Abstract
Leukocyte migration from the blood into tissues is pivotal in immune homeostasis and in inflammation. During the multistep extravasation cascade, endothelial selectins (P- and E-selectin) and vascular adhesion protein-1 (VAP-1), a cell-surface–expressed oxidase, are important in tethering and rolling. Here, we studied the signaling functions of the catalytic activity of VAP-1. Using human endothelial cells transfected with wild-type VAP-1 and an enzymatically inactive VAP-1 point mutant, we show that transcription and translation of E- and P-selectins are induced through the enzymatic activity of VAP-1. Moreover, use of VAP-1–deficient animals and VAP-1–deficient animals carrying the human VAP-1 as a transgene show a VAP-enzyme activity–dependent induction of P-selectin in vivo. Up-regulation of P-selectin was found both in high endothelial venules in lymphoid tissues and in flat-walled vessels in noninflamed tissues. VAP-1 activity in vivo led to increased P-selectin–dependent binding of lymphocytes to endothelial cells. These data show that the oxidase reaction catalyzed by VAP-1 alters the expression of other molecules involved in the leukocyte extravasation cascade. Our findings indicate cross-talk between adhesion molecules involved in the tethering and rolling of leukocytes and show that VAP-1–dependent signaling can prime the vessels for an enhanced inflammatory response.
Introduction
Coordinated function of the multistep leukocyte extravasation cascade is a prerequisite for leukocyte emigration from the blood into the tissue. Many adhesion and signaling molecules have well-established roles in this process.1,2 On endothelial cells, selectins (P-selectin [CD62P] and E-selectin [CD62E]) mediate tethering of bloodborne cells to vascular endothelium, and the subsequent rolling along the endothelial lining in a shear-dependent manner.3 The rolling cells can be exposed to activating stimuli, such as chemokines, which can trigger firm, integrin-dependent adhesion of the leukocytes in the vessel. Finally, the leukocytes diapedese through the vessel wall using adhesion molecules from immunoglobulin and other superfamilies as well as local protease activity.
In addition to these well-established interplayers, other molecules are involved in leukocyte trafficking. Among these, enzymes expressed on the cell surface that have their catalytic domains outside the plasma membrane (ectoenzymes) have emerging roles in leukocyte migration.4 Vascular adhesion protein-1 (VAP-1, also known as amine oxidase copper containing-3 [AOC3]) is an ectoenzyme that belongs to the specific subgroup of oxidases known as semicarbazide-sensitive amine oxidases (SSAOs).5,6 It catalyzes a reaction in which a primary amine is oxidatively deaminated into an aldehyde, and then hydrogen peroxide and ammonium are released.7,8 VAP-1/SSAO is a bifunctional molecule that can support leukocyte adhesion under shear conditions via enzyme-activity–dependent and enzyme-activity–independent ways.4 Monoclonal anti–VAP-1 antibodies that do not block its oxidase activity effectively block lymphocyte and granulocyte binding to endothelial cells in vitro and in vivo. Small-molecule SSAO enzyme inhibitors, on the other hand, are equally effective in perturbing leukocyte-endothelial contacts in vitro and in vivo.9–14 The ability of the oxidase reaction to regulate the expression and/or function of other molecules involved in the emigration process is largely unknown.
We hypothesized that the different adhesion molecules needed to execute the complex leukocyte extravasation cascade could act in concert. However, there is very little evidence on the cross-talk of endothelial adhesion molecules during the first steps of the leukocyte extravasation cascade. Since all end products of the VAP-1/SSAO catalyzed reaction are biologically active substances, we postulated that they could have signaling effects that could regulate the expression and/or function of endothelial selectins. We found that both E- and P-selectin are induced through VAP-1–dependent signaling, and that this cross-talk has functional consequences also in vivo. These data show that the oxidase activity of VAP-1 can orchestrate the priming of the tethering and rolling steps of the extravasation cascade, and thus can serve to target the inflammatory response to appropriate sites.
Materials and methods
All animal experiments described were approved by the Committee for Animal Experimentation, University of Turku, Finland.
