Abstract
Severe congenital neutropenia (SCN) is an inborn disorder of granulopoiesis. Like most other bone marrow failure syndromes, it is associated with a marked propensity to transform into a myelodysplastic syndrome (MDS) or acute leukemia, with a cumulative rate of transformation to MDS/leukemia that exceeds 20%. The genetic (and/or epigenetic) changes that contribute to malignant transformation in SCN are largely unknown. In this study, we performed mutational profiling of 14 genes previously implicated in leukemogenesis using 14 MDS/leukemia samples from patients with SCN. We used high-throughput exon-based resequencing of whole-genome–amplified genomic DNA with a semiautomated method to detect mutations. The sensitivity and specificity of the sequencing pipeline was validated by determining the frequency of mutations in these 14 genes using 188 de novo AML samples. As expected, mutations of tyrosine kinase genes (FLT3, KIT, and JAK2) were common in de novo AML, with a cumulative frequency of 30%. In contrast, no mutations in these genes were detected in the SCN samples; instead, mutations of CSF3R, encoding the G-CSF receptor, were common. These data support the hypothesis that mutations of CSF3R may provide the “activated tyrosine kinase signal” that is thought to be important for leukemogenesis.
Introduction
Bone marrow failure syndromes are characterized by a deficiency of 1 or more hematopoietic lineage. A common feature of both congenital and acquired forms of bone marrow failure is a marked propensity to develop acute myeloid leukemia (AML) or a myelodysplastic syndrome (MDS). The cumulative risk of developing AML or MDS in patients with chronic bone marrow failure ranges from 2% to greater than 20%.1-7 Distinct genetic changes are associated with AML arising in the setting of bone marrow failure. The most prevalent genetic changes are abnormalities of chromosome 7; while only 5% of de novo AML samples display a loss of some or all of chromosome 7, they are present in 35% to 68% of AML arising in the setting of bone marrow failure.3,8-14 These data suggest that distinct genetic mechanisms mediate leukemogenesis in patients with bone marrow failure syndromes.
Severe congenital neutropenia (SCN) is a congenital bone marrow failure syndrome characterized by severe neutropenia present from birth, an arrest of myeloid differentiation at the promyelocyte/myelocyte stage, and frequent infections.15,16 Treatment with granulocyte colony-stimulating factor (G-CSF) is effective in increasing neutrophil counts in most patients.4,17 Like other bone marrow failure syndromes, SCN is associated with a marked propensity to develop MDS or AML. The French Neutropenia Registry reported a cumulative incidence of MDS or AML in patients with SCN of 10.8% at 20 years of age.18 A recent update of the Severe Chronic Neutropenia International Registry (SCNIR) showed that the cumulative incidence of MDS or AML was 21% after 10 years of G-CSF therapy.4 Moreover, no plateau in the incidence of AML or MDS was observed, suggesting that the cumulative risk of progression may be even higher.
The molecular mechanisms that mediate leukemic transformation in SCN are poorly understood. Transformation has been associated with acquired clonal cytogenetic changes, most commonly involving the partial or complete loss of chromosome 7, activating RAS mutations, or abnormalities of chromosome 21.3,10 In addition, acquired mutations of the CSF3R gene encoding the G-CSF receptor (G-CSFR) are present in a subset of patients with SCN and are strongly associated with the development of AML or MDS.19-23 However, the contribution of these CSF3R mutations to leukemogenesis remains unclear, since transgenic mice expressing mutant G-CSFR do not develop AML or MDS,24,25 despite displaying a hyperproliferative response to G-CSF.
In the present study, the frequency of mutations in 14 genes previously implicated in the pathogenesis of AML was determined in 14 SCN patients who had transformed to MDS or acute leukemia. A high-throughput sequencing pipeline was established using exon-based resequencing of whole-genome–amplified genomic DNA (since sample abundance was extremely low for these samples) and a semiautomated method to detect mutations. The sensitivity and specificity of the resequencing pipeline was validated by determining the frequency of mutations in these 14 genes in 188 de novo AML samples. As expected, FLT3, NPM1, and CEBPA were the most frequently mutated genes in the de novo AML samples, but no mutations in these genes were detected in the SCN samples. The pattern of gene mutations in SCN-MDS/AML has implications both for the design of targeted therapies and for our understanding of the mechanisms of leukemic transformation in SCN patients.
Patients, materials, and methods
Human subjects
Ninety-four adult patients with de novo AML (Discovery set) were enrolled in a study at Washington University to identify genetic factors contributing to leukemia initiation and progression. Approval was obtained from the Washington University institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki. Eligibility criteria included age greater than 18, more than 30% myeloblasts in the bone marrow, and the absence of antecedent chemotherapy, radiation therapy, or history of myelodysplasia. Samples with 2 or fewer clonal cytogenetic abnormalities were given preference for inclusion in the study. Peripheral blood and bone marrow were obtained for analysis of tumor cells, and a 6-mm punch biopsy of skin was obtained for analysis of unaffected somatic cells. DNA was prepared from both tissues using standard protocols. Ninety-four de novo AML samples from patients with the same entry criteria were also obtained from Cancer and Leukemia Group B (CALGB); similar numbers of patients with the M0/1, M2, M3, and M4 French-American-British (FAB) subtype were selected for analysis.26 The SCN bone marrow samples were obtained from the SCNIR and French Neutropenia Registry. The institutional review boards of all participating institutions approved this study.
Sequencing
Due to the limited amounts of starting material available for many samples, Phi29-based whole-genome amplification was performed on genomic DNA samples (both SCN and de novo AML samples) using the Qiagen Repli-G service (Qiagen, Valencia, CA).27 Gene sequences were extracted from 2 public draft human genome databases, GenBank28 and Ensembl,29 and used as reference sequences for assembly and primer construction. The 500-bp 5′ flanking region and exonic regions, including the exon/intron boundaries, were amplified by polymerase chain reaction (PCR) to generate amplicons for resequencing. Universal tails were added to the 5′ ends of amplification primers to serve as the sequencing primer sites. The primers were either designed by ABI VariantSEQr (www.ncbi.nlm.nih.gov/projects/genome/probe/doc/ProjVariantSEQr.shtml) or with scripts that are based on the use of the Primer 3 program version 1.1.1.30 Amplicons were purified by Exonuclease/SAP treatment and then directly sequenced using BigDye Terminator chemistry on an ABI3730 automated sequencer (Applied Biosystems, Foster City, CA).
Sequence analysis
The sequence traces were assembled and scanned for variations from the reference sequence using 2 parallel mutation detection pipelines: the mutation profiling pipeline and the PolyScan (Washington University, St Louis, MO) informatics suite.31 The mutation profiling pipeline consists of the following steps: (1) analyze data using Phred version 0.20425.c to determine sequence quality;32,33 (2) assemble sequence into contigs using Phrap version 0.990329 (http://www.phrap.org/phredphrapconsed.html); (3) align contigs to the reference sequence using Needle (Staden's Package version 1.6.0);34 and (4) perform automated mutation detection using Informax Automated Sequence Assembly and Analysis Pipeline (ASAAP) version 1.2 (Invitrogen, Carlsbad, CA). To manually confirm the tagged sequence variations, we developed a new graphical user interface called Mutation Viewer (MV), where the mutations/polymorphisms are painted onto a DNA schematic showing protein motifs (eg, kinase domain). This program also prioritizes mutations based on their potential functional significance (conservative vs nonconservative substitutions), frequency, and whether they are known single-nucleotide polymorphisms (SNPs; derived by scanning the NCBI28 and Ensembl29 SNP databases). Finally, specimens from the same patient (eg, tumor versus germ line) were covisualized, allowing for the rapid identification of somatic mutations. All gene mutations reported in the literature (eg, NRAS G12D) or discovered in our initial analysis were considered “hot spots,” and the relevant sequence tracings were manually reviewed for all other samples. Detailed protocols and primer sequences are available through the Washington University School of Medicine Genome Sequencing Center (GSC) website.35 The primary sequence data are available at the Bioinformatics Core website.36
Sequence coverage for each amplicon was assessed as follows. Sequence traces for each amplicon were aligned with the reference sequence; alignments with a BLAST expect score of greater than 1 × 10−35 indicate poor sequence quality and were considered inadequate coverage. For amplicons in which more than 15% of samples failed, primers were redesigned and new amplicons were generated until adequate coverage for all amplicons was obtained.
Statistical analysis
Pairwise mutation analyses were done by permutation testing. Each pair of genes was tabulated to determine the frequency of the number of cases with both mutations present. Permutations were done by shuffling the mutation present/absent values within each gene independently and recalculating how often both genes were mutated within a single case. This null distribution was used to determine the significance value of the unpermuted number of double mutations. The Fisher exact test was used to test the independence of NPM1 mutations from mutations in the grouped tyrosine kinase pathway genes.
Results
Patient characteristics
A total of 188 de novo AML samples were analyzed (Table 1). For the Discovery set of 94 de novo AML samples obtained at Washington University, both skin (“germ line”) and leukemic cell genomic DNA were obtained. This allowed us to determine whether an observed nucleotide change in a leukemic sample was somatically acquired. Of note, most of the de novo AML samples displayed normal or simple cytogenetic abnormalities (Table 1); abnormalities of chromosomes 5 or 7 were rare.
Clinical characteristics of the de novo AML samples
| . | Discovery . | CALGB . |
|---|---|---|
| Cytogenetic subgroup (%) | ||
| Normal | 35 (37.2) | 41 (43.6) |
| t(15;17) only | 12 (12.8) | 20 (21.3) |
| t(8;21) only | 1 (1.1) | 3 (3.2) |
| inv (16) only | 2 (2.1) | 6 (6.4) |
| Trisomy 8 only | 5 (5.3) | 0 (0.0) |
| 5q-/-5 only | 1 (1.1) | 0 (0.0) |
| 7q-/-7 only | 1 (1.1) | 0 (0.0) |
| Complex karyotype, typical* | 2 (2.1) | 0 (0.0) |
| Complex karyotype, atypical* | 10 (10.6) | 0 (0.0) |
| Other | 25 (26.6) | 24 (25.5) |
| FAB subtype (%) | ||
| M0 | 6 (6.4) | 5 (5.3) |
| M1 | 18 (19.1) | 18 (19.1) |
| M2 | 21 (22.3) | 23 (24.5) |
| M3 | 17 (18.1) | 23 (24.5) |
| M4 | 18 (19.1) | 25 (26.6) |
| M5 | 9 (9.6) | 0 (0.0) |
| M6 | 3 (3.2) | 0 (0.0) |
| M7 | 2 (2.1) | 0 (0.0) |
| Age, y (range) | 52.8 (16-81) | 40.3 (22-70) |
| Sex, no. (%) | ||
| Male | 56 (59.6) | 54 (57.4) |
| Female | 38 (40.4) | 40 (42.6) |
| Ethnicity, no. (%) | ||
| Asian | 1 (1.1) | 4 (4.2) |
| Black | 9 (9.6) | 6 (6.4) |
| White | 83 (88.3) | 70 (74.5) |
| Hispanic | 1 (1.1) | 11 (11.7) |
| Other | 0 (0.0) | 3 (3.2) |
| . | Discovery . | CALGB . |
|---|---|---|
| Cytogenetic subgroup (%) | ||
| Normal | 35 (37.2) | 41 (43.6) |
| t(15;17) only | 12 (12.8) | 20 (21.3) |
| t(8;21) only | 1 (1.1) | 3 (3.2) |
| inv (16) only | 2 (2.1) | 6 (6.4) |
| Trisomy 8 only | 5 (5.3) | 0 (0.0) |
| 5q-/-5 only | 1 (1.1) | 0 (0.0) |
| 7q-/-7 only | 1 (1.1) | 0 (0.0) |
| Complex karyotype, typical* | 2 (2.1) | 0 (0.0) |
| Complex karyotype, atypical* | 10 (10.6) | 0 (0.0) |
| Other | 25 (26.6) | 24 (25.5) |
| FAB subtype (%) | ||
| M0 | 6 (6.4) | 5 (5.3) |
| M1 | 18 (19.1) | 18 (19.1) |
| M2 | 21 (22.3) | 23 (24.5) |
| M3 | 17 (18.1) | 23 (24.5) |
| M4 | 18 (19.1) | 25 (26.6) |
| M5 | 9 (9.6) | 0 (0.0) |
| M6 | 3 (3.2) | 0 (0.0) |
| M7 | 2 (2.1) | 0 (0.0) |
| Age, y (range) | 52.8 (16-81) | 40.3 (22-70) |
| Sex, no. (%) | ||
| Male | 56 (59.6) | 54 (57.4) |
| Female | 38 (40.4) | 40 (42.6) |
| Ethnicity, no. (%) | ||
| Asian | 1 (1.1) | 4 (4.2) |
| Black | 9 (9.6) | 6 (6.4) |
| White | 83 (88.3) | 70 (74.5) |
| Hispanic | 1 (1.1) | 11 (11.7) |
| Other | 0 (0.0) | 3 (3.2) |
CALGB indicates Cancer and Leukemia Group B.
Defined based on the criteria outlined by Bacher et al.37
A total of 17 samples from 14 patients with SCN were analyzed (Table 2); 2 of the patients (nos. 14251 and 14252) have been described previously.18 Nine patients had AML, 3 had MDS, 1 had acute lymphocytic leukemia (ALL), and 1 had a mixed AML/ALL picture. Most of the SCN samples were obtained from children (age range, 2.9-49.3 y; median, 10.8 y). Consistent with previous reports,3,10 abnormalities of chromosome 7 were common, observed in 6 of the 13 samples where cytogenetics was available. The dose and duration of G-CSF therapy were variable.
SCN patient characteristics
| Patient ID . | Age, y . | Sex . | Diagnosis . | Cytogenetics . | G-CSF dose, μg/kg/per day . | G-CSF duration, y . |
|---|---|---|---|---|---|---|
| 12397 | 15 | M | AML (M5) | 45, XY, −7 | 1.9 | 6.3 |
| 17390 | 7.8 | F | AML (M2) | 46,XX,ins(1,?7)(q25;q31q36) t(8,21)(q22;qq22)[20] | 3.8 | 3.7 |
| 12374 | 49.3 | M | AML (M7) | 45, XY, −7, del(6)(q21) | 2.4 | 4.8 |
| 17392 | 5 | F | AML (M2) | 46, XY, inv (16), t(5:6)(q31:q27) | 13.5 | 3.7 |
| 12377 | 20.3 | F | MDS (RAEB) | 46, XX, add(2)(q37), add 7(q22) | 9 | 8 |
| 17393 | 18.3 | M | MDS | NA | 5 | 11.4 |
| 17394 | 13.4 | M | AML (M1) | 46, XY | 3.7 | 13.5 |
| 12400 | 7.8 | M | AML | 46, XY | 5.2 | 6.8 |
| 13462 | 16.8 | F | AML (M5) | 45, XX, −7/4n | 2.6 | 8.5 |
| 13464 | 2.9 | M | AML/B-ALL | 46 XY,add (21)(q22); XY, +21 | 10-120 | 2.8 |
| 13476 | 7 | F | AML (M0) | 45,XX,−7[12]/46,XX[11]. nuc ish (D7S486x1) | 0.72 | 6.8 |
| 13995 | 15.6 | F | AML (M2) | del(10)(q32) | 48 | 10.2 |
| 14251 | 11.8 | F | ALL | 48,XX,del(5)(q21q34),+21,+22[16]/46, XX[8] | 26 | 6.8 |
| 14252 | 7.4 | M | MDS | 47,XY,−7,+21,+21[9]/46, XY[5] | 18 | 4.4 |
| Patient ID . | Age, y . | Sex . | Diagnosis . | Cytogenetics . | G-CSF dose, μg/kg/per day . | G-CSF duration, y . |
|---|---|---|---|---|---|---|
| 12397 | 15 | M | AML (M5) | 45, XY, −7 | 1.9 | 6.3 |
| 17390 | 7.8 | F | AML (M2) | 46,XX,ins(1,?7)(q25;q31q36) t(8,21)(q22;qq22)[20] | 3.8 | 3.7 |
| 12374 | 49.3 | M | AML (M7) | 45, XY, −7, del(6)(q21) | 2.4 | 4.8 |
| 17392 | 5 | F | AML (M2) | 46, XY, inv (16), t(5:6)(q31:q27) | 13.5 | 3.7 |
| 12377 | 20.3 | F | MDS (RAEB) | 46, XX, add(2)(q37), add 7(q22) | 9 | 8 |
| 17393 | 18.3 | M | MDS | NA | 5 | 11.4 |
| 17394 | 13.4 | M | AML (M1) | 46, XY | 3.7 | 13.5 |
| 12400 | 7.8 | M | AML | 46, XY | 5.2 | 6.8 |
| 13462 | 16.8 | F | AML (M5) | 45, XX, −7/4n | 2.6 | 8.5 |
| 13464 | 2.9 | M | AML/B-ALL | 46 XY,add (21)(q22); XY, +21 | 10-120 | 2.8 |
| 13476 | 7 | F | AML (M0) | 45,XX,−7[12]/46,XX[11]. nuc ish (D7S486x1) | 0.72 | 6.8 |
| 13995 | 15.6 | F | AML (M2) | del(10)(q32) | 48 | 10.2 |
| 14251 | 11.8 | F | ALL | 48,XX,del(5)(q21q34),+21,+22[16]/46, XX[8] | 26 | 6.8 |
| 14252 | 7.4 | M | MDS | 47,XY,−7,+21,+21[9]/46, XY[5] | 18 | 4.4 |
All patients included in this table are white. The clinical status at the time the sample was obtained is shown, including the diagnosis, major cytogentic abnormality (if any), and duration and dose of G-CSF.
RAEB indicates refractory anemia with excess of blasts; NA, not available.
Sequencing strategy
The genetic progression factors that contribute to leukemogenesis in the setting of SCN are largely unknown. We reasoned that genes that are frequently mutated in de novo AML might also contribute to leukemic transformation in SCN. A total of 14 genes were chosen for resequencing; they included tyrosine kinase genes and genes involved in the transmission of tyrosine kinase signals (FLT3, KIT, CSF1R, JAK2, NRAS, KRAS, and PTPN11), myeloid transcription factors (RUNX1, CEBPA, and SPI1), and NPM1 and TP53. In addition, we sequenced the ELA2 and CSF3R genes, since mutations of these genes have been implicated in leukemic transformation in SCN. The specific exons that were sequenced for each gene are shown in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
A high-throughput sequencing pipeline was employed using whole-genome–amplified genomic DNA and a semiautomated method to detect mutations. The amount of tumor and/or skin DNA available for many of these samples was very limited, necessitating whole-genome amplification to create adequate amounts of template for exonic amplification and resequencing. Since large-scale resequencing with whole-genome–amplified DNA has not previously been reported, it was first necessary to “credential” this material for analysis by assessing the frequency of known mutations in a large cohort of de novo AML patients.
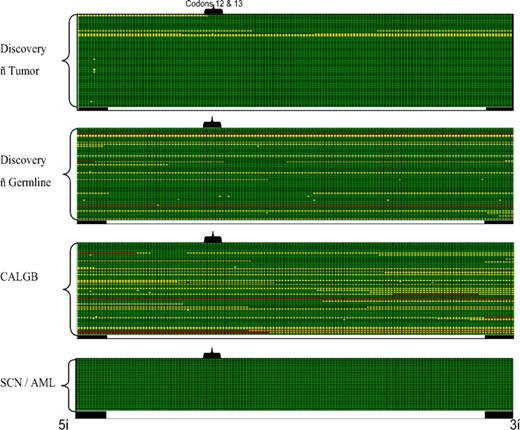
A crucial factor in assessing mutation frequency is the quality and coverage of the sequences obtained. Therefore, we developed an automated method to rapidly assess sequence quality and coverage. Figure 1 contains a representative sequence coverage map for exon 2 of NRAS. High-quality double-stranded (green) or single-stranded (yellow) sequence was observed for nearly all of the samples. For the purposes of this study, a given sample had to meet 2 criteria to be considered to have adequate sequence coverage. First, for genes with mutational hot spots (eg, codons 12, 13, and 64 for NRAS), this region had to have at least single-stranded sequence coverage. Second, sequence gaps (if any) in the coding region had to be less than 10 nucleotides in length. Based on these criteria, only 5 of 210 tumor samples (both de novo AML and SCN samples) had inadequate sequence coverage for NRAS (Figure 1). In fact, the sequence quality and coverage was high for all analyzed genes, with an average of 95% plus or minus 4.3% of samples having adequate coverage (range, 86%-100%). In each case where inadequate sequence coverage was obtained, the sample was eliminated from the final analysis of the relevant gene mutation frequency.
NRAS exon 2 sequence coverage maps. Sequence coverage for exon 2 of NRAS is shown for the Discovery set of 94 AML samples (both tumor and skin), the 94 CALGB AML samples, and the 22 SCN-AML samples; each row represents an individual sample. Each column represents a single nucleotide position starting from the 5′-intronic region (■), through exon 2 of NRAS (□) and 3′-intronic region (■). The position of codons 12 and 13 of NRAS is highlighted. High-quality double-stranded sequence is shown in green, single-stranded coverage in yellow, and no coverage in red.
NRAS exon 2 sequence coverage maps. Sequence coverage for exon 2 of NRAS is shown for the Discovery set of 94 AML samples (both tumor and skin), the 94 CALGB AML samples, and the 22 SCN-AML samples; each row represents an individual sample. Each column represents a single nucleotide position starting from the 5′-intronic region (■), through exon 2 of NRAS (□) and 3′-intronic region (■). The position of codons 12 and 13 of NRAS is highlighted. High-quality double-stranded sequence is shown in green, single-stranded coverage in yellow, and no coverage in red.
Frequency of gene mutations in de novo AML
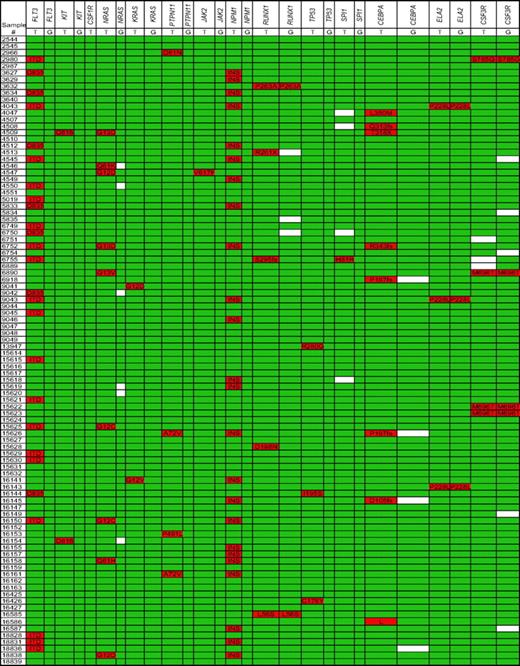
To assess the sensitivity and specificity of our sequencing pipeline, we first sequenced the 188 de novo AML samples, since the frequency of mutations in these genes had previously been established. Figure 2 summarizes the nucleotide deletions, insertions, and nonsynonymous single-nucleotide changes that were detected in the Discovery set of 94 de novo AML samples; single-nucleotide changes present in the NCBI28 or Ensembl29 SNP databases were excluded from analysis. Consistent with previous reports, the 4 genes most often mutated were FLT3,38 NPM1,39 NRAS,37,40 and CEBPA,41 with frequencies in our Discovery set of 27.7%, 25.5%, 9.6%, and 5.3%, respectively (Table 3). As reported previously, somatic mutations of KIT,42 KRAS,40 PTPN11,43 JAK2,44 RUNX1,45 TP53,46 and SPI147,48 were observed but infrequent (< 5%). No somatic mutations of CSF1R (encoding c-fms), ELA2, or CSF3R were detected. Very similar data were obtained with the CALGB set of de novo AML samples (Figure S1). To conserve resources, CSF1R and CSF3R were not sequenced in this set. Collectively, the frequency of gene mutations in our de novo AML samples was comparable to that reported in the literature, validating our use of whole-genome–amplified DNA for the resequencing pipeline (Table 3).
Nucleotide changes for the discovery set of de novo AML samples. Nonsynonymous nucleotide changes and gene deletions or insertions are highlighted in red; white boxes indicate missing sequence data. Known SNPs are excluded from this analysis. Both leukemia (T) and germline (G) samples for each patient were sequenced. ITD indicates internal tandem duplication (FLT3); INS, tetranucleotide insertion of exon 12 (NPM1).
Nucleotide changes for the discovery set of de novo AML samples. Nonsynonymous nucleotide changes and gene deletions or insertions are highlighted in red; white boxes indicate missing sequence data. Known SNPs are excluded from this analysis. Both leukemia (T) and germline (G) samples for each patient were sequenced. ITD indicates internal tandem duplication (FLT3); INS, tetranucleotide insertion of exon 12 (NPM1).
Mutation frequency in SCN-AML versus de novo AML
| Gene . | SCN . | Discovery . | ||||
|---|---|---|---|---|---|---|
| Mutation . | n . | Frequency, % . | Mutation . | n . | Frequency, % . | |
| FLT3 | 0 | 10 | 0.0 | 51 | 185 | 27.6 |
| KIT | 0† | 10 | 0.0 | 4 | 188 | 2.1 |
| CSF1R* | 0 | 9 | 0.0 | 0 | 94 | 0.0 |
| NRAS | 0 | 10 | 0.0 | 17 | 182 | 9.3 |
| KRAS | 0 | 10 | 0.0 | 3 | 187 | 1.6 |
| PTPN11 | 0 | 10 | 0.0 | 5 | 183 | 2.7 |
| JAK2 | 0 | 10 | 0.0 | 2 | 187 | 1.1 |
| NPM1 | 0 | 10 | 0.0 | 43 | 180 | 23.9 |
| RUNX1 | 0 | 10 | 0.0 | 9 | 182 | 4.9 |
| TP53 | 0 | 10 | 0.0 | 3 | 183 | 1.6 |
| SPI1 | 0 | 9 | 0.0 | 2 | 184 | 1.1 |
| CEBPA | 0 | 10 | 0.0 | 11 | 186 | 5.9 |
| ELA2 | 3 | 10 | 30.0 | 0 | 188 | 0.0 |
| CSF3R* | 3 | 10 | 30.0 | 0 | 91 | 0.0 |
| Gene . | SCN . | Discovery . | ||||
|---|---|---|---|---|---|---|
| Mutation . | n . | Frequency, % . | Mutation . | n . | Frequency, % . | |
| FLT3 | 0 | 10 | 0.0 | 51 | 185 | 27.6 |
| KIT | 0† | 10 | 0.0 | 4 | 188 | 2.1 |
| CSF1R* | 0 | 9 | 0.0 | 0 | 94 | 0.0 |
| NRAS | 0 | 10 | 0.0 | 17 | 182 | 9.3 |
| KRAS | 0 | 10 | 0.0 | 3 | 187 | 1.6 |
| PTPN11 | 0 | 10 | 0.0 | 5 | 183 | 2.7 |
| JAK2 | 0 | 10 | 0.0 | 2 | 187 | 1.1 |
| NPM1 | 0 | 10 | 0.0 | 43 | 180 | 23.9 |
| RUNX1 | 0 | 10 | 0.0 | 9 | 182 | 4.9 |
| TP53 | 0 | 10 | 0.0 | 3 | 183 | 1.6 |
| SPI1 | 0 | 9 | 0.0 | 2 | 184 | 1.1 |
| CEBPA | 0 | 10 | 0.0 | 11 | 186 | 5.9 |
| ELA2 | 3 | 10 | 30.0 | 0 | 188 | 0.0 |
| CSF3R* | 3 | 10 | 30.0 | 0 | 91 | 0.0 |
Only those SCN samples with AML were included. The Discovery and CALBG de novo AML data sets were combined. Germ line nonsynonymous mutations and samples with inadequate sequence coverage were excluded.
Not sequenced in the CALBG samples.
The T801I KIT SNP was excluded because it has been shown to be a nonactivating mutation.
The multiplexed gene sequencing approach allowed us to look for associations between gene mutations; 2 significant associations were observed (Figure 2). Although the numbers were small, a significant positive association between KIT mutations and RUNX1 mutations was observed (P = .027). As reported previously,49 a trend toward a positive association between FLT3 mutations and NPM1 mutations was also observed (P = .076). Indeed, after grouping the tyrosine kinase pathway genes (FLT3, KIT, CSF1R, NRAS, KRAS, PTPN11, and JAK2) together, the association with mutations of NPM1 was significant (P = .049). A negative association of tyrosine kinase pathway gene mutations with each other was observed, but it was not statistically significant (ie, mutations of FLT3, KIT, JAK2, or PTPN11 were never seen in the same sample; Figure 2). The same pattern does not appear to hold true for RAS mutations, since NRAS and KRAS mutations were seen in association with FLT3 or KIT mutations.
Frequency of gene mutations in SCN AML/ALL/MDS
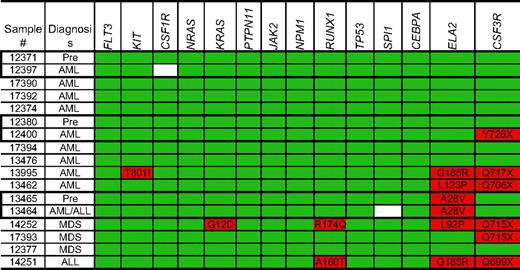
For the SCN samples, only tumor (bone marrow) samples were available, so we were unable to directly address whether the observed nucleotide changes were acquired. As expected, over 40% of patients (6 of 14) with SCN carried mutations (presumably germ line) in ELA2 (Figure 3). Consistent with a previous report,18 G185R ELA2 mutations are frequent in patients with SCN who have progressed to leukemia or AML. CSF3R mutations were observed in 6 of 14 patients (42.8%). In 1 case (no. 12400), the CSF3R mutation was clearly acquired, since it was not present in a preleukemic sample (no. 12380) from the same patient. In contrast to the de novo AML samples, no mutations of FLT3, NRAS, CEBPA, or NPM1 were detected. Nonsynonymous single-nucleotide changes of RUNX1 were detected in 2 SCN samples. Although the R174Q mutation is associated with AML,45 the significance of the A160T sequence change is less clear, since it has not previously been reported in AML or MDS. A single SCN-AML sample was found to have a homozygous T801I substitution in KIT; however, this substitution does not result in constitutive c-kit activation50 and has not previously been detected in AML or MDS51 ; its significance is therefore unclear.
Nucleotide changes in the SCN samples. Nonsynonymous nucleotide changes and gene deletions or insertions are highlighted in red; white boxes indicate missing sequence data. Known SNPs are excluded from this analysis. Pre- and post-leukemic samples from the same patient are outlined.
Nucleotide changes in the SCN samples. Nonsynonymous nucleotide changes and gene deletions or insertions are highlighted in red; white boxes indicate missing sequence data. Known SNPs are excluded from this analysis. Pre- and post-leukemic samples from the same patient are outlined.
To compare mutation frequencies with de novo AML, we limited our analysis to the 10 SCN samples with AML and eliminated those samples with inadequate sequence coverage (Table 3). Compared with the de novo AML samples, there was a nonsignificant trend toward a reduced frequency of mutations of FLT3 (27.6% vs 0%; P < .07) and NPM1 (23.9% vs 0%; P = .12) in the SCN-AML samples. Consistent with previous reports, activating mutations of the G-CSFR were specifically associated with SCN-AML (0% vs 30%; P < .01).
Discussion
In this study, we used high-throughput exonic resequencing to determine the mutation frequency of 14 AML-associated genes in patients with SCN-associated AML. To validate our resequencing methodologies, we sequenced the regions containing these common mutations in 188 de novo AML samples and found that our pipeline detected all of these mutations at the expected frequencies. The pattern of mutations in the SCN-MDS/leukemia samples was distinct, since no mutations in NPM1, CEBPA, or FLT3 were detected. As expected, we found that the CSF3R gene was frequently mutated in SCN-AML but not in de novo AML. These results therefore suggest that the genetic basis of SCN-AML is different from that of de novo AML. However, it is important to note that de novo AML samples analyzed in this study had certain favorable biologic features compared with the SCN samples, including the lack of antecedent myelodysplasia and relatively few cytogenetic abnormalities. These features make comparisons of the 2 groups difficult, but the new information regarding the SCN-AML patients is still informative regarding their pathophysiology.
Mutations of CSF3R are present in a substantial proportion of patients with SCN. In the largest published series, which included 121 patients with SCN, CSF3R mutations were detected in 41%.23 These mutations are acquired, typically heterozygous, and nearly always introduce a premature stop codon resulting in the truncation of the distal cytoplasmic portion of the G-CSFR.19-22 CSF3R mutations are strongly associated with the development of AML/MDS. Germeshausen et al23 reported that the incidence of CSF3R mutations was 78% (18/23) in individuals with SCN and monosomy 7, MDS, or AML compared with 34% (43/125) in patients without MDS or AML. In the present study, CSF3R mutations were detected in 43% (6/14) of SCN patients with malignant transformation. The lower incidence of CSF3R mutations in our study is likely due to differences in the methods used to detect CSF3R mutations. In the study by Germeshausen et al,23 sequencing was performed on PCR-amplified cDNA after reverse transcription of hematopoietic cell RNA; using this approach, they were able to detect CSF3R mutations in samples in which only 5% of the cells contained this mutation. In contrast, our method of direct amplification of tumor DNA to generate amplicons for resequencing has been shown to reliably detect mutations that are present in at least 33% of cells (this applies to all genes sequenced in this study).26 Consistent with previous reports,23,52,53 no CSF3R mutations were detected in the de novo AML samples, suggesting that CSF3R mutations are rare in de novo AML.
Accumulating data suggest that activating mutations of tyrosine kinase genes may play a key role in leukemogenesis. Indeed, we detected mutations of tyrosine kinase genes (FLT3, KIT, and JAK2) in 30% of de novo AML samples. There is evidence suggesting that the truncation mutations of CSF3R observed in SCN are likewise activating. Expression of mutant G-CSFR in myeloid cell lines results in enhanced proliferative and survival signals.19,54,55 Moreover, transgenic mice carrying truncating mutations of CSF3R display a hyperproliferative response to G-CSF.24,25 Since the G-CSFR and receptor tyrosine kinases share many signaling pathways, these observations suggest that the mutant G-CSFR present in many cases of SCN-AML may provide the “activated tyrosine kinase signal [pathway].” This hypothesis predicts that mutations of tyrosine kinase genes associated with de novo AML would not be required for disease progression. Consistent with this prediction, no activating mutations of the tyrosine kinase genes were observed in the SCN samples (0/14; P = .007 compared with de novo AML). The rarity of tyrosine kinase gene mutations in SCN is unlikely to be related to the age of the patients or the complexity of karyotypic abnormalities. The mutation frequency of FLT3 and KIT in childhood AML is 19% and 3%, respectively56,57 ; similar to that reported for adult AML. Of particular relevance to SCN, the frequency of FLT3 mutations in AML with karyotypic abnormalities of chromosome 7 is 5.8%.38 In addition, since therapy-related MDS/AML shares many features with SCN-AML (including a high incidence of chromosome 7 abnormalities and a history of antecedent MDS), it is noteworthy that the reported frequency of FLT3 mutations in therapy-related MDS/AML ranges from 4.3% to 7.8%.58,59 Finally, a recent study reported that activating KIT mutations were present in 19% of patients with AML arising in the setting of MDS (MDS-AML), another condition associated with a high frequency of chromosome 7 abnormalities.61 Collectively, these data suggest that the rarity of tyrosine kinase gene mutations is a unique finding in SCN-MDS/AML and support the hypothesis that CSF3R mutations provide the activated tyrosine kinase signal that is thought to be important for leukemogenesis.
In the present study, only a single NRAS or KRAS mutation was detected in the 14 SCN-AML/MDS samples. Similarly, a preliminary study reported a 7% frequency of RAS mutations (1/14 cases of SCN AML/MDS).61 In contrast, an earlier study reported a RAS mutation frequency of 38% (5/13 cases of SCN AML/MDS).10 Interestingly, most of the patients in this earlier study were older and had not been treated with G-CSF from infancy. Consequently, it is possible that long-term G-CSF therapy selects for CSF3R mutations, reducing the need for RAS mutations during disease progression. Consistent with this possibility, resequencing of 3 of the original 5 cases of SCN AML/MDS with RAS mutations revealed that none of these cases had mutations of CSF3R.61 The mutual exclusivity of activating RAS and CSF3R mutations suggests that these genes may act on a shared pathway; however, it should be noted thatthe sole SCN sample in our study with a KRAS mutation also had a CSF3R mutation. In any case, combining data from all of these studies, the frequency of RAS mutations in SCN MDS/leukemia is approximately 15%, similar to that reported for de novo AML.
The frequency of NPM1 mutations has been reported to be approximately 35% in adult de novo AML39 and 6.5% in pediatric de novo AML.63 In the present study, no NPM1 mutations were detected in the 14 SCN samples versus 24% in the de novo AML samples (P = .04). Of note, NPM1 mutations are more frequently detected in AML samples with a normal karyotype; the frequency of NPM1 mutations in de novo AML with an abnormal karyotype is only 6.4%.63 Since most cases of SCN-MDS/AML are associated with an abnormal karyotype, this may partially account for the reduced NPM1 mutation frequency in these samples. Nonetheless, the data suggest that mutations of the NPM1 are rare during leukemic progression in SCN patients.
There is accumulating evidence that the genetic changes that contribute to secondary leukemias, including therapy-related AML and AML arising in the setting of MDS (MDS-AML), are distinct from those that contribute to the pathogenesis of de novo AML. The present data suggest that the mutations that contribute to SCN-AML are more similar to those of the secondary AML syndromes. Similar to therapy-related AML and MDS-AML, mutations of NPM1 and CEBPA are rare in SCN-MDS/AML, whereas abnormalities of chromosomes 5 and 7 are more common than in de novo AML. The one outlier is CSF3R, where mutations appear to be uniquely associated with SCN. Given the poor prognosis of SCN-AML and other secondary leukemia syndromes, it will be important to identify the genes that contribute to transformation under these circumstances. These data will improve our understanding of the mechanisms of leukemic transformation and may provide new targets for drug design.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant CA101937, by US Public Health Service grants CA31946 and CA101140, and by the Leukemia Clinical Research Foundation (CALGB).
National Institutes of Health
Authorship
Contribution: G.K., Y.K., Y.Z., T.M., M.D.M., R.E.R., and D.K. generated and/or analyzed the data. W.S. and J.B. performed the statistical analyses. D.C.D., A.A.B., L.A.B., K.W., C.Z., J.D., C.B.-C., J.W.V., M.A.C., and C.D.B. provided crucial reagents. D.C.L., R.N., J.F.D., M.H.T., T.A.G., P.W., M.W., E.R.M., R.K.W., and T.J.L. designed the experiment. D.C.L. and T.J.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel C. Link, Division of Oncology, Department of Medicine, 660 S Euclid Avenue, Campus Box 8007, St Louis, MO 63110; e-mail: dlink@im.wustl.edu.