Abstract
Reduced lymphopoiesis during aging contributes to declines in immunity, but little consideration has been given to its effect on the development of hematologic disease. This report demonstrates that age-related defects in lymphopoiesis underlie the myeloid dominance of adult leukemia. Using a murine model of chronic myeloid leukemia, an adult-onset malignancy that arises from transformation of hematopoietic stem cells by the BCR-ABLP210 oncogene, we demonstrate that young bone marrow (BM) cells that were transformed with BCR-ABLP210 initiated both a myeloproliferative disorder (MPD) and B-lymphoid leukemia, whereas BCR-ABLP210–transformed old BM cells recapitulated the human disease by inducing an MPD with rare lymphoid involvement. In addition, the lesser severity of MPDs initiated from old BCR-ABLP210–transduced BM cells revealed unappreciated defects in aged myeloid progenitors. These data demonstrate that aging affects patterns of leukemogenesis and indicate that the effects of senescence on hematopoiesis are more extensive than previously appreciated.
Introduction
Hematopoietic stem cells (HSCs) are the precursors from which all mature blood cells are generated.1,2 HSCs normally maintain the hematopoietic system through balanced differentiation into common myeloid progenitors (CMPs) and lymphoid-specified progenitors. CMPs, the earliest myeloid-specified progenitors to be defined, subsequently differentiate into granulocyte-macrophage progenitors (GMPs) and megakaryocyte-erythroid progenitors, from which most myeloid and erythroid cells, respectively, arise.3 Although there is uncertainty regarding the characteristics of the most HSC-proximal lymphoid-specified progenitors,4 there is general agreement that common lymphoid progenitors (CLPs)5 are a canonical intermediate through which B-cell development progresses. The progeny of CLPs include pre-pro-B and pro-B cells, and after successful rearrangement of immunoglobulin (Ig) heavy-chain genes, pre-B cells that express Igμ heavy chain in their cytoplasm are produced. Surface IgM+ B cells are generated from pre-B cells after rearrangement and expression of Ig light-chain genes.6,7
Recent analyses have shown that the balance of hematopoietic cell production becomes severely perturbed during aging. B-lymphocyte development begins to decline early in adult life, and the production of B cells is dramatically diminished.8,9 This decline is manifest at all stages of B-cell development; both the frequency and number of CLPs and their downstream pre-pro-B-cell, pro-B-cell, and pre-B-cell progeny are significantly reduced with age.10–14 Because HSCs accumulate multiple functional defects with increasing age,15–18 it has been proposed that the decline in B lymphopoiesis is a result of age-related deficiencies in the potential of HSCs to generate lymphoid progeny19,20 and of proliferative and differentiative defects intrinsic to lymphoid intermediates.9,21 Despite increasing evidence that aged HSCs do not proliferate and differentiate as efficiently as their young counterparts,22 myelopoiesis has been reported to be unaffected by aging.11,19 This conclusion was based on the finding that the frequency of CMPs and their downstream progeny remains normal or is increased in old mice.
The consequences of HSC aging and reduced lymphocyte production have, for the most part, been considered in the context of their impact on the quality of the adaptive immune response, which is diminished in elderly people.23 Less attention has been given to how these age-related alterations influence disease within the hematopoietic system (hematopoietic malignancies, in particular).24 For instance, the majority of leukemias that present in children involve lymphoid cells, and these leukemias occur at a time when lymphoid progenitor number and proliferation are highest.8,11 Conversely, myeloid leukemias tend to predominate in elderly people when lymphopoiesis is waning.19
Chronic myeloid leukemia (CML), the most common myeloproliferative disorder (MPD) in humans, typifies this pattern.25 CML presents as a myeloid hyperplasia that occurs almost exclusively in adults, and its incidence increases with age. More than 90% of patients with CML possess the Philadelphia chromosome,26,27 a reciprocal translocation t(9q34;22q11)28 that fuses the breakpoint cluster region (BCR) and Abelson tyrosine kinase (ABL) genes29–31 and encodes a 210-kDa chimeric protein with constitutive tyrosine kinase activity.30,32 The occurrence of the BCR-ABLP210 translocation in HSCs33–36 has led to CML being classified as a disease of stem cell origin.37 Because HSCs have multilineage differentiation potential,38 their transformation would be expected to result in disease with representation of both the myeloid and lymphoid lineages. However, this is not the case, because BCR-ABLP210–induced leukemia predominantly presents as CML and rarely causes disease with lymphoid involvement.
This clinical presentation of CML, combined with the fact that it is a disease of middle and old age,39 led us to hypothesize that the age-related decline in lymphopoiesis is a factor that contributes to the myeloid predominance of this and other adult-onset leukemias. To test this premise, we examined the pattern of disease induced by BCR-ABLP210 transformation of bone marrow (BM) cells from young and old mice. The results of these experiments support the validity of our hypothesis and further reveal that, in contrast to what has been generally accepted, myelopoiesis is also compromised by aging. Taken together, the results from this study demonstrate that immune senescence contributes to the myeloid dominance of adult leukemia and indicate that the effects of aging on blood cell development are more extensive than is appreciated currently.
Materials and methods
Mice
Four- to 7-week-old C57BL/6J (B6) and B6.129S7-Rag1tm1Mom/J (Rag1−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and B6.SJL mice were obtained from Taconic Farms (Germantown, NY). Ten- to 24-month-old B6 mice were purchased from the National Institute on Aging (Bethesda, MD) colony. Animals were housed in the vivarium of the University of California, Los Angeles, Division of Laboratory Animal Medicine. Experiments were conducted according to the guidelines of the University of California, Los Angeles, Institutional Animal Care and Use Committee.
B6, B6.SJL, and Rag1−/− recipients were preconditioned with 500 R from a 137Cs irradiator (120 R/min; Mark I-68A; JL Shepperd and Associates, San Fernando, CA) 12 to 30 hours before intravenous injection of transformed cells.
Generation of retroviral stocks
The retroviral vector pMSCV,40 which contained a 5′ long-terminal repeat–driven BCR-ABL internal ribosome entry site (IRES) enhanced green fluorescence protein (EGFP) or a 5′ long-terminal repeat–driven IRES EGFP, was used to generate high-titer helper-free retrovirus after transient cotransfection of 293T cells. 293T cells were grown on poly-l-lysine (Sigma, St Louis, MO)–coated 10-cm tissue culture–treated plates (Becton Dickinson, San Jose, CA) in Iscove modified Dulbecco minimum essential medium (IMDM; Mediatech, Herndon, VA) supplemented with 10% fetal calf serum (FCS; HyClone Laboratories, Logan, UT), 1 mM l-glutamine (Gibco, Gaithersburg, MD), 100 U/mL streptomycin (Gibco), and 100 μg/mL penicillin (complete IMDM; Gibco). Transfections were performed by coprecipitating 15 μg of retroviral vector with 15 μg of an ecotropic packaging vector41 with the CalPhos mammalian transfection kit (BD Biosciences, San Diego, CA). Medium was replaced every 12 hours for 3 days with complete IMDM. Viral stocks were prepared by pooling supernatants collected at 36, 48, and 60 hours after transfection. Viral titers were determined after infection of 3T3 cells with serial dilutions of the pooled virus supernatant and found to range between 2 × 106 and 7 × 106 virus particles per milliliter.
Retroviral transduction and BM transplantation
Young mice were administered a single intravenous dose of 5-fluorouracil (5-FU [Sigma]; 150 mg/kg body weight). Because of their higher susceptibility to 5-FU, middle-aged and old mice were administered a dose of 115 mg/kg body weight. On the eighth day after 5-FU administration, BM cell suspensions were prepared as described.42 Cells were distributed in 5-mL polystyrene tubes (Becton Dickinson) and incubated with 1 mL of retrovirus supplemented with 10% horse serum (HyClone Laboratories), 1 mM l-glutamine, 100 U/mL streptomycin, and 100 μg/mL penicillin, 8 μg/mL polybrene (Sigma), 50 μM β-mercaptoethanol (Sigma), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [Gibco]), 100 ng/mL stem cell factor (SCF [Biosource, Camarillo, CA]), 100 ng/mL Flt-3L (R&D Systems, Minneapolis, MN), and 10 ng/mL interleukin (IL)–11 (R&D Systems) for 2 hours at 37°C in 5% CO2 and air and constant humidity. Cells were centrifuged for 5 minutes at 400g, and viral supernatant was replaced with 1 mL of virus stock supplemented as described above. This procedure was repeated twice. After 6 hours, cells were washed, counted, and resuspended in phosphate-buffered saline (PBS). Purified pro/pre-B cells were infected in a modified progenitor cell-culture system.42 One million cells were seeded in 6-well plates in RPMI 1640 (Gibco) supplemented with 10% FCS, 1 mM l-glutamine, 100 U/mL streptomycin, 100 μg/mL penicillin, 8 μg/mL polybrene, 50 μM β-mercaptoethanol, 50 μg/mL gentamicin (Sigma), 20 ng/mL SCF, 20 ng/mL IL-3, 20 ng/mL IL-6 (Biosource), 10 ng/mL Flt-3L, and 10 ng/mL IL-7. Inserted into each well were 0.4-μm transwell inserts in which confluent S17 stromal layers had been preestablished. Retrovirus was added to the bottom well 4 times over a 24-hour period, at the end of which cells were washed, counted, and resuspended in PBS. Five- to 7-week-old irradiated B6, B6.SJL, or Rag1−/− mice received an intravenous injection of 2 × 105 transduced BM cells or 105 pro/pre-B cells per mouse. For secondary transplants, 5 × 106 splenocytes from primary diseased mice were injected intravenously into irradiated 5- to 7-week-old B6 recipients.
Immunophenotypic analysis of leukemic cells and cell sorting
BM- and spleen-cell suspensions were prepared as described previously.42 Cell suspensions were incubated with anti-CD16/32 (FcγRII-III; clone 2.4G2 [eBiosciences, San Diego, CA]) or total mouse IgG (for CMP and GMP stains only; Axell Accurate Chemical and Scientific, Westbury, NY) to reduce nonspecific labeling. Cells were incubated with combinations of antibodies to the following cell-surface determinants, conjugated to fluoro-isothiocyanate, phycoerythrin, tricolor, indodicarbocyanine, biotin, or allophycocyanin: CD3ϵ (clone KT31.1), CD4 (clone GK1.5), CD8α (clone 53-6.7), CD11b (clone M1/70), CD16/32 (FcγRII/III; clone 2.4G2), CD19 (clone 1D3), CD45.1 (clone A20), CD45.2 (clone 104), CD45R (B220, clone RA3-6B2), CD117 (c-Kit, clone 2B8), CD127 (IL-7Rα, clone A7R34), Ter119 (clone Ter-119), T-cell receptor (TCR)β (clone H57-597), TCRγδ (clone UC7-13D5), natural killer 1.1 (NK1.1; clone PK136), Ly-6C (clone AL-21), AA4.1 (clone C1qRp), Sca-1 (clone E13-161.7), IgM (clone II/41), and Gr-1 (clone RB6-8C5). Biotinylated cells were visualized by incubation with tricolor-, allophycocyanin-, or allophycocyanin-Alexa Fluor 750–conjugated streptavidin. All reagents were obtained from Becton Dickinson or eBiosciences except for goat antimouse IgM (Southern Biotech, Birmingham, AL) and tricolor-conjugated CD11b and allophycocyanin-Alexa Fluor 750–conjugated streptavidin (Caltag Laboratories, Burlingame, CA). All incubations were for approximately 30 minutes at 4°C. After the last wash, live cells were acquired with Cell Quest software (Becton Dickinson) on a flow cytometer (FACScan, FACSCalibur, or Cytek modified FACSscan [BD Biosciences]) located at the Flow Cytometry Core of the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles.
Disease phenotype was established by determining the frequencies of BM or spleen cells coexpressing GFP (BCR-ABLP210) and lineage-specific cell surface antigens (myeloid: CD11b+Gr-1+; B lymphoid: CD45R+CD19+; erythroid: Ter119+; T-lymphoid: CD4+ and/or CD8+). Disease was considered present when at least 7.5% of the total GFP+ cells in either the BM or spleen coexpressed the specific cell-surface antigens.
Populations enriched for CMPs and GMPs3 were defined as Lin− (Lin=CD3ϵ, CD8α, CD45R, Gr-1, TER-119, TCRβ, TCRγδ, and NK1.1) Sca-1−CD127−CD16/32+/LoCD117Hi and Lin−Sca-1−CD127−CD16/32HiCD117Hi, respectively. Populations enriched for HSCs38 were defined as Lin− (Lin=CD3ϵ, CD8α, CD11b, CD45R, Gr-1, TER-119, TCRβ, TCRγδ, and NK1.1) Sca-1HiCD117Hi. Pre-pro-B cells and pro/pre-B cells43 were defined as Lin− (Lin=CD8α, CD11b, Gr-1, TER-119, Ly-6C, IgM, TCRβ, TCRγδ, and NK1.1) CD45R+CD19−AA4.1+ and Lin− CD45R+CD19+AA4.1+, respectively.
Cells to be purified were resuspended in minimum essential medium-α (αMEM; Gibco) supplemented with 2% FCS, 25 mM HEPES, 1 mM l-glutamine, 100 U/mL streptomycin, 100 μg/mL penicillin, and 50 μg/mL gentamicin before being sorted on a FACSaria (Becton Dickinson). Sorted fractions were examined by reanalysis and were routinely 95% pure.
B-cell progenitor cultures
BM cells from day-8 5-FU–treated mice or purified pro/pre-B cells were retrovirally transduced as described above. BM cells (3 × 105) or GFP+ pro/pre-B cells (3.8 × 105) that had been expanded for 1 week in the modified progenitor assay described above were seeded onto S17 stromal cells44 in T12.5 flasks (Becton Dickinson) in RPMI 1640 supplemented with 5% FCS, 50 μM β-mercaptoethanol, 1 mM l-glutamine, 100 U/mL streptomycin, and 100 μg/mL penicillin.45 Cultures were incubated at 37°C in 5% CO2 and air and constant humidity and fed twice weekly for up to 3 weeks.
Myeloid cultures
Myeloid colony assays were performed by resuspending target cells in 1 mL of methylcellulose supplemented with 30% FCS, 40% αMEM, 50 μM β-mercaptoethanol, 1 mM l-glutamine, 100 U/mL streptomycin, 100 μg/mL penicillin, 50 μg/mL gentamicin, 20 ng/mL SCF, 10 ng/mL granulocyte-macrophage colony-stimulating factor (Biosource), 30 ng/mL IL-3, and 10 ng/mL IL-11 in 3.5-cm Petri dishes (Becton Dickinson) in triplicate.3 Colonies were counted on day 8. Individual colonies were picked under a dissecting microscope, resuspended in 300 μL of PBS, and analyzed for GFP expression on a FACScan for 30 seconds at a fixed flow rate of 60 μL/min. Live cells were gated, and total cell numbers were calculated as [total live events/(flow rate × acquisition time)] × sample volume.
Liquid suspension cultures were initiated by seeding 1.5 × 105 whole BM cells or 2 × 103 sorted CMPs in complete IMDM supplemented with 50 μM β-mercaptoethanol, 50 μg/mL gentamicin, 20 ng/mL SCF, 10 ng/mL granulocyte-macrophage colony-stimulating factor, 30 ng/mL IL-3, 30 ng/mL IL-6, and 10 ng/mL IL-11 at 37°C in 5% CO2 and air and constant humidity. Cultures were fed on day 4, and on day 6 cells were harvested and assessed by immunostaining with Gr-1 and CD11b as described above.
Statistical analysis
Unless indicated otherwise, data are expressed as a mean plus or minus SEM. Differences between groups were tested by a 2-tailed, unpaired t test, with an α value of 0.05.