Quantitative PCR
Adenoviral constructs encoding wild-type VAP-1 and enzymatically inactive VAP-1 mutant (Tyr471Phe) have been described.12 Human umbilical vein endothelial cells (HUVECs; third passage) were grown to confluency and infected with the constructs at multiplicity of infection of 300. After a 5-hour culturing, the medium was changed, and the cells were subjected to 1 mM methylamine (a VAP-1/SSAO substrate) for 0, 0.5, 1, 2, 4, 8, and 12 hours. Total RNA was then isolated from the cells using Qiagen RNeasy Mini kit (Qiagen, Valencia, CA). After a DNAse treatment (RQ1 DNase; Promega, Madison, WI), cDNA synthesis was performed using iScript cDNA synthesis Kit from Bio-Rad (Hercules, CA). Taqman Fluorogenic 5′ nuclease assays using gene-specific 5′ FAM-labeled probes run on an ABI Prism 7700 sequence detector (also from Applied Biosystems, Foster City, CA) were used to analyze relative expression levels of E- and P-selectin mRNA. Human GAPDH was used as an internal control to which the threshold cycle (Ct) values of the target gene were normalized. Differential expression levels were calculated according to the 2−ΔΔCt method.
Formaldehyde, ammonium, and hydrogen peroxide are produced during the VAP-1–catalyzed deamination of methylamine.7 To study the role of these end products in induction of selectins, nontransfected (VAP-1−) HUVECs were exposed to 1 and 10 μM hydrogen peroxide, ammonium, or formaldehyde for 4 hours before the cells were processed for mRNA analyses as in the previous paragraph. We also subjected cells to repeated dosing of H2O2 (8 × 10 μM at 30-minute intervals) and to a combination of all 3 end products (single dose).
FACS stainings
Cell-surface stainings of HUVECs were performed using anti–VAP-1 (TK8–14), anti–P-selectin (WAPS2.12), anti–E-selectin (P2H3), and negative control (3G6) mAbs. FITC-conjugated rabbit anti-mouse IgG was used as the second-step reagent.
For platelet analyses, platelet-rich plasma was separated form citrate-anticoagulated mouse blood by low-speed centrifugation as described.15 Nonpermeabilized cells were stained for mouse P-selectin (anti-CD62P–FITC), gp IIb (anti-CD41–FITC), and negative controls (rat IgG1-FITC). All cells were analyzed using FACSCalibur and Cellquest software (Becton Dickinson, San Jose, CA).
Metabolic labelings
Pulse-chase experiments with starved HUVECs and human dermal microvascular endothelial cells (HDMECs) were done using a 45-minute pulse with [35S]-Translabel (ICN, Irvine, CA) followed by a chase in a methionine-containing RPMI as described.16 At the different time points, the supernatants were collected. The labeled cells were then washed and lysed in the immunoprecipitation buffer. The supernatant and lysate samples were precleared with protein A beads. Thereafter, the samples were incubated with protein A beads armed with specific mAbs against P- and E-selectin. After washings the antigens were eluted, loaded on SDS-PAGE gels, and processed for autoradiography. In all experiments, irrelevant antibodies were used as negative controls.
VAP-1–deficient and humanized VAP-1 mice
VAP-1–deficient mice (129S6 background), and VAP-1 transgenic animals (FVB/N background) expressing human VAP-1 on the endothelial cells under the control of the mouse tie-1 promoter have been described.17,18 VAP-1–deficient mice expressing human VAP-1 were produced by crossing these 2 strains. Heterozygote offspring animals (mouse VAP-1+/−, human VAP-1+/−) were identified using polymerase chain reaction (PCR) and backcrossed once with the VAP-1–deficient mice. The offspring deficient for the mouse VAP-1 but carrying the human transgene were then mated with those lacking both the mouse and human VAP-1 for further breedings. Sex- and age-matched mouse VAP-1−/−, human VAP-1−/− (VAP-1 knockout [KO]) animals and their mouse VAP-1−/−, human VAP-1+/− (VAP-1 KO-transgenic [TG]) littermates were then used in the in vivo studies.
VAP-1–dependent signaling in vivo
Methylamine (0.4%), a VAP-1 substrate, was administered in the drinking water to the VAP-1 KO and VAP-1 KO-TG animals for 3 or 14 days. After killing, multiple tissues were excised and snap-frozen. Frozen sections were cut and stained using indirect immunofluorescence for human VAP-1 (TK8–14), mouse P-selectin (polyclonal anti-human CD62P [Becton Dickinson] and RB40.34 [Pharmingen, San Diego, CA]) and mouse E-selectin (sc-14011; Santa Cruz Biotechnology, Santa Cruz, CA) along with isotype-matched control antibodies and appropriate FITC-conjugated second-stage reagents using a previously described protocol.5 Images were obtained using an Olympus BX60 microscope (Olympus Optical, Hamburg, Germany) equipped with a UPlanF1 20×/0.50 numeric aperture (NA) Ph1 or UPlanF1 40×/0.75 NA Ph2 objective. Images were acquired using a ColorView 12 camera (Olympus Soft Imaging Solutions, Münster, Germany). The sections were scored blindly and semiquantitatively for the expression of selectins in vessels (scale 0-3; 0 = negative, 3 = many positive vessels).
Binding assays
In vitro frozen section binding assays were done as described19 using lymph nodes from VAP-1 KO and VAP-1 KO-TG mice (see “VAP-1–deficient and humanized VAP-1 mice”) that either received or did not receive methylamine in the drinking water. When indicated, the tissue sections were pretreated with function-blocking anti–P-selectin and control mAbs for 30 minutes before adding wild-type lymphocytes isolated from mesenteric lymph nodes. The number of high endothelial venule (HEV)–bound lymphocytes was counted, and expressed as percentage of control binding. The number of lymphocytes bound per HEV in VAP-1 KO mice not treated with methylamine (4.9 ± 0.4 lymphocytes/HEV [mean ± SEM]) was chosen to define 100% binding. At least 100 HEVs in each group were counted per assay.
Statistical analyses
The numbers of independent experiments and the numbers of mice/group are indicated in the figures and legends. Student t test (unpaired, 2-tailed) was used to compare numerical variables between 2 groups.
Results
Time-dependent induction of P- and E-selectin mRNA by VAP-1/SSAO activity
To study the signaling effects of VAP-1/SSAO, we took advantage of adenoviral constructs encoding for wild-type and enzymatically inactive VAP-1. The complete loss of catalytic activity of VAP-1 is obtained through a point mutation (Tyr471 into Phe), and it does not affect the overall structure or the short cytoplasmic tail of VAP-1.12 Primary human endothelial cells infected with the viruses showed VAP-1 expression on the cell surface on more than 95% of the cells, and the mean fluorescence intensity (MFI) of the VAP-1 signal was similar with both constructs (MFI, 221 ± 39 for wild-type and 213 ± 38 for the enzymatically inactive mutant; n = 10 for both groups; Figure 1A). The cells were then subjected to a VAP-1/SSAO substrate (methylamine), and analyzed for induction of endothelial selectins at the mRNA level. This approach allows exclusion of any potential confounding effects caused by introduction of a chemical, such as VAP-1/SSAO substrate or inhibitor, to living cells.
Time-dependent induction of E- and P-selectin transcription through VAP-1/SSAO signaling. (A) HUVECs were infected with wild-type (VAP-1) or an enzymatically inactive mutant of VAP-1 (VAP-1 Y471F) and stained with anti–VAP-1 and negative control mAbs for fluorescence-activated cell sorter (FACS) analyses. (B) The transfected cells were analyzed for E-selectin and P-selectin mRNA expression after methylamine administration using real-time PCR. Relative expression levels (means ± SEM) at the indicated time points are shown. Three samples of HUVECs from different individuals were used for each time point. *P < .05
Time-dependent induction of E- and P-selectin transcription through VAP-1/SSAO signaling. (A) HUVECs were infected with wild-type (VAP-1) or an enzymatically inactive mutant of VAP-1 (VAP-1 Y471F) and stained with anti–VAP-1 and negative control mAbs for fluorescence-activated cell sorter (FACS) analyses. (B) The transfected cells were analyzed for E-selectin and P-selectin mRNA expression after methylamine administration using real-time PCR. Relative expression levels (means ± SEM) at the indicated time points are shown. Three samples of HUVECs from different individuals were used for each time point. *P < .05
As shown in Figure 1B, a 5-fold increase in E-selectin mRNA was detectable after a 2-hour incubation of the HUVECs expressing the enzymatically active VAP-1 with the substrate but not in cells expressing the enzymatically inactive form of VAP-1. VAP-1/SSAO–dependent induction of E-selectin was still seen at the 4-hour time point. The increase was transient, however, since at later time points methylamine had no VAP-1–dependent effects on E-selectin mRNA synthesis any longer. P-selectin mRNA synthesis was also transiently elevated by more than 100% 4 hours after the administration of the substrate (Figure 1B), although the response was more variable between different HUVEC isolates. Induction of E- and P-selectin mRNA was also observed upon methylamine treatment in HDMECs transfected with the wild-type VAP-1 (data not shown). Thus, VAP-1/SSAO signaling results in a transient rise in the transcription of P- and E-selectin mRNA in primary human endothelial cells after 2 to 4 hours.
VAP-1–generated hydrogen peroxide induces expression of selectins
We hypothesized that the effects of VAP-1 on induction of the selectins is mediated via the enzymatic activity of VAP-1. Therefore, we studied the effects of methylamine metabolites (hydrogen peroxide, ammonium, and formaldehyde) produced via VAP-1–catalyzed reaction in a system that circumvents VAP-1 activity. To that end we treated normal HUVECs, which do not express VAP-1 on the surface and which are devoid of all VAP-1/SSAO activity,12 with these methylamine metabolites. The results showed that E-selectin expression was not affected by single low doses of hydrogen peroxide, ammonium, or formaldehyde. However, E-selectin was slightly induced by H2O2 when it was administered repeatedly (to compensate for the extremely short half-life of exogenously administered H2O2 in biological systems). Notably, the combination of all 3 end products induced prominent (> 5-fold) E-selectin expression even when used at 1 μM.
The ability of the end products of the VAP-1/SSAO–catalyzed reaction to induce P-selectin was weaker and more variable. However, repeated doses of H2O2 caused a small induction (Figure 2B). It was comparable with the level seen when cells transfected with enzymatically active VAP-1 were exposed to methylamine (Figure 1C). Thus, hydrogen peroxide or low doses of hydrogen peroxide, ammonium, and aldehyde in combination are the most likely mediators of VAP-1/SSAO enzyme activity in the induction of selectins.
End products of VAP-1 enzyme activity regulate expression of endothelial selectins. Nontransfected HUVECs were treated with single doses of hydrogen peroxide, ammonium, and formaldehyde (the end products of VAP-1–mediated oxidative deamination of methylamine) at the indicated concentrations for 4 hours. When indicated, the cells were treated repeatedly with 10 μM H2O2 (8 times at 30-minute intervals) or with the combination of all 3 end products (each at 1 or 10 μM, single dose). The cells were then analyzed for (A) E-selectin and (B) P-selectin mRNA expression using quantitative PCR. Data points (○) and means (bars) from 4 to 6 samples of HUVECs from different individuals are shown. *P < .05, **P < .01
End products of VAP-1 enzyme activity regulate expression of endothelial selectins. Nontransfected HUVECs were treated with single doses of hydrogen peroxide, ammonium, and formaldehyde (the end products of VAP-1–mediated oxidative deamination of methylamine) at the indicated concentrations for 4 hours. When indicated, the cells were treated repeatedly with 10 μM H2O2 (8 times at 30-minute intervals) or with the combination of all 3 end products (each at 1 or 10 μM, single dose). The cells were then analyzed for (A) E-selectin and (B) P-selectin mRNA expression using quantitative PCR. Data points (○) and means (bars) from 4 to 6 samples of HUVECs from different individuals are shown. *P < .05, **P < .01
VAP-1/SSAO–dependent synthesis of P- and E-selectin proteins
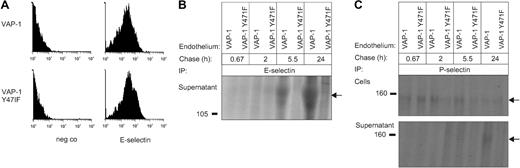
We then determined whether the VAP-1/SSAO–triggered increase in mRNA synthesis of endothelial selectins translates into the protein level. To our surprise, we did not find any VAP-1–dependent increase in E-selectin expression on either HUVECs or HDMECs upon methylamine treatment (Figure 3A). Pulse-chase assays showed that there is no detectable increase in the amount of E-selectin inside the cells either (data not shown). In contrast, a time-dependent induction in the formation of soluble E-selectin in the cell-culture supernatants from cells expressing the wild-type VAP-1 but not in those from cells expressing the enzymatically inactive VAP-1 was evident (Figure 3B). Similar results were obtained using HDMECs (data not shown). However, VAP-1/SSAO–triggered induction of E-selectin was clearly weaker than that observed by TNF-α induction (data not shown).
VAP-1/SSAO–dependent induction of selectin proteins in primary human endothelial cells. (A) HDMECs transfected with wild-type (VAP-1) or an enzymatically inactive mutant of VAP-1 (VAP-1 Y471F) were stained for cell-surface E-selectin and analyzed using FACS. neg co indicates negative control. (B) E-selectin was immunoprecipitated (IP) from the cell-culture supernatant of the transfected cells after a 45-minute pulse and chase of the indicated length. (C) P-selectin was immunoprecipitated from the cell lysate and from the cell-culture supernatant after a 45-minute pulse and chase of the indicated time. Arrows on the right point to the specific immunoprecipitates, and molecular weight markers in kDa are shown on the left.
VAP-1/SSAO–dependent induction of selectin proteins in primary human endothelial cells. (A) HDMECs transfected with wild-type (VAP-1) or an enzymatically inactive mutant of VAP-1 (VAP-1 Y471F) were stained for cell-surface E-selectin and analyzed using FACS. neg co indicates negative control. (B) E-selectin was immunoprecipitated (IP) from the cell-culture supernatant of the transfected cells after a 45-minute pulse and chase of the indicated length. (C) P-selectin was immunoprecipitated from the cell lysate and from the cell-culture supernatant after a 45-minute pulse and chase of the indicated time. Arrows on the right point to the specific immunoprecipitates, and molecular weight markers in kDa are shown on the left.
P-selectin, on the other hand, was mainly induced within the endothelial cells. In this case, the peak of up-regulation occurred after a 2- to 5-h chase in the cells transfected with the wild-type VAP-1 when compared with cells transfected with the catalytically inactive VAP-1 mutant (Figure 3C). Moreover, a small amount of soluble P-selectin was also recovered from the culture supernatant of cells expressing the active VAP-1 at later time points (Figure 3C). These data thus show that the protein synthesis of both E- and P-selectin is triggered in primary endothelial cells in a VAP-1/SSAO–dependent manner.
VAP-1/SSAO enzyme activity induces endothelial P-selectin expression in vivo
To elucidate the in vivo relevance of the VAP-1/SSAO–dependent induction of selectins, we used VAP-1–deficient mice (VAP-1 KO) and generated a VAP-1–deficient mouse line carrying the human VAP-1 cDNA as a transgene in endothelial cells (VAP-1 KO-TG). As expected, VAP-1 KO animals lack all VAP-1 protein. In contrast, in VAP-1 KO-TG animals, human VAP-1 is prominently expressed on many, but not all vessels, thus recapitulating the in vivo situation in normal animals (Figure 4A). Using this genetic model system, we were able to control for all possible nonspecific effects that may potentially take place when an enzyme substrate or inhibitor is used in vivo.
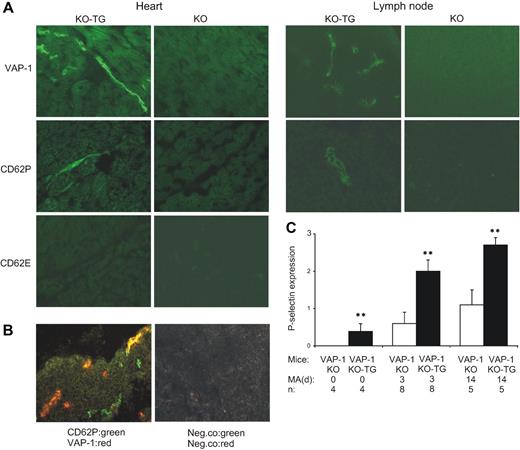
In vivo induction of endothelial P-selectin protein by VAP-1/SSAO. (A) VAP-1–deficient mice (KO) and VAP-1–deficient mice expressing human VAP-1 as a transgene (KO-TG) were fed 0.4% methylamine for 3 days in their drinking water. The expression of human VAP-1, mouse CD62P (P-selectin), and mouse CD62E (E-selectin) protein was stained using immunohistochemistry from the heart and mesenteric lymph node. (B) Partial overlap in VAP-1 and P-selectin expression (yellow) is seen in 2-color immunofluorescence images. (C) Quantitation of P-selectin expression in the mesenteric lymph nodes of VAP-1 KO mice and VAP-1 KO-TG mice that received methylamine (MA) in the drinking water for the indicated time period (days). Data (means ± SEM) are shown as semiquantitative scores ranging from 0 (negative) to 3 (high expression). n = number of mice per group. **P < .01.
In vivo induction of endothelial P-selectin protein by VAP-1/SSAO. (A) VAP-1–deficient mice (KO) and VAP-1–deficient mice expressing human VAP-1 as a transgene (KO-TG) were fed 0.4% methylamine for 3 days in their drinking water. The expression of human VAP-1, mouse CD62P (P-selectin), and mouse CD62E (E-selectin) protein was stained using immunohistochemistry from the heart and mesenteric lymph node. (B) Partial overlap in VAP-1 and P-selectin expression (yellow) is seen in 2-color immunofluorescence images. (C) Quantitation of P-selectin expression in the mesenteric lymph nodes of VAP-1 KO mice and VAP-1 KO-TG mice that received methylamine (MA) in the drinking water for the indicated time period (days). Data (means ± SEM) are shown as semiquantitative scores ranging from 0 (negative) to 3 (high expression). n = number of mice per group. **P < .01.
Endogenous VAP-1 substrates circulate in blood at low concentration.20 To test if the presence of VAP-1 substrates could alter selectin expression in vivo, we gave additional substrate (methylamine) to the mice in drinking water. After 3 days, a prominent induction of P-selectin expression was evident in VAP-1 KO-TG mice, but not in VAP-1 KO mice (Figure 4A). P-selectin was found in the small vessels of several organs, including heart and intestine. In peripheral lymph nodes, P-selectin expressing HEVs were detected. Two-color immunostainings showed that many P-selectin+ vessels coexpressed VAP-1, but that not all VAP-1+ vessels became P-selectin+ (Figure 4B). When the expression of P-selectin was semiquantitatively scored in the lymph nodes of the methylamine-treated animals, the VAP-1 KO-TG animals scored more than 3 times higher than VAP-1 KO animals (Figure 4C). When the animals received methylamine for 14 days in the drinking water, persistent P-selectin expression was still evident in the heart and other organs. Even under normal conditions (without methylamine treatment), more P-selectin expression was found in VAP-1 KO-TG mice than in VAP-1 KO mice.
Occasional E-selectin+ vessels were also detected in methylamine-fed VAP-1 KO and VAP-1 KO-TG animals using immunohistochemistry. However, the induction appeared not to be VAP-1 dependent. Enzyme-linked immunosorbent assays (ELISAs) showed no increase in soluble E-selectin in serum in methylamine-fed VAP-1 KO-TG animals (34.1 ± 1.4 ng/mL in VAP-1 KO, and 37.7 ± 3.7 ng/mL in VAP-1 KO-TG; n = 3/group). Together, our data thus suggest that E-selectin can be induced via VAP-1 in human but not in mouse endothelial cells, and that VAP-1/SSAO activity mainly regulates P-selectin expression in vivo in mice.
VAP-1/SSAO activity does not alter P-selectin on platelets in vivo
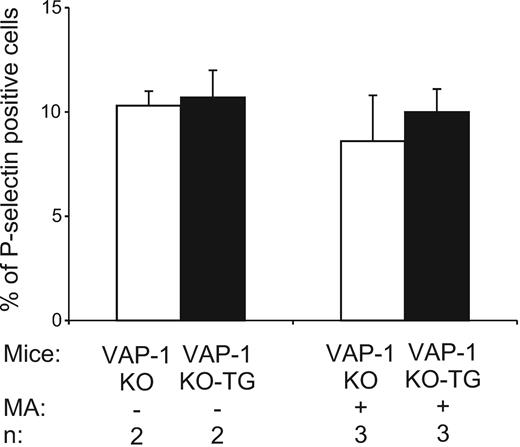
P-selectin is expressed intracellularly in all platelets, and on the platelet surface in activated cells.21 Therefore, we also isolated platelets from VAP-1 KO and VAP-1 KO-TG mice that received methylamine in vivo and analyzed their P-selectin levels. However, there was no difference in the number of platelets displaying P-selectin on their surface between the 2 groups with or without methylamine treatment (Figure 5).
VAP-1/SSAO does not induce P-selectin in platelets. Expression of P-selectin on the surface of platelets in resting and methylamine-fed (3 days) VAP-1 KO and VAP-1 KO-TG mice was determined. The data are shown as percentage of cells expressing P-selectin on the cell surface (means ± SEM).
VAP-1/SSAO does not induce P-selectin in platelets. Expression of P-selectin on the surface of platelets in resting and methylamine-fed (3 days) VAP-1 KO and VAP-1 KO-TG mice was determined. The data are shown as percentage of cells expressing P-selectin on the cell surface (means ± SEM).
VAP-1/SSAO–dependent induction of P-selectin results in enhanced leukocyte binding
We finally tested whether the in vivo induction of endothelial P-selectin expression through VAP-1 signaling is functionally relevant in terms of leukocyte trafficking. Adhesion assays showed that significantly more lymphocytes bound per HEV in lymph nodes obtained from VAP-1 KO-TG mice than in those from VAP-1 KO animals (Figure 6A). Moreover, when methylamine was administered to VAP-1 KO-TG mice, it significantly increased lymphocyte binding to HEVs compared with VAP-1 KO-TG mice that had not received the substrate. Methylamine treatment did not statistically significantly increase lymphocyte binding in VAP-1 KO mice. These data show that VAP-1/SSAO–dependent signaling increases the adhesiveness of endothelial cells for leukocytes, possibly via induction of P-selectin and other adhesion molecules.
P-selectin induced by VAP-1 is functional in vivo. (A) Frozen section binding assays were used to analyze lymphocyte binding to HEVs in mesenteric lymph nodes obtained from VAP-1 KO mice and VAP-1 KO-TG mice, which were treated or not treated with methylamine in the drinking water. Lymphocytes were from nontreated wild-type animals. The results are shown as relative adhesion ratios (mean ± SEM), in which the number of HEV-bound lymphocytes in VAP-1 KO in the absence of methylamine treatment is 1.0 by definition. (B) The function of P-selectin was blocked by preincubating the sections with anti–P-selectin antibody (or control antibody) before the binding assays. The results are shown as percentage of control binding (number of HEV-bound lymphocytes in the presence of nonblocking control mAb) (mean ± SEM). n = number of mice in each group. *P < .05, **P < .01.
P-selectin induced by VAP-1 is functional in vivo. (A) Frozen section binding assays were used to analyze lymphocyte binding to HEVs in mesenteric lymph nodes obtained from VAP-1 KO mice and VAP-1 KO-TG mice, which were treated or not treated with methylamine in the drinking water. Lymphocytes were from nontreated wild-type animals. The results are shown as relative adhesion ratios (mean ± SEM), in which the number of HEV-bound lymphocytes in VAP-1 KO in the absence of methylamine treatment is 1.0 by definition. (B) The function of P-selectin was blocked by preincubating the sections with anti–P-selectin antibody (or control antibody) before the binding assays. The results are shown as percentage of control binding (number of HEV-bound lymphocytes in the presence of nonblocking control mAb) (mean ± SEM). n = number of mice in each group. *P < .05, **P < .01.
To formally show that P-selectin contributes to the enhanced adhesion of lymphocytes to HEVs in VAP-1 KO-TG lymph nodes, the tissue sections from the methylamine-treated animals were preincubated with anti–P-selectin and control antibodies before adding the lymphocytes onto the frozen sections. These binding assays revealed that a function blocking anti–P-selectin mAb had no inhibitory effect in the VAP-1 KO lymph nodes (Figure 6B). In contrast, it efficiently inhibited the number of adhering lymphocytes in the VAP-1 KO-TG sections. These results correlate well with the observed expression pattern of P-selectin after VAP-1–triggered signaling in vivo. Moreover, they show that the in vivo induction of functional P-selectin through VAP-1–dependent mechanisms can enhance lymphocyte binding to vessels.
Discussion
We have shown here a new signaling function for the endothelial ectoenzyme VAP-1/SSAO. Provision of an amine substrate for VAP-1 results in the induction of E- and P-selectin mRNA and protein in primary human endothelial cells. Moreover, VAP-1–dependent induction of P-selectin in venules is observed in vivo in mice, and the induced P-selectin is functionally active in supporting leukocyte adhesion. Induction of endothelial selectins by the VAP-1/SSAO substrate is specific since it does not occur in the presence of enzymatically inactive VAP-1 mutant in vitro or in the absence of VAP-1 in vivo. Thus, the enzymatic activity of VAP-1 can prime the endothelial microenvironment of vessels for enhanced leukocyte extravasation.
Since the endothelial selectins are constitutively active, their function is regulated by the availability of these proteins on the endothelial cell surface.3 Both endothelial selectins are responsive to multiple soluble mediators of inflammation at the transcriptional and translational level.22–27 In addition to the soluble factors, ligation of CD40 on endothelial cells by leukocyte CD40L induces expression of E-selectin and other adhesion molecules.28,29 Our results thus indicate that the expression of these endothelial selectins can be induced through the enzymatic activity of the adhesion molecule VAP-1 that is involved in the extravasation cascade itself. Moreover, VAP-1 activity has recently also been shown to induce adhesion-related molecules in human hepatic endothelial cells.30 Interestingly, cross-talk between adhesion molecules, either through ligand-induced signaling effects or through physical interactions, has been shown to play a critical role at many stages during the later steps of the extravasation cascade. For instance, ligation of P-selectin,31 E-selectin,32 or CD3133 induces activation of leukocyte CD18 integrins. The SSAO-dependent induction of endothelial selectins now extends the cross-talk between adhesion molecules to the very first steps of the emigration cascade.
Our data show that VAP-1/SSAO dependent oxidase reaction results in induction of endothelial E- and P-selectin. The SSAO reaction results in the generation of bioactive compounds (aldehydes, ammonium, and hydrogen peroxide).7 Among the end products, hydrogen peroxide is an emerging signaling molecule.34 We show that hydrogen peroxide alone or in combination with other end products is the main mediator of VAP-1 enzyme activity on the expression of selectins at low concentrations that are produced via VAP-1 in cells. In fact, administration of exogenous hydrogen peroxide (at much higher concentrations) has been found to induce P-selectin–dependent rolling in mice,35 but the endogenous source of this reactive oxygen species has remained unknown. Moreover, antioxidants can block constitutive and cytokine-inducible P-selectin expression in human endothelial cells in vitro.36 Moreover, the absence of P-selectin induction on platelets suggests that the VAP-1–dependent induction of P-selectin is a paracellular event that does not result in general activation of cells within the vasculature.
We showed VAP-1–dependent induction of E-selectin in endothelial cells of human origin, but not in those of mouse origin. Differential responsiveness of human and mouse endothelial cells to different cytokines in terms of induction of endothelial selectins has been described earlier.27 In the case of E-selectin, the main effect of VAP-1 signaling was to induce production of the soluble form of this adhesion molecule. Soluble E-selectin can regulate leukocyte traffic by competing with the cell-membrane–bound forms for the ligands.37 However, recombinant soluble E-selectin is a relatively weak inhibitor of E-selectin–mediated adhesion.38 Therefore, the ability of soluble selectins to trigger signals in the ligand-bearing cells may be more important. Soluble E-selectin, for instance, activates neutrophil β2-integrins, resulting in increased adhesion.32 Therefore, VAP-1–dependent induction of soluble E-selectin may also serve to augment the inflammatory reaction.
VAP-1/SSAO has a cytoplasmic tail of only 4 (human) or 5 (mouse) amino acids, and it does not have classical signal transduction domains.6 Our data show that it can nevertheless be considered as a bona fide signaling molecule due to its oxidase activity. In fact VAP-1/SSAO and other ectoenzymes introduce a new dimension into the regulation of leukocyte extravasation.4 As enzymes, their function is regulated by the availability of the substrates, natural inhibitors, activity and affinity of the enzyme, and by the secondary effects of the reaction products. Moreover, the reactions are extremely fast and suitable for signal amplification. Also, ectoenzymes appear to form networks and pathways in a similar manner to intracellular enzymes, introducing multiple points of control into the extravasation process. The genetic approaches taken here are pivotal in addressing the role of SSAO activity in cell-based assays in vitro and in vivo, inasmuch as the use of chemical SSAO inhibitors and substrates inavoidably will lead to other non-SSAO–dependent effects as well.
VAP-1 can also mediate lymphocyte traffic by functioning as an adhesion molecule by itself. The evidence for the direct role of VAP-1 in adhesion comes from experiments using anti–VAP-1 mAbs. These antibodies, which do not inhibit SSAO activity at all, are nevertheless good inhibitors of leukocyte extravasation both in vitro and in vivo.9–14 Thus, VAP-1 operates both as an adhesion molecule and as an enzyme, the catalytic activity of which can regulate the expression of other adhesion molecules on the endothelial lining.
In conclusion, our data reveal a novel way of cross-talk between the different endothelial adhesion molecules involved in the multistep extravasation cascade. The cross-talk between VAP-1 and endothelial selectins opens new ways for priming and amplification of the inflammatory reaction at the site of insult. Hence, in humans, VAP-1 signaling may augment local leukocyte rolling through induction of endothelial P-selectin and leukocyte activation through binding of the induced soluble E-selectin to leukocytes. These results thus suggest that targeting of VAP-1 activity by small-molecule inhibitors could not only block the adhesive functions of VAP-1 but would also be useful in down-modulating selectin-mediated leukocyte entry at sites of inappropriate inflammation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Seppo Ylä-Herttuala for production of adenoviruses, and Ms Sari Mäki and Ellinoora Salokannel for expert technical help. Supported by the Finnish Academy and Sigrid Juselius Foundation.
Authorship
Author contributions: S.J. designed and performed research and analyzed data; M.K., N.M., K.K., T.H., K.E., and K.S. performed research; and M.S. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marko Salmi, MediCity Research Laboratory, Tykistökatu 6A, 20520 Turku, Finland; e-mail: marko.salmi@utu.fi.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal