Abstract
Janus kinase 2 (Jak2) transduces signals from hematopoietic cytokines, and a gain-of-function mutation (Jak2617V>F) is associated with myeloproliferative diseases, particularly polycythemia vera. In this study, we examined the role of jak2a in zebrafish embryos in knock-down and overexpression studies using morpholinos (MOs) targeting the 5′ untranslated region (UTR) (jak2aUTR-MO) and splice-site junction (jak2aSS-MO) of jak2a, a Jak inhibitor AG490 and a constitutive-active form of jak2a (jak2aca). At 18 and 24 hours after fertilization (hpf), jak2a is expressed predominantly in the intermediate cell mass (ICM; site of primitive hematopoiesis) of wild-type and chordin morphant embryos (characterized by expansion of ICM). Both jak2a MOs and AG490 reduced gata1+ (erythroid) cells in Tg(gata1:GFP) embryos, signal transducer and activation of transcription 5 (stat5) phosphorylation, and gene expression associated with early progenitors (scl and lmo2) and erythroid (gata1, αhe1 and βhe1) and myeloid (spi1 [early] and mpo [late]) lineages. The chordin morphant is associated with increased stat5 phosphorylation, and both jak2a MOs and treatment with AG490 significantly ameliorated ICM expansion and hematopoietic gene up-regulation in these embryos. Injection of plasmid encoding jak2aca significantly increased erythropoiesis and expression of gata1, αhe1 and βhe1, spi1, mpo, and l-plastin. In conclusion, zebrafish jak2a is involved in primitive hematopoiesis under normal and deregulated conditions.
Introduction
The Janus kinase (Jak)/signal transducer and activation of transcription (stat) cascade is a ubiquitous intracellular pathway that transduces signals from extracellular ligands. In mammals, the Jak family of protein tyrosine kinases consists of 4 members (Jak1-Jak3, Tyk2), of which Jak2 transduces signals of hematopoietic cytokines to enhance erythroid cell proliferation.1 The recent identification of a gain-of-function mutation of Jak2 in human polycythemia vera (PV) has revised the diagnosis and perhaps future treatment of this disorder.2 In PV, substitution of valine by phenylalanine at amino acid 617 in the autoregulatory JH2 domain (V617F) results in constitutive activation of Jak2 (Jak2V617F), conferring proliferative and survival advantage to erythroid progenitors.3 As a result, patients suffer from erythrocytosis and hence thrombosis and bleeding. A similar gain-of-function mutation in the Jak JH2 domain has also been described in the single Drosophila Jak, which is encoded by the hopscotch (hop) locus. A glutamic acid to lysine substitution at residue 695 (E695K) in the JH2 domain of the Hop protein resulted in hyperphosphorylation of the Drosophila Stat (D-Stat) and overproduction of primitive blood cells.4
The zebrafish has emerged as a model organism of the study of hematopoiesis during embryonic development and neoplastic transformation.5 In this organism, primitive and definitive hematopoiesis arise successively in the intermediate cell mass (ICM) and the ventral wall of the dorsal aorta in the developing embryos.6 In zebrafish, the gene encoding for Jak2 has undergone duplication and subsequent specialization: jak2a is expressed predominantly in the ICM, and jak2b is expressed predominantly in the developing eyes and pronephric ducts.7 However, functional studies of jak2a in zebrafish are lacking. We have previously demonstrated using microarray analysis that jak2a expression was significantly up-regulated in the ICM of the zebrafish chordin morphant that is characterized by expansion of primitive hematopoiesis.8 This suggested that jak2a may be involved in the hematopoietic expansion of this morphant.
In this study, we performed both qualitative and quantitative analyses to specifically examine the role of jak2a in zebrafish hematopoiesis using morpholino (MO) knock-down. Furthermore, we investigated to determine if jak2a activation is involved in the hematopoietic expansion of the chordin morphant and evaluated the effects of constitutive activation of jak2a on hematopoiesis.
Materials and methods
Zebrafish and modulation of jak2a
Danio rerio (wild-type) were obtained from a local aquarium and were maintained and raised under standard conditions at 28°C. Wild-type embryos were obtained from natural spawning and were staged according to Kimmel et al.9 Transgenic zebrafish lines Tg(gata1:GFP)10 and Tg(fli1:GFP)11 were obtained from Tsinghua University (gift from Dr Anming Meng) and Zebrafish Information Network, Eugene, Oregon). Antisense MOs (Gene-Tools, Philomath, OR) were designed to target the 5′ untranslated region (UTR) of the respective genes. MOs were also designed at the intron-exon junction (splicing site [SS]) to induce defective splicing (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Where appropriate, a random sequence MO was used as a control,12,13 which has no effect on the morphology and erythropoiesis of the developing embryos (unpublished data, A.C.H.M., January 2007). Solutions of these were prepared and injected into embryos at the 1- to 4-cell stage, which were maintained at 28°C until analyzed. In some experiments, jak2a activity was suppressed by incubating 1-cell–stage embryos with a soluble Jak2 inhibitor, AG490 (Calbiochem, San Diego, CA)14 until analysis. Protocols for whole-mount in situ hybridization and O-dianisidine staining have been described previously.8,12,13
A wild-type zebrafish jak2a clone in the mammalian expression vector pCS2+ has been described previously.7 Mutagenesis to generate a hyperactive E629K mutant equivalent to the Drosphila hop T42 mutation4 was performed using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), following the manufacturer's recommendations. Sequence analysis confirmed that the change had been correctly introduced in the absence of extraneous mutations. For rescue experiments, RNA encoding wild-type jak2a was generated by in vitro transcription and injected as described. Embryos were examined with a stereomicroscope (Nikon SMZ800; Nikon, Kawasaki, Japan) with a P-plan 1× objective. Images were captured with Nikon Coolpix E4500 (Nikon, Hong Kong, China) and processed with Adobe Photoshop Version 7.0 (Adobe, San Jose, CA). Fluorescence images were examined with Olympus IX70 (Olympus, Tokyo, Japan) with 10×/10.3 NA objective (P.T.O.). Images were captured with Olympus DP71 and acquired with Olympus DP-BSW basic software and processed with Adobe Photoshop version 7.0.
Western blotting
Total embryo protein extract was prepared by homogenizing 50 embryos at 18 hours after fertilization (hpf) in 50 μL protein extraction buffer (Tris.HCl [63 mM; pH 6.8], glycerol [10% vol/vol], β-mercaptoenthanol [5% vol/vol], and SDS [3.5% wt/vol]) and boiling for 1 minute. Protein extracts (100 μg) were fractionated on a 10% (wt/vol) SDS polyacrylamide gel and electrotransferred to nitrocellulose membrane (Protran; PerkinElmer Life Science, Boston, MA). The membrane was then blocked in blocking buffer (5% nonfat milk in Tris-buffered saline [TBS]) for 1 hour at room temperature, followed by overnight incubation at 4°C with primary antibody, either 1:250 rabbit anti-Stat5 antibody (Cell Signaling Technology, Beverly, MA) or 1:250 rabbit anti–phospho-Stat5 antibody (Zymed Laboratories, South San Francisco, CA). Afterward, the membrane was washed in TBS plus 0.05% (vol/vol) Tween-20 (TBST) followed by incubation with horseradish peroxidase (HRP)–linked electrochemiluminescence (ECL) donkey anti–rabbit IgG antibody (1:1000) at 4°C (Amersham Biosciences, Buckinghamshire, United Kingdom). The membrane was then washed with TBST followed by TBS before detection with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and Hyperfilm ECL (Amersham Biosciences, Little Chalfont, United Kingdom). To reprobe the same membrane with another primary antibody, the membrane was incubated in stripping buffer (Tris.HCl [62.4 mM at pH 6.7], β-mercaptoenthanol [100 mM], SDS [2% wt/vol]) at 50°C for 15 minutes, followed by repeated washing in TBST.
Flow cytometry
Tg(gata1:GFP) embryos at 18 hpf were dechorionated and digested with 0.05% trypsin/EDTA solution (Invitrogen, Carlsbad, CA) for 15 minutes at 28°C, then completely dissociated to single-cell suspension by pipetting. Trypsin digestion was terminated by CaCl2 (2 mM), and the whole suspension was filtered through a 40-μm cell strainer (BD Falcon, BD Biosciences Discovery Labware, Bedford, MA). Cells were washed and harvested in phosphate-buffered saline (PBS) with 2% (vol/vol) FBS, and the percentage of GFP+ cells were enumerated by flow cytometry.
Real-time Q-RT-PCR assay
Quantitative reverse transcriptase–polymerase chain reaction (Q-RT-PCR) was performed to examine the relative expression of genes specific for early progenitor (scl, lmo-2, gata2), erythroid (gata1, alpha and beta embryonic Hb 1, and αhe1 and βhe1), early myeloid (spi1), heterophilic granulocyte (myeloperoxidase, mpo), and macrophage (l-plastin) lineages using the ABI Prism 7700 Sequence Detector (PE Biosystems, Foster City, CA). Embryos at 12 and 18 hpf were dechorionated, and RNA was extracted using Trizol reagent as per the manufacturer's protocol (Invitrogen, Carlsbad, CA). A total of 30 embryos were included in each experiment. RNA was reverse-transcribed and PCR was performed using the SYBR Green Method. Primer sequences used for Q-RT-PCR are shown in Table S1.
To ensure that the amplification efficiency for all marker genes in the Q-RT-PCR was equal, RNA was extracted before the project from a group of 40 embryos at 24 hpf followed by reverse transcription. Serial dilutions of samples ranging from 0.0025 to 125 ng input cDNA were frozen at −20°C in small aliquots (standards). Once thawed, each aliquot was used only once. Relative quantification of each gene expression was performed by the comparative CT methods.
Statistical analysis
Data are expressed as means (± SEM; calculated by dividing the SD by the square root of the number of replicate experiments). Comparisons between numerical data were evaluated by paired Student t tests. A P value less than .05 was considered statistically significant.
This study was approved by the Committee of the Use of Laboratory and Research Animals (CULATR) at the University of Hong Kong.
Results
jak2a is expressed in the ICM of wild-type embryos and chordin morphants
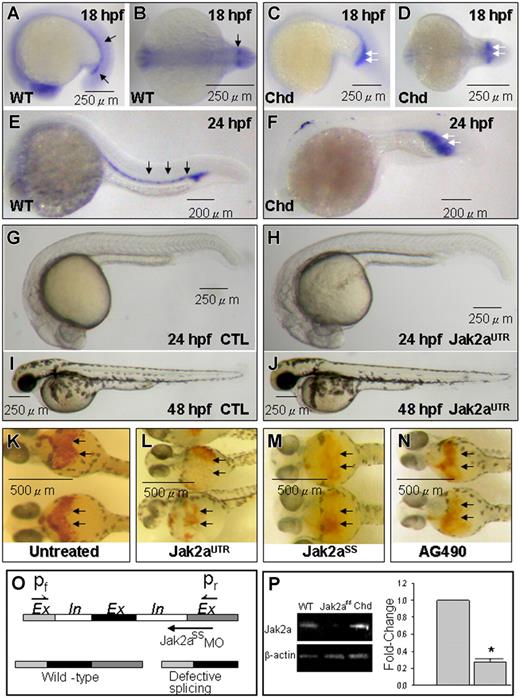
We first examined the expression of jak2a during zebrafish embryonic development using whole-mount in situ hybridization. In agreement with another study,7 jak2a was expressed predominantly in the ICM of zebrafish embryos at 18 and 24 hpf (Figure 1A,B,E). Expression of jak2a was significantly up-regulated within the expanded ICM of the chordin morphant at both time points (Figure 1C,D,F), confirming our previous observations based on microarray analysis.8
jak2a expression and effects of jak2a knock-down. (A,B,E) Whole-mount ISH for jak2a mRNA showing specific expression in the ICM (black arrows) of 18 hpf (A,B) and 24 hpf embryos (F). (C,D,F) Increased jak2a expression in the expanded ICM (white arrows) of the chordin morphant. (G-J) Knock-down of jak2a by jak2aUTR-MO had no effects on embryonic development at 24 hpf (G,H) and 48 hpf (I,J). (K-N) O-dianisidine staining (black arrows) in 48-hpf embryos showing reduced hematopoiesis by MOs against jak2a (L,M) and a soluble jak2a inhibitor AG490 (N). (O,P) MOs against an intron-exon junction in jak2a resulting in reduced levels of jak2a mRNA shown by Q-RT-PCR. Chd indicates chordin morphant; jak2aUTR and jak2aSS, embryos injected with jak2aUTR and jak2aSS MOs; CTL, embryos injected with random sequence MOs (“Materials and methods, Zebrafish and modulation of jak2a”). (A-N) Representative pictures from at least 3 separate experiments containing 10 (A-J) and 20 (K-N) embryos per experiment. The columns in panel P represent mean values of 3 separate experiments using around 20 embryos per experiment. Comparison was made between uninjected embryos and those injected with jak2aUTR MOs. (*P < .05). Error bars are SEM.
jak2a expression and effects of jak2a knock-down. (A,B,E) Whole-mount ISH for jak2a mRNA showing specific expression in the ICM (black arrows) of 18 hpf (A,B) and 24 hpf embryos (F). (C,D,F) Increased jak2a expression in the expanded ICM (white arrows) of the chordin morphant. (G-J) Knock-down of jak2a by jak2aUTR-MO had no effects on embryonic development at 24 hpf (G,H) and 48 hpf (I,J). (K-N) O-dianisidine staining (black arrows) in 48-hpf embryos showing reduced hematopoiesis by MOs against jak2a (L,M) and a soluble jak2a inhibitor AG490 (N). (O,P) MOs against an intron-exon junction in jak2a resulting in reduced levels of jak2a mRNA shown by Q-RT-PCR. Chd indicates chordin morphant; jak2aUTR and jak2aSS, embryos injected with jak2aUTR and jak2aSS MOs; CTL, embryos injected with random sequence MOs (“Materials and methods, Zebrafish and modulation of jak2a”). (A-N) Representative pictures from at least 3 separate experiments containing 10 (A-J) and 20 (K-N) embryos per experiment. The columns in panel P represent mean values of 3 separate experiments using around 20 embryos per experiment. Comparison was made between uninjected embryos and those injected with jak2aUTR MOs. (*P < .05). Error bars are SEM.
Knock-down of jak2a affects hematopoiesis
To investigate the role of jak2a in primitive hematopoiesis, we injected the jak2aUTR-MOs into zebrafish embryos at the 1- to 4-cell stage. Initial experiments showed that most embryos tolerated 7.5 ng and developed normally at 24 and 48 hpf (Figure 1G-J). However, at higher doses, this MO caused excessive toxicity and lethality. Therefore, in all subsequent experiments, the embryos were injected with a dose of 7.5 ng (referred to hereafter as jak2aUTR embryos). Erythropoiesis was reduced in these embryos at 48 hpf as shown by O-dianisidine staining (Figure 1K,L). To confirm the specificity of this result, a jak2a splice-site MO targeting an exon-intron junction (jak2aSS-MO; 2 ng) was injected. These jak2aSS embryos showed a similarly reduced erythropoiesis at 48 hpf (Figure 1M). Moreover, the efficacy of the jak2aSS-MOs could be demonstrated by RT-PCR showing significantly reduced levels of jak2a transcript (Figure 1O-P). To further correlate tyrosine kinase activity with primitive hematopoiesis, we incubated freshly spawned embryos with AG490 (50 μM), a known soluble inhibitor of Jak2 (jak2aAG embryos). At 48 hpf, O-dianisidine staining was also markedly reduced in these embryos, corroborating the proposition that jak2a regulates primitive hematopoiesis during zebrafish embryonic development (Figure 1N).
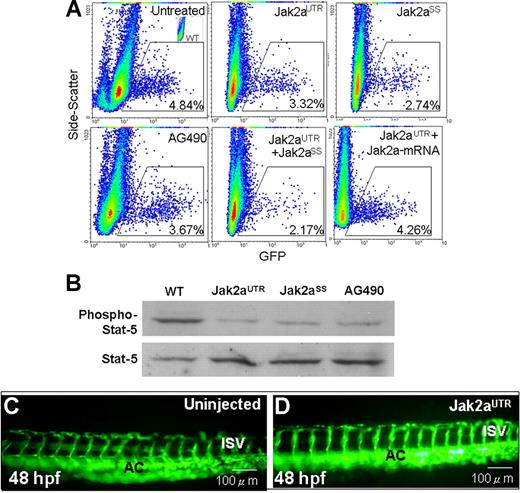
We performed quantitative analysis on the effects of jak2a knock-down on the gata1+ population using transgenic Tg(gata1:GFP) embryos at 18 hpf, before the onset of functional circulation. In these embryos, GFP+ cells represent cells committed to the erythroid lineage. In uninjected embryos, the GFP+ population constitutes 4.47% (± 0.18%) of the dissociated embryos. A significant reduction in the GFP+ population was observed in jak2aUTR (3.56% ± 0.58%; P=.017), jak2aSS (2.68% ± 0.40%; P=.01), and jak2aAG (3.31% ± 0.18%; P=.003) embryos (Figure 2A). Furthermore, coinjection of embryos with both jak2aUTR-MOs (7.5 ng) and jak2aSS-MOs (2 ng) resulted in a further reduction of erythropoiesis (2.15% ± 0.10%; P < .001) compared with either of these MOs alone, demonstrating synergism and hence specificity of these MOs on jak2a gene function. Specificity of jak2aUTR-MO was further demonstrated by a reversal of its inhibitory effect on erythropoiesis after rescue by wild-type jak2a mRNA (75 pg) coinjection (4.25% ± 0.03%; P=.86). Collectively, these observations confirmed the findings with O-dianisidine staining that jak2a knock-down resulted in a decrease in erythropoiesis.