Results
Hematopoietic cell age alters BCR-ABLP210–induced leukemia phenotype
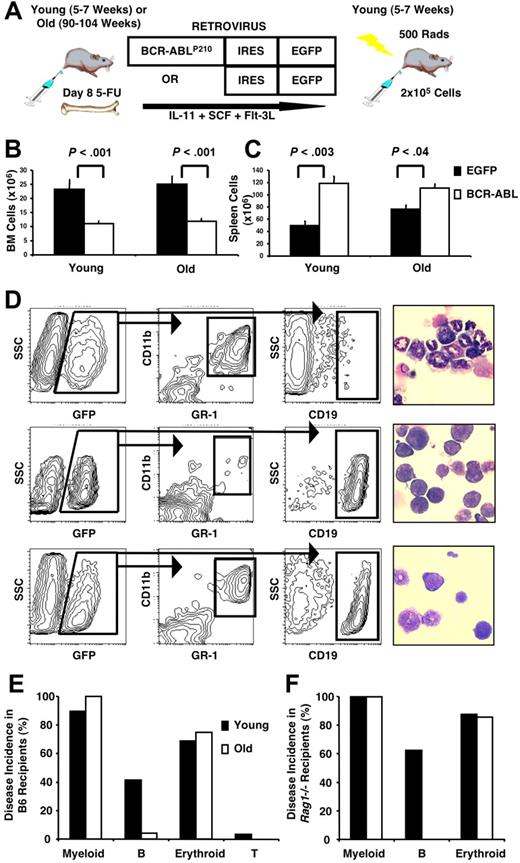
To assess the impact of aging on BCR-ABLP210–induced leukemogenesis,46 BM cells harvested from day-8 5-FU–treated young (5- to 7-week-old) or old (90- to 104-week-old) mice were infected with a retrovirus that carried a bicistronic IRES expression vector encoding BCR-ABLP210 and an EGFP reporter gene and subsequently injected into sublethally irradiated syngeneic young mice (BMBCR-ABL recipients; Figure 1A). Control animals similarly received transplants with BM cells infected with a retrovirus containing EGFP alone (BMEGFP recipients). The use of congenic CD45.1+ recipients in one experiment confirmed that GFP+ (BCR-ABL+) leukemic cells coexpressed the donor-derived CD45.2 cell surface antigen (data not shown).
Age alters the phenotype of BCR-ABLP210–induced leukemia. (A) BM from young or old B6 mice was harvested on day 8 after 5-FU treatment, infected with a retrovirus carrying a bicistronic IRES expression vector encoding BCR-ABLP210 and a reporter EGFP gene, and transplanted into sublethally irradiated, young syngeneic or congenic recipients. Control recipients received BM transduced with EGFP alone. Leukemias were accompanied by decreased BM (B) and increased splenic (C) cellularity. Cell numbers represent mean values plus or minus SEM obtained from 5 independent experiments with 29 recipients of young BMBCR-ABL, 24 recipients of old BMBCR-ABL, 9 recipients of young BMEGFP, and 5 recipients of old BMEGFP. (D) Leukemic cells in the BM were characterized by flow cytometry for expression of GFP (BCR-ABLP210) in combination with lineage-specific cell-surface antigens. Examples of recipients with an MPD (top panels), B-lymphoid leukemia (middle panels), and both an MPD and lymphoid leukemia (bottom panels) are shown. Recipients that developed an MPD had increased granulocytes and decreased lymphocytes in the spleen (top right panel), whereas the ones that developed B-lymphoid leukemia had increased lymphoid cells and blasts in the spleen (middle right panel) compared with controls (× 400 magnification). Images were viewed with a Leitz Laborlux D microscope (Leitz, Wetzlar, Germany) using a 40×/0.70 NA phase-contrast objective. Cells were deposited onto slides with a cytocentrifuge (Shandon-Elliot, Sewickley, PA) and stained with Wright-Giemsa. Coverslips were mounted with Permount (Biomeda, Foster City, CA). Micrographs were taken using an Olympus DP11 camera (Olympus, Tokyo, Japan), and prepared using Adobe Photoshop image-acquisition software (Adobe Systems, San Jose, CA). (E,F) Summary of the incidence of leukemia according to phenotype in B6 (E) and Rag1−/− (F) recipients of young and old BMBCR-ABL cells. The data shown in panel E are based on the same recipients as those shown in panels B and C, and data shown in panel F are based on 8 recipients of young and 7 recipients of old BMBCR-ABL cells.
Age alters the phenotype of BCR-ABLP210–induced leukemia. (A) BM from young or old B6 mice was harvested on day 8 after 5-FU treatment, infected with a retrovirus carrying a bicistronic IRES expression vector encoding BCR-ABLP210 and a reporter EGFP gene, and transplanted into sublethally irradiated, young syngeneic or congenic recipients. Control recipients received BM transduced with EGFP alone. Leukemias were accompanied by decreased BM (B) and increased splenic (C) cellularity. Cell numbers represent mean values plus or minus SEM obtained from 5 independent experiments with 29 recipients of young BMBCR-ABL, 24 recipients of old BMBCR-ABL, 9 recipients of young BMEGFP, and 5 recipients of old BMEGFP. (D) Leukemic cells in the BM were characterized by flow cytometry for expression of GFP (BCR-ABLP210) in combination with lineage-specific cell-surface antigens. Examples of recipients with an MPD (top panels), B-lymphoid leukemia (middle panels), and both an MPD and lymphoid leukemia (bottom panels) are shown. Recipients that developed an MPD had increased granulocytes and decreased lymphocytes in the spleen (top right panel), whereas the ones that developed B-lymphoid leukemia had increased lymphoid cells and blasts in the spleen (middle right panel) compared with controls (× 400 magnification). Images were viewed with a Leitz Laborlux D microscope (Leitz, Wetzlar, Germany) using a 40×/0.70 NA phase-contrast objective. Cells were deposited onto slides with a cytocentrifuge (Shandon-Elliot, Sewickley, PA) and stained with Wright-Giemsa. Coverslips were mounted with Permount (Biomeda, Foster City, CA). Micrographs were taken using an Olympus DP11 camera (Olympus, Tokyo, Japan), and prepared using Adobe Photoshop image-acquisition software (Adobe Systems, San Jose, CA). (E,F) Summary of the incidence of leukemia according to phenotype in B6 (E) and Rag1−/− (F) recipients of young and old BMBCR-ABL cells. The data shown in panel E are based on the same recipients as those shown in panels B and C, and data shown in panel F are based on 8 recipients of young and 7 recipients of old BMBCR-ABL cells.
Recipients of both young and old BMBCR-ABL cells exhibited weight loss, cachexia, and poor grooming and were killed between 2 and 7 weeks after transplantation. These animals had decreased BM and increased splenic cellularity compared with BMEGFP recipients (Figure 1B,C). Hematopoietic infiltration of the liver and lungs with occasional lymphadenopathy was evident at necropsy (data not shown). In contrast, all BMEGFP recipients were observed for up to 4 months after transplantation and remained healthy.
Disease patterns in BMBCR-ABL recipients were assessed by phenotypic and morphologic characterization of hematopoietic cells in their BM and spleen (Figure 1D and Table 1). Ninety percent (26 of 29) of young and 100% (24 of 24) of old BMBCR-ABL recipients developed MPDs that were characterized by the expansion of GFP+Gr-1+CD11b+ granulocytes in the BM and spleen that was frequently accompanied by the presence of GFP+ Ter119+ erythroid cells (Figure 1D,E). Similar to the situation in humans, 96% (23 of 24) of old BMBCR-ABL recipients lacked significant B-lineage involvement in their disease, as determined by the minimal frequency of GFP+CD45R+CD19+ B-lineage cells and absence of lymphoid blasts in their BM and spleen. In stark contrast, 35% (9 of 26) of young BMBCR-ABL recipients that developed MPDs concurrently developed B-lymphoid leukemia. In addition, 10% (3 of 29) of young BMBCR-ABL recipients presented with B-lymphoid leukemia without significant myeloid involvement, a disease profile that was never observed in any old BMBCR-ABL recipients.
Disease characteristics of mice that received a transplant of young or old EGFP or BCR-ABLP210–transduced BM cells in one representative experiment
| Donor . | BM . | Spleen . | Disease type . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total cells, × 106* . | GFP+, % . | GFP+ Gr-1+ CD11b+, % . | GFP+ CD45R+ CD19+, % . | Total cells, × 106 . | GFP+, % . | GFP+ Gr-1+ CD11b+, % . | GFP+ CD45R+ CD19+, % . | ||
| Young | |||||||||
| EGFP1 | 9.4 | 1.9 | 1.1 | 0.6 | 15.8 | 5.9 | 2.8 | 0.9 | None |
| EGFP2 | 26.0 | 1.5 | 0.8 | 0.2 | 52.8 | 1.8 | 0.5 | 1.3 | None |
| EGFP3 | 13.2 | 2.1 | 0.9 | 0.3 | 48.0 | 2.5 | 0.4 | 1.7 | None |
| EGFP4 | 18.8 | 1.9 | 0.9 | 0.3 | 61.6 | 5.3 | 0.3 | 1.5 | None |
| BCR1 | 9.2 | 62.0 | 39.1 | 0.2 | 52.8 | 75.3 | 55.1 | 1.0 | M |
| BCR2 | N/A | 64.1 | 12.7 | 2.5 | NA | 84.8 | 16.5 | 1.3 | M |
| BCR3 | 11.0 | 76.3 | 52.3 | 0.3 | 90.2 | 86.7 | 42.8 | 0.9 | M |
| BCR4 | 10.2 | 65.4 | 25.7 | 0.1 | 92.2 | 76.6 | 50.0 | 0.7 | M |
| BCR5 | 12.0 | 55.7 | 44.7 | 5.2 | 108.5 | 66.0 | 38.9 | 6.4 | M + B |
| BCR6 | 7.2 | 32.0 | 10.9 | 19.5 | 130.4 | 74.5 | 9.9 | 15.1 | M + B |
| BCR7 | 11.6 | 60.7 | 1.3 | 58.8 | 56.8 | 58.6 | 5.2 | 48.4 | B |
| BCR8 | 10.2 | 82.0 | 56.9 | 0.5 | 199.2 | 56.0 | 37.6 | 2.9 | M |
| Old | |||||||||
| EGFP1 | 17.8 | 2.4 | 1.7 | 0.1 | 72.0 | 1.2 | 0.7 | 0.4 | None |
| EGFP2 | 23.4 | 1.4 | 1.0 | 0.2 | 82.6 | 2.2 | 1.4 | 0.3 | None |
| BCR1 | 8.8 | 68.9 | 27.5 | 0.2 | 148.8 | 65.6 | 18.2 | 2.6 | M |
| BCR2 | 20.0 | 17.8 | 12.9 | 0.1 | 136.3 | 43.7 | 12.8 | 1.2 | M |
| BCR3 | 15.6 | 34.4 | 26.6 | 0.2 | 144.0 | 58.4 | 32.2 | 1.7 | M |
| BCR4 | 9.4 | 56.0 | 23.1 | 0.1 | 168.0 | 53.8 | 21.0 | 2.2 | M |
| BCR5 | 15.2 | 45.4 | 24.0 | 0.1 | 178.6 | 62.4 | 19.5 | 1.2 | M |
| BCR6 | 14.8 | 30.7 | 25.2 | 0.1 | 121.0 | 50.7 | 26.5 | 1.8 | M |
| BCR7 | 20.6 | 22.7 | 18.4 | 0.1 | 136.3 | 52.6 | 20.3 | 1.3 | M |
| BCR8 | 20.8 | 2.2 | 1.6 | 0.1 | 67.2 | 21.7 | 9.0 | 0.7 | M |
| Donor . | BM . | Spleen . | Disease type . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total cells, × 106* . | GFP+, % . | GFP+ Gr-1+ CD11b+, % . | GFP+ CD45R+ CD19+, % . | Total cells, × 106 . | GFP+, % . | GFP+ Gr-1+ CD11b+, % . | GFP+ CD45R+ CD19+, % . | ||
| Young | |||||||||
| EGFP1 | 9.4 | 1.9 | 1.1 | 0.6 | 15.8 | 5.9 | 2.8 | 0.9 | None |
| EGFP2 | 26.0 | 1.5 | 0.8 | 0.2 | 52.8 | 1.8 | 0.5 | 1.3 | None |
| EGFP3 | 13.2 | 2.1 | 0.9 | 0.3 | 48.0 | 2.5 | 0.4 | 1.7 | None |
| EGFP4 | 18.8 | 1.9 | 0.9 | 0.3 | 61.6 | 5.3 | 0.3 | 1.5 | None |
| BCR1 | 9.2 | 62.0 | 39.1 | 0.2 | 52.8 | 75.3 | 55.1 | 1.0 | M |
| BCR2 | N/A | 64.1 | 12.7 | 2.5 | NA | 84.8 | 16.5 | 1.3 | M |
| BCR3 | 11.0 | 76.3 | 52.3 | 0.3 | 90.2 | 86.7 | 42.8 | 0.9 | M |
| BCR4 | 10.2 | 65.4 | 25.7 | 0.1 | 92.2 | 76.6 | 50.0 | 0.7 | M |
| BCR5 | 12.0 | 55.7 | 44.7 | 5.2 | 108.5 | 66.0 | 38.9 | 6.4 | M + B |
| BCR6 | 7.2 | 32.0 | 10.9 | 19.5 | 130.4 | 74.5 | 9.9 | 15.1 | M + B |
| BCR7 | 11.6 | 60.7 | 1.3 | 58.8 | 56.8 | 58.6 | 5.2 | 48.4 | B |
| BCR8 | 10.2 | 82.0 | 56.9 | 0.5 | 199.2 | 56.0 | 37.6 | 2.9 | M |
| Old | |||||||||
| EGFP1 | 17.8 | 2.4 | 1.7 | 0.1 | 72.0 | 1.2 | 0.7 | 0.4 | None |
| EGFP2 | 23.4 | 1.4 | 1.0 | 0.2 | 82.6 | 2.2 | 1.4 | 0.3 | None |
| BCR1 | 8.8 | 68.9 | 27.5 | 0.2 | 148.8 | 65.6 | 18.2 | 2.6 | M |
| BCR2 | 20.0 | 17.8 | 12.9 | 0.1 | 136.3 | 43.7 | 12.8 | 1.2 | M |
| BCR3 | 15.6 | 34.4 | 26.6 | 0.2 | 144.0 | 58.4 | 32.2 | 1.7 | M |
| BCR4 | 9.4 | 56.0 | 23.1 | 0.1 | 168.0 | 53.8 | 21.0 | 2.2 | M |
| BCR5 | 15.2 | 45.4 | 24.0 | 0.1 | 178.6 | 62.4 | 19.5 | 1.2 | M |
| BCR6 | 14.8 | 30.7 | 25.2 | 0.1 | 121.0 | 50.7 | 26.5 | 1.8 | M |
| BCR7 | 20.6 | 22.7 | 18.4 | 0.1 | 136.3 | 52.6 | 20.3 | 1.3 | M |
| BCR8 | 20.8 | 2.2 | 1.6 | 0.1 | 67.2 | 21.7 | 9.0 | 0.7 | M |
NA indicates not applicable; M, myeloid; B, lymphoid.
Numbers of BM cells are from 2 femurs and 2 tibias.
Overall, 41% (12 of 29) of young BMBCR-ABL recipients developed B-lymphoid leukemia compared with 4% (1 of 24) of old BMBCR-ABL recipients (Figure 1E). These differences were not the result of decreased engraftment of old cells,18 because engraftment of GFP+ cells was comparable in the BM of recipients of young and old BMEGFP (Table 1), all recipients of old BMBCR-ABL developed myeloid disease, and increasing the number of transplanted old BMBCR-ABL cells did not increase the incidence of lymphoid disease in the recipients (data not shown). Therefore, these data strongly indicate that BCR-ABLP210–transduced old hematopoietic cells lack significant potential to initiate B-lymphoid leukemia.
This conclusion was confirmed by transplanting BCR-ABLP210–transduced young and old BM cells into Rag1−/− recipient mice, whose lack of lymphocytes creates a more favorable environment for normal and dysplastic lymphoid development.47–49 All BMBCR-ABLRag1−/− recipients developed MPDs, and recipients of young BMBCR-ABL also developed B-lymphoid leukemia with an incidence markedly higher (63% [5 of 8]) than that observed in wild-type mice. In contrast, none (0 of 7) of the old BMBCR-ABLRag1−/− recipients showed any significant B-lineage component to their disease (Figure 1F).
The incidence of B-lymphoid leukemia correlates with age-related declines in B lymphopoiesis
The decline in B lymphopoiesis does not abruptly initiate in old age but, instead, begins in relatively young animals and progresses gradually thereafter.8 For example, middle-aged mice (42-44 weeks old) have approximately half the number of B-lineage cells as young mice (Figure 2A,B). Similarly, the frequency of pre-pro-B, pro-B, and pre-B cells is progressively reduced in the BM of 5-FU–treated mice of increasing age, which demonstrates a reduced capacity to generate B-lineage cells de novo in old mice.11
B-lymphoid leukemogenic potential declines in parallel with age-related declines in B lymphopoiesis. (A) Immunostaining used to define pre-pro-B cells (Lin− CD19−CD45R+AA4.1+) and pro/pre-B cells (Lin− CD19+CD45R+AA4.1+) in murine BM. (B) The frequency of lymphoid progenitor populations in the BM progressively declined in mice of increasing age. Groups of young (5- to 7-week-old; n=4), middle-aged (42- to 44-week-old; n=2), and old (90- to 104-week-old; n=3) mice were analyzed. Steady-state frequencies are presented as the mean plus or minus SEM. 5-FU frequencies are presented from the pooled BM of 4 young, 7 middle-aged, and 4 old mice. Total B-lineage cells represents CD19+CD45R+ cells. Pre-pro-B-cell frequencies were 0.103% in young, 0.073% in middle-aged, and 0.01% in old 5-FU–treated mice, respectively. (C) The incidence of B-lymphoid leukemia in Rag1−/− recipients of BMBCR-ABL cells was reduced with increasing BM age. Recipients of middle-aged (n=8) BMBCR-ABL cells developed B-lymphoid leukemia less frequently than recipients of young (n=8) BMBCR-ABL cells but more frequently than recipients of old (n=7) BMBCR-ABL cells. The recipients of young and old BMBCR-ABL are the same as those shown in Figure 1F.
B-lymphoid leukemogenic potential declines in parallel with age-related declines in B lymphopoiesis. (A) Immunostaining used to define pre-pro-B cells (Lin− CD19−CD45R+AA4.1+) and pro/pre-B cells (Lin− CD19+CD45R+AA4.1+) in murine BM. (B) The frequency of lymphoid progenitor populations in the BM progressively declined in mice of increasing age. Groups of young (5- to 7-week-old; n=4), middle-aged (42- to 44-week-old; n=2), and old (90- to 104-week-old; n=3) mice were analyzed. Steady-state frequencies are presented as the mean plus or minus SEM. 5-FU frequencies are presented from the pooled BM of 4 young, 7 middle-aged, and 4 old mice. Total B-lineage cells represents CD19+CD45R+ cells. Pre-pro-B-cell frequencies were 0.103% in young, 0.073% in middle-aged, and 0.01% in old 5-FU–treated mice, respectively. (C) The incidence of B-lymphoid leukemia in Rag1−/− recipients of BMBCR-ABL cells was reduced with increasing BM age. Recipients of middle-aged (n=8) BMBCR-ABL cells developed B-lymphoid leukemia less frequently than recipients of young (n=8) BMBCR-ABL cells but more frequently than recipients of old (n=7) BMBCR-ABL cells. The recipients of young and old BMBCR-ABL are the same as those shown in Figure 1F.
If the age-related decline in B-cell production underlies the reduced capacity of BMBCR-ABL cells to initiate B-lymphoid leukemia, then the incidence of BCR-ABLP210–induced lymphoid disease should decline gradually with increasing age. This hypothesis was tested by transplanting BCR-ABLP210–transduced BM cells from young, middle-aged, and old mice into Rag1−/− recipients. As shown in Figure 2C, the incidence of B-lymphoid leukemia is highest among young BMBCR-ABL recipients (63% [5 of 8]), intermediate in middle-aged BMBCR-ABL recipients (25% [2 of 8]), and nil in recipients of old BMBCR-ABL (0% [0 of 7]).
Cell-intrinsic defects diminish the leukemogenic potential of aged B-lineage cells
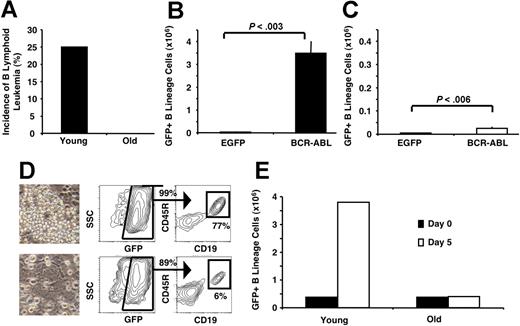
In addition to its emergence from HSCs,36 B-lymphoid leukemia can also initiate from direct transformation of committed lymphoid progenitors.50–52 This raised the possibility that the reduced incidence of B-lymphoid leukemia from aged BM cells observed in the above-mentioned study resulted from the lower number of lymphoid progenitors available for transformation (Figure 2B). However, this was not the case. When an equivalent number of BCR-ABLP210–transduced pro/pre-B cells from young and old mice were injected into sublethally irradiated Rag1−/− recipients, 25% of the recipients of young BCR-ABLP210–transduced B-lineage cells developed B-lymphoid leukemia 8 weeks later, whereas none of the recipients of old BCR-ABLP210–transduced pro/pre-B cells developed disease (Figure 3A).
Age-related intrinsic defects in B-lineage progenitors diminish their leukemogenic potential. (A) Pro/pre-B cells (Lin− CD19+CD45R+AA4.1+) purified from the BM of young and old mice were transduced with BCR-ABLP210, and the same number of young and old cells was transplanted into Rag1−/− recipients. Eight weeks later, 25% of the recipients of BCR-ABL–transduced young pro/pre-B cells (n=8) developed B-lymphoid leukemia, whereas recipients of old pro/pre-B cells (n=8) did not develop any characteristics of disease. Young (B) and old (C) BM cells (3 × 105) transduced with EGFP or BCR-ABLP210 were used to establish hematopoietic cultures in B-lineage–permissive conditions.45 Cultures were examined 3 weeks later. Young BMBCR-ABL cells expanded 100-fold, whereas only a 6-fold expansion was observed with old cells compared with controls. (D) Phenotypic and morphologic analysis of cultures described for B and C. Cultures derived from young BMBCR-ABL had increased cellularity and a higher frequency of GFP+ B-lineage cells compared with those that were initiated from old BMBCR-ABL, which produced cells primarily with a myeloid morphology. Images of live, cultured cells were viewed with a Nikon Diaphot TMD inverted phase microscope (Nikon, Tokyo, Japan) using a 20×/0.40 NA phase-contrast objective. Micrographs were taken using an Olympus DP11 camera (Olympus), and photographs were prepared using Adobe Photoshop image-acquisition software (Adobe Systems). (E) BCR-ABLP210–expressing (GFP+) young and old pro/pre-B cells (3.8 × 105) were seeded on stromal layers in B-lineage–permissive conditions.45 After 5 days, the number of GFP+ cells increased 10-fold in the cultures seeded with young pro/pre-B cells, whereas the old pro/pre-B cells did not show any significant expansion. One of 2 representative experiments is shown.
Age-related intrinsic defects in B-lineage progenitors diminish their leukemogenic potential. (A) Pro/pre-B cells (Lin− CD19+CD45R+AA4.1+) purified from the BM of young and old mice were transduced with BCR-ABLP210, and the same number of young and old cells was transplanted into Rag1−/− recipients. Eight weeks later, 25% of the recipients of BCR-ABL–transduced young pro/pre-B cells (n=8) developed B-lymphoid leukemia, whereas recipients of old pro/pre-B cells (n=8) did not develop any characteristics of disease. Young (B) and old (C) BM cells (3 × 105) transduced with EGFP or BCR-ABLP210 were used to establish hematopoietic cultures in B-lineage–permissive conditions.45 Cultures were examined 3 weeks later. Young BMBCR-ABL cells expanded 100-fold, whereas only a 6-fold expansion was observed with old cells compared with controls. (D) Phenotypic and morphologic analysis of cultures described for B and C. Cultures derived from young BMBCR-ABL had increased cellularity and a higher frequency of GFP+ B-lineage cells compared with those that were initiated from old BMBCR-ABL, which produced cells primarily with a myeloid morphology. Images of live, cultured cells were viewed with a Nikon Diaphot TMD inverted phase microscope (Nikon, Tokyo, Japan) using a 20×/0.40 NA phase-contrast objective. Micrographs were taken using an Olympus DP11 camera (Olympus), and photographs were prepared using Adobe Photoshop image-acquisition software (Adobe Systems). (E) BCR-ABLP210–expressing (GFP+) young and old pro/pre-B cells (3.8 × 105) were seeded on stromal layers in B-lineage–permissive conditions.45 After 5 days, the number of GFP+ cells increased 10-fold in the cultures seeded with young pro/pre-B cells, whereas the old pro/pre-B cells did not show any significant expansion. One of 2 representative experiments is shown.
Further in vitro analysis showed that young and old BCR-ABLP210–transduced 5-FU BM differed significantly in the potential to establish long-term B-lineage cultures. After 3 weeks in B-lymphoid–permissive conditions, recovery of GFP+ B-lineage cells in cultures initiated with young BMBCR-ABL was 100 times higher than that in cultures established with young BMEGFP cells (Figure 3B). In contrast, BCR-ABLP210–transformed old BM cells expanded only 6-fold compared with old BMEGFP cells (Figure 3C). Morphologic and phenotypic analyses confirmed these observations (Figure 3D). As in the in vivo experiments, this was not a result of differences in numbers of B-lineage progenitors present in young and old BM. When an equivalent number of young and old BCR-ABLP210–transduced pro/pre-B cells were cultured in B-lymphoid–permissive conditions, the young BCR-ABLP210–transduced pro/pre-B cells had expanded approximately 10-fold after 5 days, whereas no increase in cell number was observed in the cultures established with old B-lineage cells (Figure 3E).
Taken together, these observations indicate that BCR-ABLP210 expression cannot overcome the proliferative and differentiative defects that have accumulated in aged B-lymphoid precursors, and these defects underlie their reduced leukemogenic potential.
Decreased severity of MPDs derived from old BMBCR-ABL
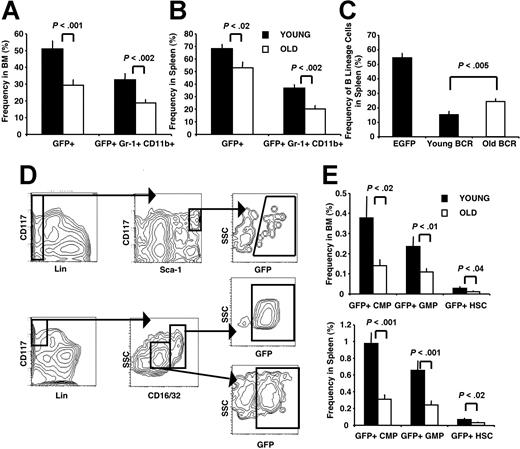
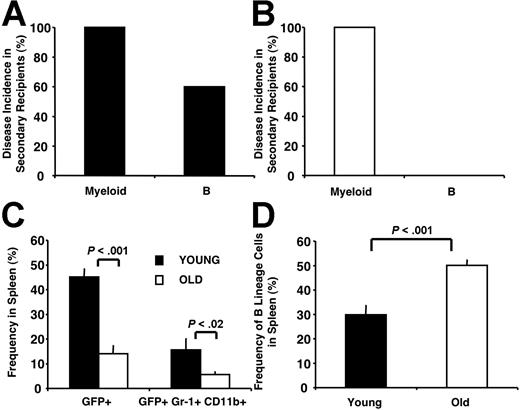
Compared with recipients of old BMBCR-ABL cells, young BMBCR-ABL recipients consistently presented with increased wasting and a more pronounced invasion of organs such as liver and lung with leukemic cells (data not shown). Although young and old BMBCR-ABL recipients had comparable numbers of cells in their BM and spleen (Figure 1B,C), the proportion of cells that were GFP+ was higher in young BMBCR-ABL recipients. For example, the proportion of GFP+ cells in the spleen of young and old BMBCR-ABL recipients was 62% and 52%, respectively, and 47% and 29% in the BM (P < .002), respectively. Because this increased tumor burden in young BMBCR-ABL recipients could have reflected the addition of B-lymphoid leukemia to their MPDs, we compared young and old BMBCR-ABL recipients that developed MPDs without B-lineage involvement. Surprisingly, the difference in tumor burden was accentuated in these animals because of a higher frequency of GFP+Gr-1+CD11b+ cells present in their BM and spleen (Figure 4A,B). This also resulted in tissue disruption as demonstrated by an enhanced displacement of endogenous B-lineage cells in the spleen (Figure 4C).
MPDs derived from old BMBCR-ABL are characterized by a reduced tumor burden. The frequency of total leukemic GFP+ (BCR-ABL+) cells and leukemic myeloid GFP+Gr-1+CD11b+ cells in the BM (A) and spleen (B) of recipients of young BMBCR-ABL cells was increased compared with recipients of old BMBCR-ABL cells. (C) Decreased frequency of B-lineage cells in the spleen of young BMBCR-ABL recipients compared with old BMBCR-ABL recipients. Cell frequency shown in panels A through C is presented as the mean frequency of cells plus or minus SEM from 17 recipients of young and 23 recipients of old BMBCR-ABL that developed MPDs analyzed in 5 independent experiments. (D) Immunostaining used to define populations enriched for leukemic HSCs (top; GFP+Lin−Sca-1HiCD117Hi), CMPs (middle; GFP+Lin−Sca-1−CD127−CD16/32+/LoCD117Hi), and GMPs (bottom; GFP+Lin− Sca-1−CD127−CD16/32+/LoCD117Hi) in the BM and spleen of BMBCR-ABL recipients. (E) Recipients of young BMBCR-ABL cells have more leukemic GFP+ HSCs, CMPs, and GMPs in their BM (top panel) and spleen (bottom panel) compared with those that received a transplant with old BMBCR-ABL cells. The frequency of GFP+ HSCs, CMPs, and GMPs is presented as the mean plus or minus SEM of 12 recipients of young and 13 recipients of old BMBCR-ABL that developed MPDs with no B-lineage involvement.
MPDs derived from old BMBCR-ABL are characterized by a reduced tumor burden. The frequency of total leukemic GFP+ (BCR-ABL+) cells and leukemic myeloid GFP+Gr-1+CD11b+ cells in the BM (A) and spleen (B) of recipients of young BMBCR-ABL cells was increased compared with recipients of old BMBCR-ABL cells. (C) Decreased frequency of B-lineage cells in the spleen of young BMBCR-ABL recipients compared with old BMBCR-ABL recipients. Cell frequency shown in panels A through C is presented as the mean frequency of cells plus or minus SEM from 17 recipients of young and 23 recipients of old BMBCR-ABL that developed MPDs analyzed in 5 independent experiments. (D) Immunostaining used to define populations enriched for leukemic HSCs (top; GFP+Lin−Sca-1HiCD117Hi), CMPs (middle; GFP+Lin−Sca-1−CD127−CD16/32+/LoCD117Hi), and GMPs (bottom; GFP+Lin− Sca-1−CD127−CD16/32+/LoCD117Hi) in the BM and spleen of BMBCR-ABL recipients. (E) Recipients of young BMBCR-ABL cells have more leukemic GFP+ HSCs, CMPs, and GMPs in their BM (top panel) and spleen (bottom panel) compared with those that received a transplant with old BMBCR-ABL cells. The frequency of GFP+ HSCs, CMPs, and GMPs is presented as the mean plus or minus SEM of 12 recipients of young and 13 recipients of old BMBCR-ABL that developed MPDs with no B-lineage involvement.
The increase in leukemic myeloid cells in young BMBCR-ABL recipients was accompanied by an increased frequency and number of GFP+ HSCs, CMPs, and GMPs in the BM and spleen (Figure 4D-E), and splenocytes from young BMBCR-ABL recipients formed more GFP+ colonies than splenocytes from old BMBCR-ABL recipients when tested in myeloid colony assays (data not shown).
This discrepancy in tumor burden did not result from an increased transduction efficiency of young BM cells. After transduction, young and old BM cells were used to establish myeloid colonies in 2 independent experiments. Eight days later, 12 colonies derived from young and old BMEGFP were examined for GFP expression by flow cytometry. The number of young and old derived colonies that contained GFP-expressing cells (8 of 12) was the same.
Identification of myelopoietic defects in old mice
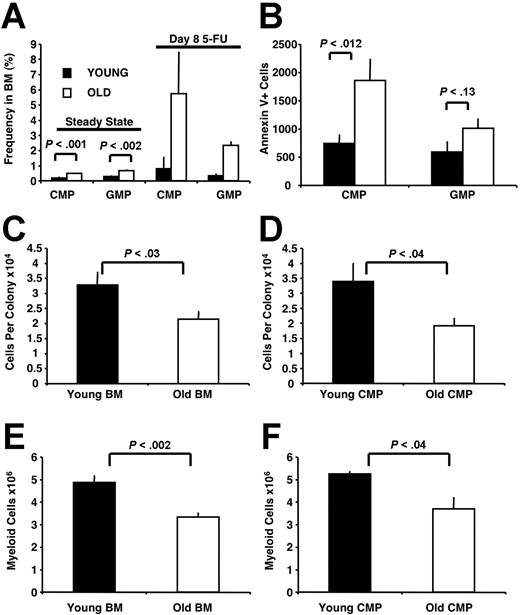
The data discussed above were surprising, because the frequency and absolute number of HSCs and myeloid progenitors was increased in the BM of old mice (Figure 5A).11,19 Therefore, we considered the possibility that aging affected the quality of old myeloid progenitors. Further analysis revealed that although old BM cells formed 1.3-fold more colonies than young BM cells, this value was less than would be predicted from the 2.3-fold increase in myeloid progenitor frequency observed by flow cytometry. This could, in part, be a result of a 2- to 3-fold increase in the number of apoptotic Annexin V+ CMPs and GMPs in old compared with young BM (Figure 5B). In addition, individual colonies derived from old BM cells contained approximately 50% fewer cells than those derived from young BM cells (Figure 5C), and old CMPs similarly generated smaller colonies than their counterparts from young mice (Figure 5D).
Myelopoietic defects are present in old mice. (A) The frequency of CMPs and GMPs is increased in the BM of old (78- to 105-week-old) compared with young (5-week-old) mice both at steady state and at day 8 after 5-FU treatment. Steady-state frequencies are presented as the mean plus or minus SEM of 16 young and 12 old mice analyzed in 4 independent experiments. 5-FU frequencies are presented from the pooled BM of 9 young and 7 old mice analyzed in 2 independent experiments. (B) The number of Annexin V+ CMPs and GMPs was increased in the BM of old compared with young mice. Cell numbers are presented as the mean plus or minus SEM of 12 young and 8 old mice analyzed in 3 independent experiments and normalized per 5 × 104 CMPs or GMPs, respectively. Myeloid colonies derived from whole BM cells (C) and sorted CMPs (D) isolated from young mice contained more cells than those derived from BM and CMPs isolated from old mice. Whole BM cells (5 × 104 per dish) and CMPs (250 per dish) were plated. Cell numbers shown in C are presented as the mean plus or minus SEM of 24 colonies derived from young BM cells and 24 colonies derived from old BM cells picked in 2 independent experiments. Cell numbers shown in D are presented as the mean of 12 colonies derived from young CMPs and 12 colonies derived from old CMPs plus or minus SEM picked in 1 of 2 representative experiments. BM cells (1.5 × 105) (E) and CMPs (2 × 103) (F) plated in liquid culture supplemented with myelopoietic cytokines isolated from young mice produced more Gr-1+CD11b+ myeloid cells compared with their old counterparts. Numbers are presented as the mean plus or minus SEM of 3 to 6 wells analyzed in 1 of 2 representative experiments.
Myelopoietic defects are present in old mice. (A) The frequency of CMPs and GMPs is increased in the BM of old (78- to 105-week-old) compared with young (5-week-old) mice both at steady state and at day 8 after 5-FU treatment. Steady-state frequencies are presented as the mean plus or minus SEM of 16 young and 12 old mice analyzed in 4 independent experiments. 5-FU frequencies are presented from the pooled BM of 9 young and 7 old mice analyzed in 2 independent experiments. (B) The number of Annexin V+ CMPs and GMPs was increased in the BM of old compared with young mice. Cell numbers are presented as the mean plus or minus SEM of 12 young and 8 old mice analyzed in 3 independent experiments and normalized per 5 × 104 CMPs or GMPs, respectively. Myeloid colonies derived from whole BM cells (C) and sorted CMPs (D) isolated from young mice contained more cells than those derived from BM and CMPs isolated from old mice. Whole BM cells (5 × 104 per dish) and CMPs (250 per dish) were plated. Cell numbers shown in C are presented as the mean plus or minus SEM of 24 colonies derived from young BM cells and 24 colonies derived from old BM cells picked in 2 independent experiments. Cell numbers shown in D are presented as the mean of 12 colonies derived from young CMPs and 12 colonies derived from old CMPs plus or minus SEM picked in 1 of 2 representative experiments. BM cells (1.5 × 105) (E) and CMPs (2 × 103) (F) plated in liquid culture supplemented with myelopoietic cytokines isolated from young mice produced more Gr-1+CD11b+ myeloid cells compared with their old counterparts. Numbers are presented as the mean plus or minus SEM of 3 to 6 wells analyzed in 1 of 2 representative experiments.
This age-related reduction in myelopoietic potential was also observed when whole BM cells or sorted CMPs isolated from young and old mice were grown in liquid culture supplemented with myelopoietic cytokines. Consistent with results from the colony assays, BM cells (Figure 5E) and CMPs (Figure 5F) from old mice produced approximately 45% fewer Gr-1+CD11b+ myeloid cells than those isolated from young animals. Taken together, these results demonstrate that aged myeloid progenitors harbor intrinsic proliferative and/or differentiative defects.
Age-related hematopoietic defects alter the potential of leukemia stem cells
Hematopoietic stem and progenitor cells play a critical role in the pathogenesis of CML. HSCs have been implicated as the leukemic cell of origin, whereas committed myeloid and lymphoid progenitors have been deemed the leukemia stem cells (LSCs) in more-advanced stages of disease.36,37,53 Consequently, we assessed whether age-related differences in the pathogenesis of BCR-ABLP210–induced leukemia was a reflection of changes intrinsic to LSCs. Because LSCs are defined by their potential to transplant disease,54,55 splenocytes isolated from diseased mice grafted with young or old BMBCR-ABL were transplanted into sublethally irradiated syngeneic mice. Disease patterns and progression were then analyzed in these secondary recipients.
Secondary recipients developed disease symptoms within 3 weeks after transplantation and were killed. All these mice (27 of 27) presented with MPDs. However, 60% (9 of 15) of the mice grafted with splenocytes from young BMBCR-ABL recipients also presented with B-lymphoid leukemia (Figure 6A). In contrast, none (0 of 12) of the mice grafted with splenocytes from old BMBCR-ABL recipients developed lymphoid disease (Figure 6B). These data suggest that either no B-lymphoid LSCs were produced after BCR-ABLP210 transduction of old hematopoietic cells or, if they were produced, they had intrinsic defects that disrupted their leukemia-initiating potential.
Age-related hematopoietic defects alter the leukemogenic potential of LSCs. (A) Secondary recipients of splenocytes from leukemic mice grafted with young BMBCR-ABL cells developed MPDs and B-lymphoid leukemia. Splenocytes (5 × 106) from primary young BMBCR-ABL recipients were transplanted into secondary recipients (n=15). (B) Secondary recipients of 5 × 106 splenocytes from leukemic mice grafted with old BMBCR-ABL cells (n=12) developed MPDs with no significant involvement of B-lineage cells. (C) The frequency of total leukemic GFP+ cells and leukemic myeloid GFP+ Gr-1+CD11b+ cells in the spleens of secondary recipients of splenocytes from tumors derived from young BMBCR-ABL cells was increased compared with secondary recipients of tumors derived from old BMBCR-ABL cells. (D) The frequency of B-lineage cells in the spleen of secondary recipients of tumors derived from young BMBCR-ABL cells was decreased compared with secondary recipients of tumors derived from old BMBCR-ABL cells. In all transfers, donor splenocytes were transplanted into at least 3 secondary recipients. At least 3 primary recipients were analyzed in each experiment. Cell frequency shown in C-D is presented as the mean frequency of cells plus or minus SEM. The mice analyzed for C-D developed MPDs with no B-lineage involvement.
Age-related hematopoietic defects alter the leukemogenic potential of LSCs. (A) Secondary recipients of splenocytes from leukemic mice grafted with young BMBCR-ABL cells developed MPDs and B-lymphoid leukemia. Splenocytes (5 × 106) from primary young BMBCR-ABL recipients were transplanted into secondary recipients (n=15). (B) Secondary recipients of 5 × 106 splenocytes from leukemic mice grafted with old BMBCR-ABL cells (n=12) developed MPDs with no significant involvement of B-lineage cells. (C) The frequency of total leukemic GFP+ cells and leukemic myeloid GFP+ Gr-1+CD11b+ cells in the spleens of secondary recipients of splenocytes from tumors derived from young BMBCR-ABL cells was increased compared with secondary recipients of tumors derived from old BMBCR-ABL cells. (D) The frequency of B-lineage cells in the spleen of secondary recipients of tumors derived from young BMBCR-ABL cells was decreased compared with secondary recipients of tumors derived from old BMBCR-ABL cells. In all transfers, donor splenocytes were transplanted into at least 3 secondary recipients. At least 3 primary recipients were analyzed in each experiment. Cell frequency shown in C-D is presented as the mean frequency of cells plus or minus SEM. The mice analyzed for C-D developed MPDs with no B-lineage involvement.
In addition, we compared secondary recipients that developed MPDs without B-lineage involvement for disease severity. Leukemic burden was 3-fold greater in secondary recipients that received transplants with young BMBCR-ABL-derived tumors compared with secondary recipients of old BMBCR-ABL-derived tumors (Figure 6C,D). These data demonstrate that myeloid LSCs derived from young BMBCR-ABL have a greater expansive potential through either enhanced self-renewal and/or increased production of mature leukemic myeloid progeny. Taken together, these data indicate that age-related intrinsic hematopoietic defects ultimately alter the leukemogenic potential of LSCs generated after transformation of hematopoietic cells by BCR-ABLP210.
Discussion
This report demonstrates that age-related intrinsic defects that accumulate in B-lineage cells limit lymphoid involvement in CML. This finding provides a biologic explanation for the myeloid predominance of adult-onset leukemia. Although we focused on B-cell development, T-cell production also declines with increasing age,21 which may explain the extreme rarity with which T-cell leukemia presents in older humans.
In the murine CML model used in this study, transformation of young BM cells with BCR-ABLP210 consistently resulted in myeloid and lymphoid disease, whereas transformation of old BM cells primarily resulted in an MPD with no lymphoid involvement. Because the 5-FU BM used in these experiments contained HSCs as well as B-lineage-specified progenitors, B-lymphoid leukemia could have developed from transformation of either population. However, the origin of the leukemia does not influence the interpretation of our results, which demonstrate that age-related intrinsic defects that accumulate in B-lineage cells limit their involvement in CML. This conclusion is supported by the observation that BCR-ABLP210–transduced old B-lineage precursors lack lymphoid-leukemia–initiating potential. Furthermore, expression of a powerful oncogene such as BCR-ABLP210, which greatly enhanced the growth of young B-lineage cells, was unable to significantly augment the growth of old B-cell progenitors in vitro. These data, combined with intrinsic age-related defects in the ability of HSCs to generate lymphoid progeny,19 can explain the clinical presentation of CML. Inefficient production of early lymphoid progenitors from older HSCs, in combination with intrinsic proliferative and developmental defects in the progenitors that are generated, would contribute to a low incidence of lymphoid leukemia and a predominance of myeloid disease in the aged. In contrast, young HSCs efficiently generate lymphoid progeny, which, in turn, are highly susceptible to the effects of BCR-ABLP210 and thereby result in MPD and B-lymphoid leukemia.56
Our finding that B-lymphoid leukemia initiated more frequently with young than old BMBCR-ABL is consistent with the clinical observation that the incidence of B-lineage leukemia is highest in children. That children are predisposed to lymphoid leukemia is consistent with results from murine studies that have demonstrated that B-lineage progenitors from neonates and young adults cycle at levels above their aged counterparts.11 Although this increased proliferation may be necessary to fill peripheral lymphoid compartments, it could increase the chance that an aberrant genetic event could occur, particularly because developing B-lineage cells possess active gene-rearrangement machinery.57 This possibility, combined with the presence of chromosomal translocations in neonatal B-lineage progenitors that cause a predisposition to leukemia development,58 may increase the likelihood of transformation during early B lymphopoiesis.
The observation that MPDs initiated from young BMBCR-ABL cells were more aggressive than those initiated from old BMBCR-ABL cells was quite unexpected. This pattern led to the discovery that defects do, in fact, accumulate in aged myeloid progenitors. Thus, it is reasonable to propose that the milder MPDs are the result of age-related defects in growth and survival of myeloid progenitors and/or their progeny. It is surprising that age-related changes in myeloid progenitors have not been reported. However, previous studies primarily examined myeloid progenitors as a population rather than on a per-cell basis, and as a result, the cell-intrinsic defects defined herein have been overlooked. The age-related defects that accumulate in myeloid progenitors are similar to the those exhibited by aged HSCs, including increased frequency and cycling, decreased survival, and diminished per-cell repopulating potential.22 These observations suggest that age-related defects that are intrinsic to myeloid progenitors may be the result of intrinsic changes in old HSCs. However, whether old HSCs and CMPs share common molecular changes59 remains to be determined.
Although the detrimental effects of advancing age and decreased lymphocyte production on the adaptive immune response have been well documented,23 the consequences of aging on the innate immune system remain largely unexplored.60 The innate immune system is a critical first responder to infection. Despite defects in old myeloid progenitors, their increased number may compensate for the fact that they do not produce progeny as efficiently as their young counterparts, which results in a relatively normal number of mature myeloid cells. However, reports of diminished function of aged neutrophils,61 macrophages,62 and dendritic cells63 indicate that the innate immune response may become significantly compromised with age. Consequently, the unexpected observation that myeloid progenitors from old mice are intrinsically defective suggests that age-related defects may contribute to the overall reduction in myeloid cell function in elderly people.
Although we used CML as a model with which to investigate the larger question of how aging affects leukemia development, the data nevertheless provide new insights into this disease. First, the demonstration that the degree of lymphoid disease mediated by BCR-ABLP210 is related to overall levels of B lymphopoiesis provides an explanation for the paradoxical observation that although CML is considered a stem cell disease, it presents as an MPD with relatively rare lymphoid involvement. Second, age-related defects in myeloid progenitors may explain, in part, why human CML presents with a chronic rather than acute course.64 Finally, we demonstrate that patterns of lymphoid and myeloid disease exhibited in primary recipients of young and old BMBCR-ABL are conserved after transplantation of leukemic cells into secondary recipients. This finding suggests an intrinsic role for senescence in governing the behavior of LSCs and demonstrates the impact of aging on disease development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) (AG-21459) and the US Department of Defense (W81XWHO410795). O.N.W is an investigator of the Howard Hughes Medical Institute. R.A.J.S. is supported by a fellowship from the California Institute for Regenerative Medicine (TI-00005). The University of California, Los Angeles, Flow Cytometry Core Facility is supported by grants from NIH (CA-16042 and AI-28697).
National Institutes of Health
Authorship
Contribution: The study was designed by R.A.J.S., E.M.-R., O.N.W., and K.D.; experiments were performed and data were analyzed by R.A.J.S. with assistance from E.M.-R.; critical reagents were provided by J.M.; and the manuscript was written by R.A.J.S., E.M.-R., and K.D.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth Dorshkind, Department of Pathology and Laboratory Medicine and the Hematopoietic Malignancies Program, Jonsson Comprehensive Cancer Center, David Geffen School of Medicine, University of California, Los Angeles, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail: kdorshki@mednet.ucla.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal