, Gaithersburg, MD).
Analyses were performed using Microsoft Excel (Microsoft Office Excel 2003) and Stata9 (StataCorp Release 9, College Station, TX). P values are 2-sided. Data are reported as odds ratios in favor of diagnosis of DC, 95% confidence intervals, sensitivity and specificity, and positive and negative predictive values (PPV and NPV) using the frequency of “very short” or “short plus very short” telomeres in each patient group. Eighteen of the total of 156 subjects did not have sufficient cells for analyses of one or more of granulocytes, B cells, or NK/NKT cells.
The features of the subjects are summarized in Tables 1 and 2. The patients with DC were on average significantly younger than their relatives: 58% of patients with DC were younger than age 18 (ie, children), versus 20% of their unaffected family members (P < .002, Fisher exact test). Similarly, the non-DC patients included 59% children versus 37% children in their relatives (P = .07). Fifteen patients with classic DC had at least 2 features of the diagnostic triad as did 3 HH and one patient with Revesz syndrome; 6 were classified as DC based on a combination of the less common findings. One 11-year-old patient with no signs or symptoms of DC had a germline mutation in TERC (see next paragraph).
DC subjects
| . | DC patient diagnosis . | DC relatives . | ||||
|---|---|---|---|---|---|---|
| DC . | HH . | RS . | Silent . | All DC . | ||
| Number | 17 | 4 | 4 | 1 | 26 | 54 |
| Age, y | ||||||
| Median | 23 | 7.5 | 4.5 | 11 | 13 | 38 |
| Range | 3-47 | 3-13 | 1-13 | — | 1-47 | 0-87 |
| Less than 18 years old, no. (%) | 7 | 4 | 4 | 1 | 15 (58) | 11 (20)‡ |
| DC triad, no. | 15 | 3 | 1 | 0 | 19 | 0 |
| Mutations | ||||||
| DKC1 | 2 | 3 | 0 | — | 5 | 4* |
| TERC | 4 | 0 | 0 | 1† | 5 | 0 |
| TERT | 2 | 0 | 0 | — | 2 | 0 |
| Unknown | 9 | 1 | 4 | — | 14 | |
| BMF | ||||||
| None | 0 | 0 | 0 | 1 | 1 | 54 |
| Moderate | 7 | 3 | 0 | — | 10 | 0 |
| Severe | 10 | 1 | 4 | — | 15 | 0 |
| . | DC patient diagnosis . | DC relatives . | ||||
|---|---|---|---|---|---|---|
| DC . | HH . | RS . | Silent . | All DC . | ||
| Number | 17 | 4 | 4 | 1 | 26 | 54 |
| Age, y | ||||||
| Median | 23 | 7.5 | 4.5 | 11 | 13 | 38 |
| Range | 3-47 | 3-13 | 1-13 | — | 1-47 | 0-87 |
| Less than 18 years old, no. (%) | 7 | 4 | 4 | 1 | 15 (58) | 11 (20)‡ |
| DC triad, no. | 15 | 3 | 1 | 0 | 19 | 0 |
| Mutations | ||||||
| DKC1 | 2 | 3 | 0 | — | 5 | 4* |
| TERC | 4 | 0 | 0 | 1† | 5 | 0 |
| TERT | 2 | 0 | 0 | — | 2 | 0 |
| Unknown | 9 | 1 | 4 | — | 14 | |
| BMF | ||||||
| None | 0 | 0 | 0 | 1 | 1 | 54 |
| Moderate | 7 | 3 | 0 | — | 10 | 0 |
| Severe | 10 | 1 | 4 | — | 15 | 0 |
Features described in “Patients, materials, and methods.”
BMF indicates bone marrow failure; HH, Hoyeraal-Hreidarsson; RS, Revesz Syndrome; —, not applicable; and no., number.
3 mothers and 1 sister of males with DKC1 mutations were heterozygous carriers of this X-linked recessive gene, 1 mother was not a carrier.
1 silent carrier son of an affected mother with TERC mutation.
A larger proportion of DC patients was less than 18 years old compared with DC relatives (P < .002, Fisher exact).
Non-DC subjects
| Diagnosis . | FA patients . | Non-FA, non-DC . | All non-DC . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FA . | BMT . | Mosaic . | FA Total . | DBA . | SDS . | Other . | Non-DC patients . | Non-DC relatives . | |
| Number | 13 | 1 | 3 | 17 | 14 | 5 | 10 | 46 | 35 |
| Age, y | |||||||||
| Median | 18 | 15 | 27 | 18 | 13.5 | 12 | 5.5 | 15 | 31 |
| Range | 5-42 | — | 27-33 | 5-42 | 3-43 | 8-42 | 1-21 | 1-43 | 5-72 |
| Less than 18 y, no. (%) | 6 | 1 | 0 | 7 (41) | 8 (57) | 4 (80) | 8 (80) | 27 (59) | 13 (37)‡ |
| Mutations | |||||||||
| FANCA | 8 | 1 | 3 | 12 | RPS19 4 | SBDS 4 | 0 | 20 | — |
| FANCC | 2 | 0 | 0 | 2 | — | — | — | — | — |
| FANCF | 1 | 0 | 0 | 1 | — | — | — | — | — |
| Unknown | 2 | 0 | 0 | 2 | 10 | 1 | 10 | 23 | — |
| BMF | |||||||||
| None | 3 | 1* | 3 | 7 | 1 | 0 | 2† | 7 | 35 |
| Moderate | 4 | 0 | 0 | 4 | 2 | 5 | 6 | 30 | 0 |
| Severe | 6 | 0 | 0 | 6 | 11 | 0 | 2 | 8 | 0 |
| Diagnosis . | FA patients . | Non-FA, non-DC . | All non-DC . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FA . | BMT . | Mosaic . | FA Total . | DBA . | SDS . | Other . | Non-DC patients . | Non-DC relatives . | |
| Number | 13 | 1 | 3 | 17 | 14 | 5 | 10 | 46 | 35 |
| Age, y | |||||||||
| Median | 18 | 15 | 27 | 18 | 13.5 | 12 | 5.5 | 15 | 31 |
| Range | 5-42 | — | 27-33 | 5-42 | 3-43 | 8-42 | 1-21 | 1-43 | 5-72 |
| Less than 18 y, no. (%) | 6 | 1 | 0 | 7 (41) | 8 (57) | 4 (80) | 8 (80) | 27 (59) | 13 (37)‡ |
| Mutations | |||||||||
| FANCA | 8 | 1 | 3 | 12 | RPS19 4 | SBDS 4 | 0 | 20 | — |
| FANCC | 2 | 0 | 0 | 2 | — | — | — | — | — |
| FANCF | 1 | 0 | 0 | 1 | — | — | — | — | — |
| Unknown | 2 | 0 | 0 | 2 | 10 | 1 | 10 | 23 | — |
| BMF | |||||||||
| None | 3 | 1* | 3 | 7 | 1 | 0 | 2† | 7 | 35 |
| Moderate | 4 | 0 | 0 | 4 | 2 | 5 | 6 | 30 | 0 |
| Severe | 6 | 0 | 0 | 6 | 11 | 0 | 2 | 8 | 0 |
FA indicates Fanconi anemia; BMT, bone marrow transplant; —, not applicable.
History of severe BMF pre-BMT. Not included in subsequent statistical analyses, but data included in figures. Severity defined in “Patients, materials, and methods.”
Two patients with physical findings (one sibling of a subject with thrombocytopenia absent radii, one with skin poikiloderma and macrocytic red cells) and normal blood counts.
‡The trend was for a larger proportion of non-DC subjects less than 18 years old compared with non-DC relatives (P = .07, Fisher exact).
Two patients with DC and 3 siblings with HH had mutations in DKC1, 4 patients had mutations in TERC, and 2 in TERT.19 The 11-year-old silent carrier inherited a mutation in TERC from his symptomatic mother. During 4 years of follow up he developed mild thrombocytopenia, decreasing bone marrow cellularity, and a stable cytogenetic clone; he is now considered clinically affected. Fifteen of the 26 patients with DC had no detectable DC gene mutations. Half of the patients with DC had severe cytopenias and were receiving transfusions, androgens, and/or granulocyte-colony stimulating factor; 6 were considering hematopoietic stem cell transplantation at the time of the telomere length study.
Three mothers and one sister of male patients with mutations in the X-linked DKC1 gene shared the proband's mutation; one mother did not. Two brothers (who had no physical or hematologic DC stigmata) were being evaluated as hematopoietic stem cell donors for their sister with severe AA; their father and his twin brother had died from complications of DC, and several additional relatives had signs of DC. All other relatives of patients with DC were well and lacked the family mutation and any DC-associated clinical findings.
All 17 patients with FA had increased chromosome breakage in blood T-lymphocytes or in fibroblasts; their relatives had normal chromosome breakage. One patient with FA had received a bone marrow transplant from his sister 3 years previously and 3 were hematopoietic somatic mosaics with normal blood counts (skin fibroblasts confirmed the FA diagnoses).21,25 Results from the FA patient who had received a transplant are included in the graphs but not in the statistical analyses. Six patients with FA had severe pancytopenia and were receiving transfusions and/or androgens and/or granulocyte-colony stimulating factor, whereas 4 had abnormal values in at least one cell line, usually platelets.
Fourteen patients were clinically diagnosed with DBA. Four belonged to one family with an RPS19 mutation26 ; this gene was wild-type in the others. Eleven were receiving transfusions or corticosteroids, 2 had mild macrocytic anemia, and one patient with an RPS19 mutation had normal blood counts. Five patients with exocrine pancreatic insufficiency and neutropenia had clinical SDS27 ; 4 had mutations in SBDS.28 The 10 “other” patients included those with single or multiple cytopenias and/or abnormal fingernails or skin pigmentation sufficient to warrant considering the diagnosis of DC. Although not every patient was directly examined by the research team, clinical summaries, laboratory results, and high-quality photographs were available, and the consensus was that these patients did not have DC.
Results
Comparison of patients with dyskeratosis congenita with their relatives
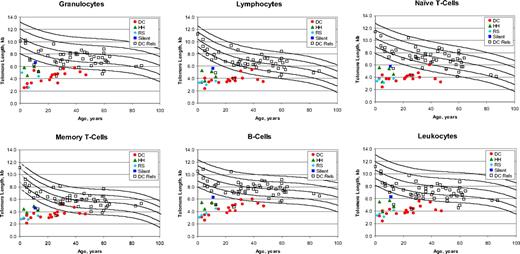
Telomere length was reported as the median value in kilobytes (kb) for each cell type. The results for patients with DC and their relatives are shown in Figure 1 and summarized in Table 3 and Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Because the DC patient group was significantly younger than the unaffected DC relatives, the median telomere lengths cannot be directly compared (Table S1). Total leukocyte telomeres were shorter than the age-matched first percentile of control cells in all 26 patients whose DC diagnosis preceded the telomere study. Five clinically unaffected relatives also had total leukocyte telomeres shorter than the first percentile; however, 2 relatives had telomeres in all cell subsets that were as short as in the majority of the known patients, suggesting that they might be silent carriers. The total leukocyte telomere assay was 100% sensitive, but only 91% specific (Table 3). Assay performance characteristics were improved when several leukocyte subsets were examined individually. Granulocytes were sensitive (96%) but not specific (84%) for distinguishing patients with DC from their relatives. However, lymphocytes, naive T cells, and B cells all had sensitivities and specificities greater than 90%, whereas memory T cells and NK/NKT cells were less sensitive (85% and 72%, respectively). The combinations of very short telomeres in granulocytes and lymphocytes, granulocytes plus naive T cells plus B cells, and lymphocytes plus naive T cells plus B cells were 92% sensitive and 96% specific. The PPV was 92% or greater for lymphocytes, memory T cells, NK/NKT cells, and all the combinations mentioned, but was only 75% for granulocytes, 84% for total leukocytes, and 89% for naive T cells. The NPV was very high in all cell types. Relaxing the cutoff to include very short and short telomeres, that is, less than the tenth percentile of normal, improved the sensitivities and NPVs for all cell types but markedly reduced the specificities and PPVs (Table S1).
Telomere length according to age in patients with dyskeratosis congenita and their relatives. The vertical axis represents telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Symbols represent subjects: 17 patients with dyskeratosis congenita (red solid circle), 4 Hoyeraal-Hreidarsson variant (green solid triangle), 4 Revesz syndrome (turquoise solid diamond), one silent carrier (blue solid square), and 54 relatives (open square). (Top panels) Granulocytes, lymphocytes, and CD45RA-positive/CD20-negative naive T cells. (Bottom panels) CD45RA-negative memory T cells, CD20-positive B cells, and total leukocytes.
Telomere length according to age in patients with dyskeratosis congenita and their relatives. The vertical axis represents telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Symbols represent subjects: 17 patients with dyskeratosis congenita (red solid circle), 4 Hoyeraal-Hreidarsson variant (green solid triangle), 4 Revesz syndrome (turquoise solid diamond), one silent carrier (blue solid square), and 54 relatives (open square). (Top panels) Granulocytes, lymphocytes, and CD45RA-positive/CD20-negative naive T cells. (Bottom panels) CD45RA-negative memory T cells, CD20-positive B cells, and total leukocytes.
Telomere lengths in DC patients compared with DC relatives
| Cell type . | DC patients . | DC relatives . | DC patients vs DC relatives . | |||||
|---|---|---|---|---|---|---|---|---|
| N abnormal . | N abnormal . | OR . | 95% CI . | Sens (%) . | Spec (%) . | PPV (%) . | NPV (%) . | |
| Granulocytes | 24/25 | 8/51 | 129 | 15-5419 | 96 | 84 | 75 | 98 |
| Lymphocytes* | 24/26* | 2/54* | 312* | 34-3694* | 92* | 96* | 92* | 96* |
| CD45RA+/CD20− naïve T cells* | 25/26* | 3/54* | 425* | 37-17734* | 96* | 94* | 89* | 98* |
| CD45− memory T cells | 22/26 | 2/54 | 143 | 21-1443 | 85 | 96 | 92 | 93 |
| CD20+ B-cells* | 24/26* | 4/54* | 150* | 22-1508* | 92* | 93* | 86* | 96* |
| CD57+ NK/NKT cells | 18/25 | 1/50 | 129 | 14-5347 | 72 | 98 | 95 | 88 |
| Total leukocytes | 26/26 | 5/54 | ∞ | — | 100 | 91 | 84 | 100 |
| Granulocytes, lymphocytes | 23/25 | 2/51 | 282 | 30-3349 | 92 | 96 | 92 | 96 |
| Granulocytes, naïve T cells, B cells | 23/25 | 2/51 | 282 | 30-3349 | 92 | 96 | 92 | 96 |
| Lymphocytes, naïve T cells, B cells* | 24/26* | 2/54* | 312* | 34-3694* | 92* | 96* | 92* | 96* |
| Cell type . | DC patients . | DC relatives . | DC patients vs DC relatives . | |||||
|---|---|---|---|---|---|---|---|---|
| N abnormal . | N abnormal . | OR . | 95% CI . | Sens (%) . | Spec (%) . | PPV (%) . | NPV (%) . | |
| Granulocytes | 24/25 | 8/51 | 129 | 15-5419 | 96 | 84 | 75 | 98 |
| Lymphocytes* | 24/26* | 2/54* | 312* | 34-3694* | 92* | 96* | 92* | 96* |
| CD45RA+/CD20− naïve T cells* | 25/26* | 3/54* | 425* | 37-17734* | 96* | 94* | 89* | 98* |
| CD45− memory T cells | 22/26 | 2/54 | 143 | 21-1443 | 85 | 96 | 92 | 93 |
| CD20+ B-cells* | 24/26* | 4/54* | 150* | 22-1508* | 92* | 93* | 86* | 96* |
| CD57+ NK/NKT cells | 18/25 | 1/50 | 129 | 14-5347 | 72 | 98 | 95 | 88 |
| Total leukocytes | 26/26 | 5/54 | ∞ | — | 100 | 91 | 84 | 100 |
| Granulocytes, lymphocytes | 23/25 | 2/51 | 282 | 30-3349 | 92 | 96 | 92 | 96 |
| Granulocytes, naïve T cells, B cells | 23/25 | 2/51 | 282 | 30-3349 | 92 | 96 | 92 | 96 |
| Lymphocytes, naïve T cells, B cells* | 24/26* | 2/54* | 312* | 34-3694* | 92* | 96* | 92* | 96* |
N indicates number; abnormal, below 1st percentile (numerator and denominator indicate the number abnormal/number assayed for each cell type); OR, odds ratio in favor of being a DC patient; CI, confidence interval; Sens, sensitivity, the proportion of those with disease who were correctly identified; Spec, specificity, the proportion of nondiseased people who were correctly identified as negative; PPV, positive predictive value, the proportion of those who test positive who actually have the disease; NPV, negative predictive value, the proportion of those who test negative who do not have the disease; —, not applicable.
The leukocyte subsets with the best performance characteristics.
Two patients with DC did not have universally very short telomeres (Figure 1). A 39-year-old TERC mutation-positive patient with mild pancytopenia associated with hypocellular MDS had telomeres that were below the first percentile in total leukocytes and granulocytes; between the first and tenth percentiles in total lymphocytes, naive T cells, memory T cells, and B cells; and above the tenth percentile in NK/NKT cells. A 31-year-old patient from a large, mutation-unknown dominant family with classic DC had leukoplakia, dysplastic nails, mild thrombocytopenia, and a hypocellular marrow; telomeres were at or below the first percentile in all cell types except lymphocytes, memory T cells, and NK/NKT cells. All other patients with DC had very short telomeres in at least 6 different subsets.
Two clinically normal DC relatives (members of the large family mentioned previously) had very short telomeres in all cell types; they may be silent carriers of a mutation in a new DC gene. Seven relatives from other families had very short telomeres in one to 3 leukocyte subsets, particularly granulocytes and total leukocytes.
In patients with DC, there was no correlation between telomere length and severity of bone marrow failure, presence of the diagnostic triad, or less common physical findings (data not shown). Telomeres were shorter in patients with DC lacking a mutation in the known DC genes than in those with mutations (data not shown).
Non–dyskeratosis congenita patients and their relatives
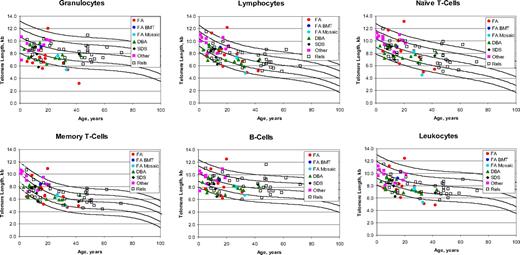
Telomere lengths were generally above the first percentile in the relatives of non-DC patients (Figure 2). Only 3 relatives had very short telomeres in up to 3 cell types. However, several of the non-DC IBMFS patients had very short telomeres, particularly in total leukocytes (6 of 44 patients) and granulocytes (11 of 42 patients). Only 2 of these patients had very short telomeres in at least 5 cell subsets and only one in the combination of lymphocytes plus naive T cells plus B cells.
Telomere length according to age in non–dyskeratosis congenita patients and their relatives. The vertical axis represents telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Symbols represent subjects: 13 Fanconi anemia (FA) patients (red solid circle), one FA postbone marrow transplant (blue solid circle), 3 FA mosaics (turquoise solid circle), 14 Diamond-Blackfan anemia (green solid triangle), 5 Shwachman-Diamond syndrome (black solid diamond), 10 other (magenta solid square), and 35 relatives (open square).
Telomere length according to age in non–dyskeratosis congenita patients and their relatives. The vertical axis represents telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Symbols represent subjects: 13 Fanconi anemia (FA) patients (red solid circle), one FA postbone marrow transplant (blue solid circle), 3 FA mosaics (turquoise solid circle), 14 Diamond-Blackfan anemia (green solid triangle), 5 Shwachman-Diamond syndrome (black solid diamond), 10 other (magenta solid square), and 35 relatives (open square).
Diamond-Blackfan anemia.
One transfusion-dependent patient had very short telomeres in all 6 analyzed cell types; telomeres were also very short in the granulocytes and memory T cells of his transfusion-dependent fraternal twin. RPS19, DKC1, and TERC were wild-type in the first twin (with microcephaly, developmental delay, and growth retardation but no components of the DC diagnostic triad), who might have the HH variant of DC. One other patient with DBA had very short telomeres only in granulocytes.
Fanconi anemia.
Seven patients had very short telomeres in at least one cell type. Granulocytes were very short in one FANCF and one FANCA patient. Two patients had very short telomeres in granulocytes and total leukocytes (FANCA) and granulocytes and memory T cells (FANC unclassified). Two patients (FANCA and FANCC) had very short telomeres in 3 cell types, and one FANCA patient with hematopoietic somatic mosaicism (no chromosome breakage in peripheral blood; positive breakage in fibroblasts) surprisingly had very short telomeres in total leukocytes, granulocytes, lymphocytes, and naive T cells. This patient had previously received full-dose radiation therapy for head and neck squamous cell carcinoma21 as had one of the FANCC patients with very short telomeres for vaginal squamous cell carcinoma,29 which might have affected the rate of division of hematopoietic stem cells during recovery from radiation-induced suppression.30
Shwachman-Diamond syndrome and others.
One patient with SDS had unexplained very short telomeres in granulocytes, lymphocytes, memory T cells, B cells, and total leukocytes. One “other” patient with pancytopenia had very short telomeres only in B cells; red cell- and platelet transfusion-dependent bone marrow failure has spontaneously improved. None of the other patients in this group had very short telomeres in any leukocyte subset.
Comparison of patients with dyskeratosis congenita with non–dyskeratosis congenita patients
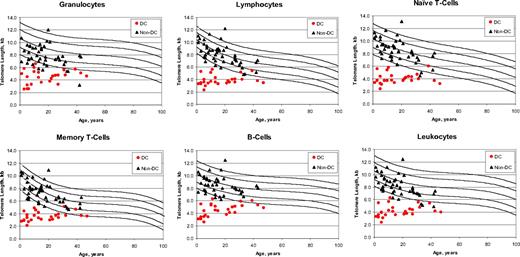
Although the DC and non-DC IBMFS patients were similar in age (Tables 1 and 2), the telomere lengths in each cell type were significantly shorter in the patients with DC (Figure 3, Tables 4 and S2). The performance characteristics of the telomere length assay in distinguishing patients with DC from non-DC patients were similar to those distinguishing patients with DC from their own relatives; sensitivities for each cell type exceeded 90% for all cell types except memory T cells and NK/NKT cells. However, the specificities and PPVs were lower in granulocytes, B cells, and total leukocytes. The combinations of cell types that performed well for patients with DC versus their relatives also did so for patients with DC versus non-DC patients, that is, very short granulocytes and lymphocytes, granulocytes plus naive T cells plus B cells, and lymphocytes plus naive T cells plus B cells were all 92% sensitive and 93% to 98% specific, with PPVs of 88% to 96% and NPVs of 95%.
Telomere length according to age in dyskeratosis congenita and non–dyskeratosis congenita patients. The vertical axis represents telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Symbols represent subjects: 26 patients with dyskeratosis congenita (red solid circle), 46 non–dyskeratosis congenita patients (black solid triangle).
Telomere length according to age in dyskeratosis congenita and non–dyskeratosis congenita patients. The vertical axis represents telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Symbols represent subjects: 26 patients with dyskeratosis congenita (red solid circle), 46 non–dyskeratosis congenita patients (black solid triangle).
Telomere lengths in DC patients compared with non-DC patients
| Cell type . | DC patients . | Non-DC patients . | DC patients vs non-DC patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N abnormal . | N abnormal . | OR . | 95% CI . | Sens (%) . | Spec (%) . | PPV (%) . | NPV (%) . | |
| Granulocytes | 24/25 | 11/42 | 68 | 8-2867 | 96 | 74 | 69 | 97 |
| Lymphocytes* | 24/26* | 4/44* | 120* | 17-1215* | 92* | 91* | 86* | 95* |
| CD45RA+/CD20− naïve T cells* | 25/26* | 3/45* | 350* | 31-14665* | 96* | 93* | 89* | 98* |
| CD45− memory T cells | 22/26 | 4/45 | 56 | 11-325 | 85 | 91 | 85 | 91 |
| CD20+ B cells* | 24/26* | 5/41* | 86* | 13-855* | 92* | 88* | 83* | 95* |
| CD57+ NK/NKT cells | 18/25 | 0/42 | 1/∞ | — | 72 | 100 | 100 | 86 |
| Leukocytes | 26/26 | 6/45 | ∞ | — | 100 | 87 | 81 | 100 |
| Granulocytes, lymphocytes | 23/25 | 3/42 | 150 | 19-1592 | 92 | 93 | 88 | 95 |
| Granulocytes, naïve T cells, B cells | 23/25 | 1/41 | 460 | 33-19338 | 92 | 98 | 96 | 95 |
| Lymphocytes, naïve T cells, B cells* | 24/26* | 1/41* | 480* | 35-20144* | 92* | 98* | 96* | 95* |
| Cell type . | DC patients . | Non-DC patients . | DC patients vs non-DC patients . | |||||
|---|---|---|---|---|---|---|---|---|
| N abnormal . | N abnormal . | OR . | 95% CI . | Sens (%) . | Spec (%) . | PPV (%) . | NPV (%) . | |
| Granulocytes | 24/25 | 11/42 | 68 | 8-2867 | 96 | 74 | 69 | 97 |
| Lymphocytes* | 24/26* | 4/44* | 120* | 17-1215* | 92* | 91* | 86* | 95* |
| CD45RA+/CD20− naïve T cells* | 25/26* | 3/45* | 350* | 31-14665* | 96* | 93* | 89* | 98* |
| CD45− memory T cells | 22/26 | 4/45 | 56 | 11-325 | 85 | 91 | 85 | 91 |
| CD20+ B cells* | 24/26* | 5/41* | 86* | 13-855* | 92* | 88* | 83* | 95* |
| CD57+ NK/NKT cells | 18/25 | 0/42 | 1/∞ | — | 72 | 100 | 100 | 86 |
| Leukocytes | 26/26 | 6/45 | ∞ | — | 100 | 87 | 81 | 100 |
| Granulocytes, lymphocytes | 23/25 | 3/42 | 150 | 19-1592 | 92 | 93 | 88 | 95 |
| Granulocytes, naïve T cells, B cells | 23/25 | 1/41 | 460 | 33-19338 | 92 | 98 | 96 | 95 |
| Lymphocytes, naïve T cells, B cells* | 24/26* | 1/41* | 480* | 35-20144* | 92* | 98* | 96* | 95* |
N indicates number; abnormal, below 1st percentile (numerator and denominator indicate the number abnormal/number assayed for each cell type); OR, odds ratio in favor of being a DC patient; CI, confidence interval; Sens, sensitivity, the proportion of those with disease who were correctly identified; Spec, specificity, the proportion of nondiseased people who were correctly identified as negative; PPV, positive predictive value, the proportion of those who test positive who actually have the disease; NPV, negative predictive value, the proportion of those who test negative who do not have the disease; —, not applicable.
The leukocyte subsets with the best performance characteristics.
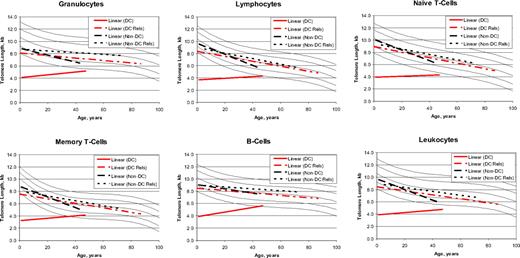
Telomere length versus age
The cross-sectional patterns of age-related telomere lengths (Figure 4) in the non-DC IBMFS patients as well as the DC and non-DC relatives showed the expected decreasing median telomere lengths with increasing age.8 In contrast, telomeres were very short in patients with DC of all ages, slightly increasing with age, which reached statistical significance in the B cells (P for slope = .008). These unexpected cross-sectional results may not reflect individual changes with age, but the distinctive trend in DC is tantalizing. The median intercept with age zero for telomere length in lymphocytes was 3.7 kb for patients with DC, 9.7 kb for non-DC patients, and 8.3 and 8.8 kb in the respective relatives. At age 40, the comparable telomere lengths were 4.2, 6.0, 6.7, and 7.0 kb, respectively.
Regression lines for telomere length according to age. The vertical axis represents the median telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Patients with dyskeratosis congenita (DC) (red solid line), relatives with dyskeratosis congenita (red dashed line), non–dyskeratosis congenita patients (black long dashed line), and non–dyskeratosis congenita relatives (black short dashed line).
Regression lines for telomere length according to age. The vertical axis represents the median telomere length in kilobytes. Lines in the figures indicate the first, tenth, 50th, 90th, and 99th percentiles of results from 400 normal control subjects. Patients with dyskeratosis congenita (DC) (red solid line), relatives with dyskeratosis congenita (red dashed line), non–dyskeratosis congenita patients (black long dashed line), and non–dyskeratosis congenita relatives (black short dashed line).
Discussion
We addressed 2 major questions: (1) Can we distinguish patients with DC from their relatives on the basis of telomere length and can we identify clinically silent carriers of DC in the absence of a mutation in a known DC gene? (2) Can we diagnose DC in patients with an IBMFS that does not meet diagnostic criteria for one of the defined IBMFS or in patients with apparently sporadic AA, including those who fail to respond to immunosuppressive therapy?31
Nearly all of the known patients with DC in our series had very short telomeres in the majority of their leukocyte subsets (Tables 3,Table 4-5), clearly distinguishing them from the other groups and demonstrating that very short telomeres are a surrogate marker for DC. Management decisions in families without mutations in known DC genes must be made without genetic identification of the specific family members at risk, complicated by variable expression and age-related penetrance of the DC clinical phenotype; analysis of telomere length may be informative. Silent carriers may be at risk of subsequently developing DC-related complications such as bone marrow failure, MDS, acute myeloid leukemia, premalignant leukoplakia, or solid tumors. They may benefit from appropriate genetic counseling regarding their own future health risks and the risk of DC in their children. If very short telomeres represent a reliable indicator of DC, then designating such persons as affected can markedly enhance the statistical power of linkage analysis aimed at identifying new DC genes in mutation-negative families. It is also essential to exclude as BMT donors family members who appear well but have very short telomeres, because their cells may fail to engraft.32
Percent of patients with very short or short telomeres in each leukocyte subset
| Diagnosis . | Granulocytes . | Lymphocytes . | Naïve T cells . | Memory T cells . | B cells . | NK/NKT cells . | Leukocytes . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | |
| DC patients* | 96* | 4* | 92* | 8* | 96* | 4* | 85* | 8* | 92* | 8* | 72* | 20* | 100* | 0* |
| DC relatives* | 16* | 22* | 4* | 15* | 6* | 13* | 4* | 13* | 4* | 15* | 2* | 10* | 9* | 39* |
| FA† | 40 | 33 | 13 | 20 | 13 | 19 | 6 | 19 | 14 | 14 | 0 | 27 | 27 | 20 |
| DBA | 21 | 21 | 7 | 36 | 7 | 29 | 14 | 14 | 7 | 29 | 0 | 62 | 7 | 50 |
| SDS | 20 | 40 | 20 | 20 | 0 | 40 | 20 | 0 | 20 | 20 | 0 | 60 | 20 | 40 |
| Other | 13 | 13 | 0 | 10 | 0 | 0 | 0 | 10 | 10 | 0 | 0 | 0 | 0 | 10 |
| All non-DC patients* | 26* | 26* | 9* | 23* | 7* | 20* | 9* | 13* | 12* | 16* | 0* | 36* | 14* | 30* |
| Non-DC relatives | 3 | 20 | 0 | 17 | 0 | 17 | 0 | 20 | 3 | 14 | 3 | 20 | 3 | 20 |
| Diagnosis . | Granulocytes . | Lymphocytes . | Naïve T cells . | Memory T cells . | B cells . | NK/NKT cells . | Leukocytes . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | <1% . | 1-10% . | |
| DC patients* | 96* | 4* | 92* | 8* | 96* | 4* | 85* | 8* | 92* | 8* | 72* | 20* | 100* | 0* |
| DC relatives* | 16* | 22* | 4* | 15* | 6* | 13* | 4* | 13* | 4* | 15* | 2* | 10* | 9* | 39* |
| FA† | 40 | 33 | 13 | 20 | 13 | 19 | 6 | 19 | 14 | 14 | 0 | 27 | 27 | 20 |
| DBA | 21 | 21 | 7 | 36 | 7 | 29 | 14 | 14 | 7 | 29 | 0 | 62 | 7 | 50 |
| SDS | 20 | 40 | 20 | 20 | 0 | 40 | 20 | 0 | 20 | 20 | 0 | 60 | 20 | 40 |
| Other | 13 | 13 | 0 | 10 | 0 | 0 | 0 | 10 | 10 | 0 | 0 | 0 | 0 | 10 |
| All non-DC patients* | 26* | 26* | 9* | 23* | 7* | 20* | 9* | 13* | 12* | 16* | 0* | 36* | 14* | 30* |
| Non-DC relatives | 3 | 20 | 0 | 17 | 0 | 17 | 0 | 20 | 3 | 14 | 3 | 20 | 3 | 20 |
Data are percentage of patients in the diagnostic group who had telomeres less than 1st percentile (<1%), or between 1st and 10th percentile (1-10%). Telomeres that were normal or longer than normal (ie, 10th percentile or above) are not included.
The important groups for this study: DC patients, DC relatives, and all non-DC patients.
FA includes 1 patient after bone marrow transplant whose telomeres were normal, and 3 with hematopoietic somatic mosaicism.
Identification of individuals with very short telomeres within mutation-positive DC families would alter the risk–benefit assessment with regard to whether to undergo clinical germline mutation testing. Confirmation of DC in silent carriers identified with very short telomeres will expand our understanding of the clinical and hematologic spectrum and the natural history of DC and facilitate insights into gene–gene or gene–environment interactions, which result in bone marrow failure or malignant progression in only some patients with DC.
The correct attribution of bone marrow failure, MDS, acute myeloid leukemia, or a solid tumor (upper or lower gastrointestinal tract carcinomas) to DC has major management implications. DC-related AA is more likely to respond to treatment with androgens or hematopoietic growth factors and unlikely to respond to immunosuppressive therapy.23,31,33 The protocol for BMT may require modification, because patients with DC are at particular risk of pulmonary and hepatic fibrosis and other transplant-related complications.34 Treatment of leukemia or solid tumors may also require modification, because all cells share the defect in telomere maintenance and associated genomic instability. The failure to recognize DC in patients (particularly older patients) with marrow failure or typical cancers may be more common than generally appreciated as a result of its rarity, variable clinical phenotype, and variable age at onset.
This study is the first to systematically examine leukocyte telomere length by flow FISH in patients with a non-DC IBMFS. Although we agree with earlier reports of “short telomeres” in groups of patients with FA and SDS, our analysis of multiple leukocyte subsets in individuals and use of a larger cohort of age-matched control subjects led us to identify important differences in the pattern of telomere abnormality in DC versus other IBMFS. We found that very short telomeres (explicitly defined as below the first percentile of normal) were restricted to a small subset of patients in the non-DC IBMFS group (Figure 2, Table 5) and were primarily observed in total leukocytes and granulocytes. Only one of 14 patients with DBA had very short telomeres in all leukocyte subsets; although he met standard diagnostic criteria for DBA, he also had some soft features of DC such as microcephaly and developmental delay. It is theoretically possible that he has both genetic conditions. Identification of new genes for both these disorders may resolve this diagnostic dilemma.
One of 5 patients with SDS also had very short or short telomeres in 5 white cell subsets. This patient has both clinical and molecular evidence of SDS and no DC phenotype; sequencing of known DC genes is underway. Three of 16 patients with FA without prior BMT had very short telomeres in 3 or 4 white cell subsets. Two of these patients had received radiation therapy for cancer (which might have affected their hematopoietic cell cycle kinetics), whereas the third had mild neutropenia and thrombocytopenia and required no treatment. One of the patients with prior radiation therapy had somatic hematopoietic mosaicism; excessive cell division resulting from clonal expansion of a genetically corrected stem cell might have resulted in short telomeres.21 However, the other 2 mosaic patients had normal telomeres.
There are several possible explanations for very short telomeres in some non-DC IBMFS patients in addition to the coincidence of 2 very rare conditions. Unlike the pattern in DC, telomere length was not equally short in all cell types among the non-DC IBMFS patients. Very short telomeres were more frequent in total leukocytes and granulocytes than in lymphocytes or lymphocyte subsets in this patient subset. Very short telomeres in patients with DC support an etiologic role for mutations in genes in the telomere maintenance pathway. In contrast, very short telomeres in non-DC patients may be a consequence of hematopoietic failure or stress or a result of treatment (eg, radiation).
The unexpected rise in age-related telomere length in DC (Figure 4) contrasts with the expected decrease in the non-DC patients and in all the relatives.8 Interpretation is complicated by the cross-sectional nature of the data. Patients with DC may be born with hematopoietic stem cells with very short telomeres, and it is possible that cells with telomeres below a specific length do not survive. Younger patients with DC were clinically more severe (this younger group includes the 4 patients with HH and the 4 with Revesz syndrome phenotypes) than older patients with DC and had the shortest telomeres. Patients who were identified as DC when they were older may have milder forms of DC. Thus, the aberrant pattern of telomere length in successive subgroups of patients with DC by age may reflect the type of patients available for analysis at each age.
The analysis of leukocyte subsets within the same blood sample by flow FISH is a powerful tool for addressing our clinical and experimental questions. The median value for telomere length in each cell type is plotted on a graph, which contains the range for a large number of normal, age-matched control subjects, permitting easy comparison of the patient's results with those of the control subjects. The definition of abnormal, or very short, as less than the first percentile is arbitrary, but provides good sensitivity and specificity for the important distinction between patients with DC and their unaffected relatives. The performance characteristics for distinguishing between DC and non-DC IBMFS patients were slightly less satisfactory with good sensitivity but less specificity in individual leukocyte subsets. However, the combination of very short telomeres in the combination of lymphocytes, naive T cells, and B cells was similar in the distinction of patients with DC from their relatives or from non-DC patients with sensitivity 92%, specificity 96% to 98%, PPV 92% to 96%, and NPV 95% to 96%. In our experience, all but 2 patients with DC had very short telomeres in at least 5 white cell types, whereas the 2 relatives with DC with this result were presumably silent carriers; all other relatives had very short telomeres in less than 4 cell types (most had no subsets with very short telomeres). Only 4 patients with non-DC disorders had very short telomeres in more than 3 leukocyte subsets; most had no cells with very short telomeres (Figure S1).
Using intact cells in the highly sensitive and reproducible flow FISH system provides a more precise measure of telomere length than Southern blots, which estimate median telomere length from an electrophoretic pattern. Although no single cell type was perfectly sensitive and specific, the most suitable cell types appear to be total lymphocytes, naive T cells, and B cells, alone, or particularly, in combination. NK/NKT cells and memory T cells lack sensitivity and granulocytes and total leukocytes lack specificity. Total leukocytes are a heterogeneous cell population with the proportions of each cell type specific to each patient, thus providing less consistency than the individual analyses of defined leukocyte subsets. For example, in acquired AA, the telomere length in granulocytes may be very short, whereas the telomere length in lymphocytes is less affected.13 Most likely this reflects sustained destruction of hematopoietic stem cells resulting in an increased turnover of the remaining hematopoietic stem cells. However, patients with DC have short telomeres in hematopoietic cell subsets from birth; thus, even long-lived cells with physiologically slow turnover such as lymphocytes are diagnostic. Eleven of the non-DC IBMFS patients had very short granulocyte telomeres, and 6 had short total leukocyte telomeres, contributing to the lack of specificity in these cells.
A limitation of our analysis is the lack of mutations in known genes in 60%, 70%, and 20% of the DC, DBA, and SDS patients, respectively. Although the diagnosis of FA is definitive in the presence of an abnormal chromosome breakage test, diagnosis of the other disorders in the absence of a deleterious mutation is less certain. Furthermore, we have no clear diagnoses for some of the “other” patients. Strengths of our study include the classification of disease status in patients and relatives before performance of the telomere length assay, the large number of patients with DC and other IBMFS, the large number of normal relatives and control subjects, and the evaluation of multiple leukocyte subsets at the individual level.
We propose that telomere length measurement by automated multicolor flow FISH in leukocyte subsets has great potential in the clinical evaluation of patients in whom DC is suspected. The result can be obtained rapidly, and the assay is particularly useful in families without mutations in the known DC genes. Asymptomatic family members with very short telomeres can be excluded as transplantation donors as a result of anticipated failure of engraftment,32,35 and patients with bone marrow failure of unclear etiology may be classified as DC or DC may be excluded, thus permitting syndrome-appropriate management. Our data suggest that the diagnostic algorithm for patients with marrow failure can now be expanded: chromosome breakage for FA, red cell adenosine deaminase for DBA, serum trypsinogen and isoamylase for SDS, and telomere length of leukocyte subsets by automated multicolor flow FISH for DC.
Presented in abstract form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 11, 2006 (Abstract 183).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (B.P.A., S.A.S., S.J.C., J.A.P., N.G.) and by contracts N02-CP-91026, N02-CP-11019 and HHSN261200655001C with Westat, Inc. G.M.B. was supported by the Swiss National Foundation and the Bernese Cancer League. Work in the laboratory of P.M.L. is supported by grants from the National Institutes of Health (AI29524), The Canadian Institute of Health Research (MOP38075 and GMH79042), and the National Cancer Institute of Canada with funds from the Terry Fox Foundation.
We thank Mark H. Greene, MD, and Philip S. Rosenberg, PhD, for helpful discussions; Irma Vulto for excellent technical assistance; and Lisa Leathwood, RN, Ann Carr, MS, CGC, Sara Glashofer, MS, and Luda Brener, and the other members of the Inherited Bone Marrow Failure Syndromes (IBMFS) team at Westat for their extensive efforts. We are extremely grateful to all the patients for their enthusiastic participation in the IBMFS study.
National Institutes of Health
Authorship
Contribution: B.P.A., G.M.B., and P.M.L. participated in designing and performing the research; G.M.B. and P.M.L. blindly interpreted the telomere results; S.A.S. and S.J.C. performed analyses of DC genes; B.B.W. and J.P.W. referred atypical important patients; N.G. and J.A.P. provided clinical and genetic counseling support to all patients in the study. B.P.A., G.M.B., and P.M.L. wrote the paper; and all authors checked the final version of the manuscript.
B.P.A. and G.M.B. contributed equally to this work.
Conflict-of-interest disclosure: P.M.L. is a founding shareholder in Repeat Diagnostic Inc., a company specializing in leukocyte telomere length measurements using flow FISH. All other authors declare no competing financial interests.
Correspondence: Blanche P. Alter, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 6120 Executive Blvd., Executive Plaza South, Room 7020, Rockville, MD 20852-7231; e-mail: alterb@mail.nih.gov.