Effects of jak2a knock-down on erythropoiesis, Stat signaling, and angiogenesis. (A) Flow cytometric analyses (x-axis, GFP; y-axis, side scatter) showing the effects of jak2a knock-down using jak2aUTR and jak2aSS morpholinos as well as a soluble jak2a inhibitor AG490 on GFP+ populations in Tg(gata1:GFP) embryos. Synergistic effect was seen when the embryos were coinjected with both MOs. Effects of jak2aUTR-MOs could be rescued by wild-type jak2a mRNA. Each record was representative of 3 experiments using 20 embryos per experiment. (B) Western blotting showing reduced phospho-stat5 upon knock-down of jak2a functions. About 30 embryos were used in each experiment, and the result was representative of 3 experiments. (C,D) Fluorescent microscopy using Tg(fli1:GFP) showing knock-down of jak2a by jak2aUTR-MOs had no effects on angiogenesis. AC indicates axial circulation; ISV, intersegmental vessels. Results were representative of at least 4 separate experiments using more than 5 embryos each time.
Effects of jak2a knock-down on erythropoiesis, Stat signaling, and angiogenesis. (A) Flow cytometric analyses (x-axis, GFP; y-axis, side scatter) showing the effects of jak2a knock-down using jak2aUTR and jak2aSS morpholinos as well as a soluble jak2a inhibitor AG490 on GFP+ populations in Tg(gata1:GFP) embryos. Synergistic effect was seen when the embryos were coinjected with both MOs. Effects of jak2aUTR-MOs could be rescued by wild-type jak2a mRNA. Each record was representative of 3 experiments using 20 embryos per experiment. (B) Western blotting showing reduced phospho-stat5 upon knock-down of jak2a functions. About 30 embryos were used in each experiment, and the result was representative of 3 experiments. (C,D) Fluorescent microscopy using Tg(fli1:GFP) showing knock-down of jak2a by jak2aUTR-MOs had no effects on angiogenesis. AC indicates axial circulation; ISV, intersegmental vessels. Results were representative of at least 4 separate experiments using more than 5 embryos each time.
jak2a knock-down induces changes in stat5 phosphorylation
To confirm that the reduced erythropoiesis in zebrafish embryos after down-modulation of jak2a was related to reduction in Jak/Stat signaling, we examined the phosphorylation status of endogenous stat5 protein in these embryos. Although the amount of stat5 protein was unchanged in jak2aUTR, jak2aSS, and jak2aAG embryos, stat5 phosphorylation was significantly reduced compared with that of uninjected embryos (Figure 2B). The results supported the notion that stat5 mediates the downstream effects of jak2a.
jak2a knock-down has no effect on angiogenesis
The effects of jak2a knock-down on hematopoiesis led us to examine its effects on angiogenesis as both processes arise from a common precursor, known as the hemangioblasts.15 Moreover, in the zebrafish cloche mutant, whose blood and vascular formation are defective, jak2a expression was significantly down-regulated.7 We injected jak2aUTR-MOs into transgenic Tg(fli1:GFP) embryos at the 1- to 4-cell stage. At 48 hpf, both vasculogenesis and angiogenesis was not affected as shown by the intact axial and intersegmental vessels (Figure 2C,D). A patent circulation in these vessels was further confirmed by microangiography (data not shown).
Gene-expression analysis
In order to understand the regulatory role of jak2a in the hematopoietic hierarchy, we examined the expression of a panel of hematopoietic genes in the jak2aUTR embryos by Q-RT-PCR (Table 1). At 12 hpf, expression of scl, lmo2, gata1, αhe1, and βhe1 were significantly reduced in jak2aUTR embryos, while expression of mpo was unaffected. At 18 hpf, expression of genes encoding for all these hematopoietic genes were reduced. At both time points, spi1 appeared to be down-regulated, although the difference from uninjected embryos did not reach statistical significance. Expression of fli1 was not affected in the jak2aUTR embryos. Intriguingly, expression of l-plastin was significantly up-regulated in jak2aUTR embryos at both time points.
Differential gene expression in wild-type, jak2aUTR, chordin morphant, and chordin plus jak2aUTR embryos
| Gene . | 12 hpf . | 18 hpf . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| jak2aUTR . | P* . | chordin . | chordin plus jak2aUTR . | P† . | jak2aUTR . | P* . | chordin . | chordin plus jak2aUTR . | P† . | |
| scl0 | .47 ± 0.06 | .027 | 4.18 ± 0.33 | 0.73 ± 0.11 | .003 | 0.17 ± 0.02 | .001 | 9.56 ± 1.51 | 0.19 ± 0.02 | .000 |
| lmo-20 | .34 ± 0.02 | .002 | 1.87 ± 0.33 | 0.44 ± 0.02 | .015 | 0.66 ± 0.05 | .011 | 3.55 ± 0.30 | 0.82 ± 0.05 | .001 |
| gata10 | .55 ± 0.05 | .026 | 7.16 ± 0.52 | 2.15 ± 0.41 | .016 | 0.57 ± 0.07 | .018 | 24.89 ± 2.89 | 12.64 ± 0.77 | .002 |
| αhe10 | .59 ± 0.06 | .017 | 4.00 ± 0.33 | 0.69 ± 0.10 | .013 | 0.48 ± 0.09 | .036 | 9.24 ± 0.85 | 0.52 ± 0.13 | .006 |
| βhe10 | .58 ± 0.08 | .031 | 1.47 ± 0.44 | 0.95 ± 0.11 | .059 | 0.46 ± 0.09 | .037 | 2.37 ± 0.04 | 0.53 ± 0.08 | .005 |
| spi10 | .58 ± 0.14 | .071 | 8.79 ± 2.20 | 0.96 ± 0.26 | .016 | 0.51 ± 0.15 | .107 | 12.48 ± 2.61 | 0.61 ± 0.17 | .017 |
| mpo | 2.17 ± 0.88 | .204 | 9.63 ± 3.32 | 1.62 ± 0.19 | .025 | 0.05 ± 0.02 | .004 | 31.32 ± 6.09 | 0.03 ± 0.02 | .000 |
| fli1 | 1.05 ± 0.01 | .320 | 1.06 ± 0.02 | 1.02 ± 0.03 | .317 | 1.09 ± 0.03 | .184 | 1.11 ± 0.05 | 1.01 ± 0.02 | .067 |
| l-plastin | 2.54 ± 0.22 | .009 | 5.60 ± 0.14 | 5.71 ± 0.05 | .511 | 3.23 ± 0.22 | .002 | 9.10 ± 0.83 | 9.87 ± 1.34 | .195 |
| Gene . | 12 hpf . | 18 hpf . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| jak2aUTR . | P* . | chordin . | chordin plus jak2aUTR . | P† . | jak2aUTR . | P* . | chordin . | chordin plus jak2aUTR . | P† . | |
| scl0 | .47 ± 0.06 | .027 | 4.18 ± 0.33 | 0.73 ± 0.11 | .003 | 0.17 ± 0.02 | .001 | 9.56 ± 1.51 | 0.19 ± 0.02 | .000 |
| lmo-20 | .34 ± 0.02 | .002 | 1.87 ± 0.33 | 0.44 ± 0.02 | .015 | 0.66 ± 0.05 | .011 | 3.55 ± 0.30 | 0.82 ± 0.05 | .001 |
| gata10 | .55 ± 0.05 | .026 | 7.16 ± 0.52 | 2.15 ± 0.41 | .016 | 0.57 ± 0.07 | .018 | 24.89 ± 2.89 | 12.64 ± 0.77 | .002 |
| αhe10 | .59 ± 0.06 | .017 | 4.00 ± 0.33 | 0.69 ± 0.10 | .013 | 0.48 ± 0.09 | .036 | 9.24 ± 0.85 | 0.52 ± 0.13 | .006 |
| βhe10 | .58 ± 0.08 | .031 | 1.47 ± 0.44 | 0.95 ± 0.11 | .059 | 0.46 ± 0.09 | .037 | 2.37 ± 0.04 | 0.53 ± 0.08 | .005 |
| spi10 | .58 ± 0.14 | .071 | 8.79 ± 2.20 | 0.96 ± 0.26 | .016 | 0.51 ± 0.15 | .107 | 12.48 ± 2.61 | 0.61 ± 0.17 | .017 |
| mpo | 2.17 ± 0.88 | .204 | 9.63 ± 3.32 | 1.62 ± 0.19 | .025 | 0.05 ± 0.02 | .004 | 31.32 ± 6.09 | 0.03 ± 0.02 | .000 |
| fli1 | 1.05 ± 0.01 | .320 | 1.06 ± 0.02 | 1.02 ± 0.03 | .317 | 1.09 ± 0.03 | .184 | 1.11 ± 0.05 | 1.01 ± 0.02 | .067 |
| l-plastin | 2.54 ± 0.22 | .009 | 5.60 ± 0.14 | 5.71 ± 0.05 | .511 | 3.23 ± 0.22 | .002 | 9.10 ± 0.83 | 9.87 ± 1.34 | .195 |
Data are means plus and minus the SEM of 3 to 4 separate experiments. WT were 1.00 in all cases.
Comparison between wild-type (WT) and jak2aUTR embryos using the Student paired t test.
Comparison between chondrin and chondrin plus jak2aUTR embryos using the Student paired t test.
Hematopoietic expansion in the chordin morphant is mediated by jak2a
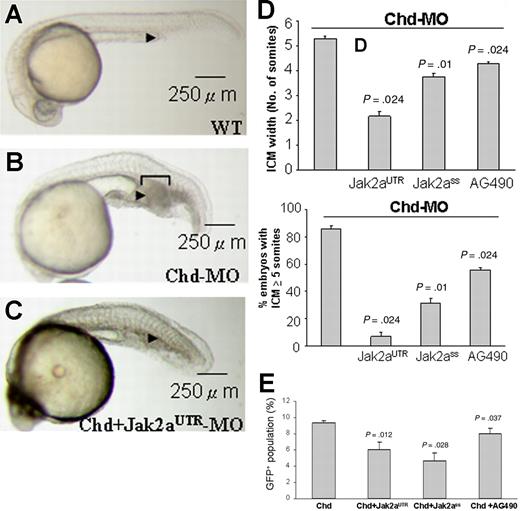
The up-regulation of jak2a expression in the chordin morphant in which there is expansion of primitive hematopoiesis led us to investigate whether increased jak2a activity may mediate the hematopoietic expansion in these embryos. Wild-type embryos injected with chordin MO (0.75 ng) induced expansion of ICM in nearly 90% of embryos (Figure 3A-B). However, coinjection of chordin and jak2aUTR-MOs resulted in significant reduction in ICM expansion both in the average size of the expansion as well as the number of embryos showing significant expansion (defined arbitrarily by ICM of 5 somites or more; Figure 3C). Similar changes were recapitulated by coinjection of chordin and jak2aSS-MOs or by incubating chordin MO–injected embryos with AG490 (Figure 3D). Using flow cytometry on Tg(gata1:GFP) embryos at 18 hpf, jak2aUTR-MOs and jak2aSS-MOs as well as AG490 were able to reduce the increase in the GFP+ population in the chordin morphant (Figure 3E). Q-RT-PCR showed that the expression of scl, lmo2, gata1, αhe1 and βhe1, spi1, mpo, and l-plastin were up-regulated in the chordin morphant at 12 and 18 hpf (Table 1). With the exception of l-plastin, the increases in gene expression were ameliorated by coinjection with Jak2UTR-MOs.
jak2a mediates the expanded ICM phenotypes in the chordin morphants. (A) Wild-type 24-hpf embryos. (B) chordin morphants at 24 hpf generated by injection of chordin MOs at the 1- to 4-cell stage. Bracket shows the extent of expanded ICM (arrowhead). (C) 24-hpf embryos coinjected with chordin and jak2aUTR-MOs showing reduced expansion of ICM. Each image is a representative picture of 3 to 4 experiments using more than 20 embryos at each time. (D) Knock-down of jak2a by both UTR and splice-site MOs and AG490 reduced the width of ICM in 24-hpf chordin morphant embryos, expressed as the width of adjacent spanning somites (left panel) and percentage of embryos with an ICM of 5 somites or more (right panel). Each column represents mean value (± SEM) of 3 to 4 experiments using more than 20 embryos at each time. (E) Average results of flow cytometry showing means (± SEM) of 3 to 4 experiments. P values at the top of each column represent the results of statistical evaluation based on the paired Student t test when compared with chordin morphant.
jak2a mediates the expanded ICM phenotypes in the chordin morphants. (A) Wild-type 24-hpf embryos. (B) chordin morphants at 24 hpf generated by injection of chordin MOs at the 1- to 4-cell stage. Bracket shows the extent of expanded ICM (arrowhead). (C) 24-hpf embryos coinjected with chordin and jak2aUTR-MOs showing reduced expansion of ICM. Each image is a representative picture of 3 to 4 experiments using more than 20 embryos at each time. (D) Knock-down of jak2a by both UTR and splice-site MOs and AG490 reduced the width of ICM in 24-hpf chordin morphant embryos, expressed as the width of adjacent spanning somites (left panel) and percentage of embryos with an ICM of 5 somites or more (right panel). Each column represents mean value (± SEM) of 3 to 4 experiments using more than 20 embryos at each time. (E) Average results of flow cytometry showing means (± SEM) of 3 to 4 experiments. P values at the top of each column represent the results of statistical evaluation based on the paired Student t test when compared with chordin morphant.
Constitutive activation of jak2a stimulates hematopoiesis
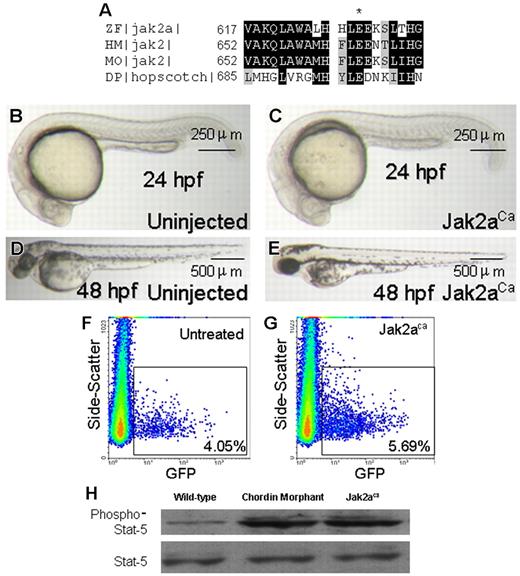
The results from the chordin morphant suggested that activation of jak2a in zebrafish embryos may increase hematopoiesis. To test this hypothesis, we injected the plasmid containing the constitutive-active form of jak2a (jak2aca, 150 pg; Figure 4A) into 1-cell–stage wild-type or Tg(gata1:GFP) embryos and examined its effects on hematopoiesis, stat5 phosphorylation, and gene expression. The jak2aca embryos had normal morphology (Figure 4B-E). When jak2aca was injected into Tg(gata1:GFP), there was a significant increase in gata1+ cells (uninjected embryos, 4.26% ± 0.06% vs jak2aca embryos, 5.26% ± 0.14%; P=.019, n=4 separate paired experiments including a total of 120 embryos; Figure 4F,G). Furthermore, stat5 phosphorylation was significantly increased in the jak2aca embryos. Similar increase was observed in the chordin morphant embryos (Figure 4H). In addition, expression of gata1, αhe1, βhe1, spi1, mpo, and l-plastin was significantly increased. On the other hand, those encoding for scl, lmo2, and fli1 did not show any changes in the jak2aca embryos (Table 2).
Constitutive activation of jak2a. (A) Amino acid sequence alignment of zebrafish (ZF) jak2a, human (HM) and mouse (MO) jak2, and Drosophila (DP) hopscotch at the JH2 domain. The conserved glutamic residue was marked with an asterisk. (B-E) Injection of jak2aca had no effect on gross morphology at 24 hpf (B,C) and 48 hpf (D,E). Each image is representative of at least 3 experiments using more than 10 embryos each time. (F-H) Quantitative analyses showed that jak2aca resulted in increased erythropoiesis (F,G) and stat5 phosphorylation (H). The latter was also increased in chordin morphant embryos. Each graph is representative of 3 separate experiments using 20 (F,G) and 30 (H) embryos each time.
Constitutive activation of jak2a. (A) Amino acid sequence alignment of zebrafish (ZF) jak2a, human (HM) and mouse (MO) jak2, and Drosophila (DP) hopscotch at the JH2 domain. The conserved glutamic residue was marked with an asterisk. (B-E) Injection of jak2aca had no effect on gross morphology at 24 hpf (B,C) and 48 hpf (D,E). Each image is representative of at least 3 experiments using more than 10 embryos each time. (F-H) Quantitative analyses showed that jak2aca resulted in increased erythropoiesis (F,G) and stat5 phosphorylation (H). The latter was also increased in chordin morphant embryos. Each graph is representative of 3 separate experiments using 20 (F,G) and 30 (H) embryos each time.
Differential gene expression in wild-type and jak2aca embryos at 18 hpf
| Gene . | jak2aCa . | P . |
|---|---|---|
| scl | 1.06 ± 0.05 | .386 |
| lmo-2 | 1.13 ± 0.05 | .248 |
| gata1 | 1.55 ± 0.06 | .001 |
| αhe1 | 1.74 ± 0.06 | .001 |
| βhe1 | 1.88 ± 0.06 | < .001 |
| spi1 | 2.13 ± 0.13 | < .001 |
| mpo | 1.30 ± 0.03 | .002 |
| fli1 | 1.05 ± 0.03 | .116 |
| l-plastin | 1.16 ± 0.02 | .006 |
| Gene . | jak2aCa . | P . |
|---|---|---|
| scl | 1.06 ± 0.05 | .386 |
| lmo-2 | 1.13 ± 0.05 | .248 |
| gata1 | 1.55 ± 0.06 | .001 |
| αhe1 | 1.74 ± 0.06 | .001 |
| βhe1 | 1.88 ± 0.06 | < .001 |
| spi1 | 2.13 ± 0.13 | < .001 |
| mpo | 1.30 ± 0.03 | .002 |
| fli1 | 1.05 ± 0.03 | .116 |
| l-plastin | 1.16 ± 0.02 | .006 |
Data are means (± SEM) of 4 separate experiments. WT were 1.00 in all cases. Comparison between wild-type (WT) and embryos injected with jak2aca were evaluated by the Student paired t test.
Discussion
In this study, we examined the role of jak2a during embryonic hematopoiesis in zebrafish. Injection of jak2aUTR-MOs significantly reduced primitive hematopoiesis, as shown qualitatively by O-dianisidine staining and quantitatively by flow cytometry of dissociated Tg(gata1:GFP) embryos and by real-time quantitative PCR. Enumeration of gata1+ cells at 18 hpf is specific for erythropoiesis, as thrombopoiesis, which is also under gata1 regulation, occurs at a later time point during embryonic development.16 Specificity of the MO was shown by the synergism between this and the jak2aSS-MO, which induced defective splicing of jak2a pre-mRNA, and by use of a pharmacologic inhibitor to reproduce the observed phenotypes. Moreover, the hematopoietic defects of the jak2aUTR embryos could be rescued by wild-type jak2a mRNA. The 2 MOs were different in their efficacies of perturbing hematopoiesis under basal and stimulated conditions. Whereas the efficacy of jak2aSS-MO could be tested by RT-PCR, that of jak2aUTR-MO was not testable due to the lack of specific antibody against zebrafish jak2a. Notwithstanding this limitation, the overall results were consistent with earlier observations showing reduced jak2a expression in the cloche mutant and corroborated with the proposition that jak2a mediates primitive hematopoiesis in zebrafish.7,17 In mice, the function of Jak2 in embryonic hematopoiesis could not be examined mechanistically because homozygous Jak2null mice are embryonic lethal at days 12.0 to 13.0 after coitus.18,19 Therefore, the zebrafish system has provided us with a unique model for the examination of this gene with hitherto uncertain function during embryonic development, and we have made a number of observations that may shed light to our understanding of jak2a during normal and deregulated hematopoiesis.
First, we demonstrated that jak2a plays a nonredundant role in the initiation of primitive hematopoiesis. In particular, genes associated with early hematopoietic specification (scl and lmo2) as well as erythroid (gata1, αhe1, and βhe1) and myeloid (spi1 [early] and mpo [late]) differentiation were down-regulated in the jak2aUTR embryos as early as 12 hpf. Therefore, jak2a might be required at the level of early hematopoietic progenitor/stem cells before they differentiate into distinct erythroid and myeloid lineages. We have recently shown that zebrafish stat5.1 is crucial for multilineage hematopoietic development (R. S. Lewis, C. Liongue, and A.C.W., manuscript submitted). Given the tight correlation between jak2a activation and stat5 phosphorylation observed in this study, it is likely that the jak2a-stat5.1 signaling module is critically involved in the cell-fate decision of early hematopoietic progenitor/stem cells. Intriguingly, l-plastin, which is associated with primitive macrophage development,20 was significantly up-regulated in jak2aUTR embryos at both 12 and 18 hpf. This lineage appears to be derived from an anterior hematopoietic precursor population distinct from the posterior ICM population.21,22 Whether the paradoxical up-regulation of l-plastin represented skewing of hematopoietic progenitor cell-fate from erythromyeloid toward a macrophage lineage, or from a posterior to an anterior precursor population, warrants further examination.
Second, we demonstrated that erythrocytosis in zebrafish embryos is mediated by jak2a activation, consistent with enforced expression of a tel-jak2a fusion.23 The proposition was first supported by the study of the chordin morphant, whose hematopoietic expansion was significantly ameliorated by jak2a knock-down. Furthermore, constitutive activation of jak2a also resulted in a significant increase (24%) in hematopoiesis, as defined quantitatively by flow cytometry in Tg(gata1:GFP) embryos. Such an increase in erythropoiesis might not manifest morphologically in the developing embryos, and nonquantitative whole-mount in situ hybridization did not show any consistent increase in gata1 staining (unpublished data, A.C.H.M., February 2007). However, consistent with the data from flow cytometry, we were able to demonstrate both an increase in erythroid (gata1, αhe1, and βhe1) and myelomonocytic gene expression (spi1, mpo and l-plastin) using quantitative real-time PCR as well as an increase in stat5 phosphorylation with Western blotting. Notably, constitutive activation of stat5.1 has also been shown to increase gata1+ and spi1+ populations in zebrafish embryos.21 The jak2aca used in the present study was generated based on the Drosophila HopT42 mutant carrying a gain-of-function mutation (E695K) at the JH2 domain.4 In this mutant, proliferation of primitive hematopoietic cells was increased, and the Drosophila Jak/Stat pathway was activated.24 Our observations therefore highlight a conserved phenomenon in which gain-of-function mutations of the JH2 domain in Jak2 induce hematopoietic proliferation in Drosophila, zebrafish, and human via activation of Stat signaling. Intriguingly, constitutive jak2a activation had no effect on scl or lmo2 expression. Therefore, the effects of jak2a stimulation (presumably via stat5.1) appear to be restricted to the lineage-committed erythroid/myeloid precursors rather than the early hematopoietic progenitor cells.
Third, we demonstrated that neither knock-down or constitutive activation of jak2a had any effect on endothelial cell specification, as shown by Q-RT-PCR for fli-1 and the intact vasculature in Tg(fli1:GFP) embryos injected with jak2aUTR-MOs. This issue has not been addressed in murine model, as Jak2 knock-out results in early embryonic lethality.18,19 Remarkably, the jak2aUTR embryos showed specific reduction of gene expression associated with early hematopoiesis but not vasculogenesis, and may be useful as a tool whereby these hitherto intertwined processes can be dissected. Our data also suggested that jak2a functions at a higher level in the hematopoietic hierarchy than phospholipase C gamma 1 (PLCγ1) during development, as specific knock-down of PLCγ1 reduces gata1, αhe1, and βhe1, but not scl or lmo2 expression.13
In the present study, the use of flow cytometry in dissociated transgenic embryos and Q-RT-PCR has enabled us to examine zebrafish hematopoiesis objectively and quantitatively. In fact, the differential gene expression induced by jak2aUTR-MOs could not be differentiated reliably by whole-mount in situ hybridization (unpublished data, A.C.H.M., February 2007), which is nonquantitative and is subject to biases due to variations in embryo staining. Therefore, quantitative analyses such as those described herein represent a useful supplement to nonquantitative tests in future study of zebrafish embryonic hematopoiesis.
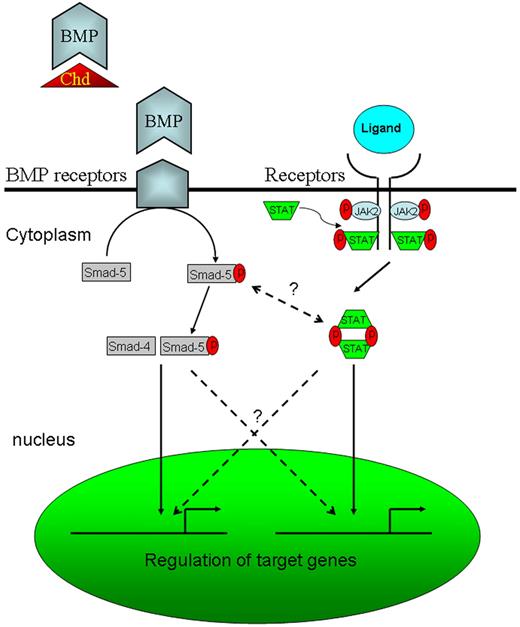
The results of this study are of clinical relevance. Recent identification of Jak2V617F in PV23 suggested that specific targeting of Jak2 signaling might lead to better treatment for this disorder. The jak2aca embryos may therefore shed light on our understanding of human PV and provide us with a robust model for the screening of potential therapeutic agents used for this and other Jak2-related blood diseases. A zebrafish equivalent of the jak2V617F mutation has also been generated (unpublished data, A.C.W., January 2007), and this model will enable us to dissect the pathogenesis of jak2V617F-induced neoplastic transformation. On the other hand, the up-regulation of jak2a in chordin morphants and the amelioration of expanded hematopoiesis by jak2a knock-down have provided us with 2 possible leads for further studies. First, a possible link between bone morphogenetic protein (BMP) and jak2a signaling should be examined (Figure 5). In particular, whether the increase in jak2a signaling in the embryos was due to a cross-talk between Smad and stat pathways as reported in neural stem cells25 or a simple increase in hematopoietic cell population in the embryos should be further studied. Second, previous studies have demonstrated the pivotal role of BMP4 in mesoderm induction and hematopoietic differentiation during embryonic development,26,27 and whether and how deregulation of BMP/Smad signaling might link to human leukemia would have to be further evaluated.
Diagrammatic representation of BMP and Jak/Stat signaling pathways. Chordin (Chd), together with other BMP antagonists such as twisted gastrulation, blocks the binding of BMP to its receptor. Therefore, knock-down of Chd with MO enhances BMP signaling.8 Potential cross-talk between BMP and Jak/Stat signaling pathways are shown by the dotted lines.
Diagrammatic representation of BMP and Jak/Stat signaling pathways. Chordin (Chd), together with other BMP antagonists such as twisted gastrulation, blocks the binding of BMP to its receptor. Therefore, knock-down of Chd with MO enhances BMP signaling.8 Potential cross-talk between BMP and Jak/Stat signaling pathways are shown by the dotted lines.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Anming Meng (Tsinghua University, China) for the generous gift of the Tg(gata1:GFP) fish line. We also thank Ms Jessie Fu, Babs Kwok, and Rachel Lin for performing some of the microinjection experiments; Dr Andrew Oates for the jak2a clone; and Mr Howard Chow and Ms Rowena Lewis for part of the molecular studies.
This work was supported by Research Grants Council grant (University of Hong Kong 7488/04M and 7520/06M) and small project funding from The University of Hong Kong.
Authorship
Contribution: A.C.H.M. conducted the study and wrote the manuscript. A.C.W. conducted part of the experiments. R.L. analyzed the data and wrote the manuscript. A.Y.H.L. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anskar Y. H. Leung, Rm K418, K Block, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Pok Fu Lam Rd, Hong Kong, China; e-mail: ayhleung@hku.hk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal