Abstract
An HIV antibody (Ab) against platelet integrin GPIIIa49-66 induces complement-independent platelet particle formation by the elaboration of reactive oxygen species (ROS) downstream of the activation of the platelet NADPH oxidase by the 12-lipoxygenase (12-LO) product 12(S)-HETE. To determine whether other inducers of platelet particle formation also function via the induction of ROS, we examined the effects of the Ca2+ ionophore A23187 and phorbol myristate acetate (PMA). Both agents induced oxidative platelet particle formation in an identical fashion as Ab, requiring Ca2+ flux and 12(S)-HETE production as well as intact NADPH oxidase and 12-LO pathways. Since HIV-ITP patients with this Ab correct their platelet counts with dexamethasone (Dex), we examined the role of this steroid in this unique autoimmune disorder. Dex at therapeutic concentrations inhibited Ab-, A23187-, or PMA-induced platelet particle formation by inhibiting platelet PLA2, 12-LO, and NADPH oxidase. The operational requirement of translocation of PLA2, 12-LO, and NADPH oxidase components (p67 phox) from cytosol to membrane for induction of ROS was both inhibited and partially reversed by Dex in platelets. We conclude that (1) platelet particle formation can be induced by the generation of ROS; and (2) platelet PLA2, 12-LO, NADPH oxidase, and cytosol membrane translocation, requirements for ROS production, are inhibited by Dex.
Introduction
Patients with HIV-1–related thrombocytopenia (HIV-ITP) have a unique immunodominant antibody (Ab) against the platelet surface glycoprotein GPIIIa49-66. The presence of this Ab induces human and mouse platelet fragmentation (particle formation) induced by oxidative/fragmentation in vitro and in vivo in the absence of complement1 and correlates inversely with platelet count (r=0.7).2 Rabbit Ab raised against this epitope induces platelet particle formation that is indistinguishable from that induced by HIV–ITP anti–GPIIIa49-66 Ab.1 Platelet oxidation is induced by H2O2 generated by platelet NADPH oxidase, a pathway that is downstream of the platelet 12-lipoxygenase (12-LO).3 Ab-induced platelet oxidation/fragmentation and thrombocytopenia does not occur in mice that are deficient in the NADPH oxidase (p91phox−/−) or lack 12-LO (12 LO−/−). 12(S)-HETE, the 12-LO product, alone is sufficient to induce oxidative/fragmentation and particle formation in normal platelets but fails to do so in platelets from NADPH oxidase–deficient mice. In contrast, 12(S)-HETE–induced particle formation is normal in platelets from 12-LO–deficient animals.3
The NADPH oxidase of granulocytes/macrophages is composed of 5 major components that coalesce onto the cell or vacuolar membrane to form an active electron donor that generates superoxide (O2−).4 Three cytoplasmic phox components (p47phox, p67phox, and p40phox) translocate to the cytoplasmic surface of the membrane5–9 in independent association with activated Rac G protein. Rac binds to p67phox10 and they then bind to 2 membrane-localized components, gp91phox and p22phox, the α and β subunits of the cytochrome b complex.11,12 This complex can bind NADPH and flavin adenine dinucleotide (FAD).4,13,14 Activation of several signaling enzymes is required to activate the oxidase after binding of various physiologic ligands (fMLP, C5a, PAF, leukotriene B4 [LTB4], IL-8) to pertussis toxin (PTX)–sensitive G-protein–coupled receptors.15 Furthermore, activation depends upon lipid mediators such as phosphatidic and arachidonic acids and phosphatidylinositol.16–18 The enzymes involved include PI3K, whose products form a scaffold for membrane attachment of p40phox and p47phox19–21 ; protein kinase C, which phosphorylates p47phox, enabling its translocation to the membrane16,22,23 ; and cytosolic phospholipase A2 (cPLA2), which generates arachidonic acid from membrane phospholipids,24 serving to activate the association of p47phox with p22phox.16 Extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (p38 MAPK) are required for the phosphorylation and activation of cPLA2.25–28 In both phagocytic and nonphagocytic cells, cPLA2 participates in the generation of LTB4, which appears to be required for reactive oxygen species (ROS) generation and chemotaxis.29 Leukotrienes are products of arachidonic acid metabolism by the 5-lipoxygenase (5-LO). The relationship between LTB4 and NADPH oxidase is poorly understood. Platelets, unlike granulocytes, do not have a 5-LO and thus do not produce leukotrienes.30 However, platelets do express the 12-LO, which produces 12(S)-HETE from arachidonic acid.30 This product is related to the activation of the oxidase in these cells.
We recently provided preliminary evidence that both phorbol myristate acetate (PMA) and the Ca2+ ionophore A23187 induced platelet particle formation that was dependent upon NADPH oxidase and 12-LO activation.31 These observations raised the possibility that Ab-induced particle formation could result from intracellular Ca2+ signaling and protein kinase C (PKC) activation.
In classic autoimmune idiopathic thrombocytopenia (AITP), Ab-opsonized platelets are destroyed by interacting with macrophage Fc-γ receptors. These patients respond well to glucocorticoids. Patients with HIV-ITP also respond dramatically to treatment with glucocorticoids,32 although the mechanism that leads to platelet destruction in this case clearly differs from AITP. Therefore, we hypothesized that H2O2-induced particle formation induced by Ab, A23187, or PMA might also be inhibited by glucocorticoids. Such proved to be the case. The present report demonstrates that (1) platelet particle formation is also induced by A23187 as well as PMA; (2) Ab-induced particle formation requires intracellular Ca2+ flux and PKC activation; and (3) dexamethasone inhibits particle formation induced by ROS through inhibition of PLA2 and 12-LO as well as translocations of cytosol components to membrane, activity requirements for the generation of 12(S)-HETE, and subsequent activation of platelet NADPH oxidase.
Patients, materials, and methods
Human population
Patient sera were obtained from early-onset HIV-1–infected thrombocytopenic patients without AIDS and from control subjects (healthy laboratory personnel). These studies were approved by the New York University Medical Center Institutional Review Board (IRB). The IRB has approved the use of stored frozen sera from patients.
Mice
Materials
All reagents were obtained from Sigma-Aldrich (St Louis, MO) unless otherwise designated. Reagents include PMA, the Ca2+ ionophore A23187, EGTA, BAPTA, bisindolylmaleimide, bromophenacyl bromide (BPB), dexamethasone, corticosterone, RU 38486, catalase, diphenyleneiodonium, and annexin V–FITC. 12(S)-HETE was obtained from Biomol Research Laboratories (Plymouth Meeting, PA). DCFH diacetate (DCFH-DA) was obtained from Molecular Probes (Eugene, OR). [3H] arachidonic acid (1 Ci/mmol [3.7 × 1010 Bq/mmol]) was obtained from Amersham Biosciences (Piscataway, NJ). Goat polyclonal Ab against p67phox was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody (mAb) against 12-LO was obtained from Alexis Biochemicals (San Diego, CA).
Affinity purification of antiplatelet GPIIIa49-66
IgG was isolated from polyethylene glycol–precipitable immunocomplexes35 followed by protein A affinity chromatography, G-200 gel filtration, affinity chromatography, and affinity purification on 2% paraformaldehyde-fixed platelets as described.36 The antiplatelet Ab was eluted with 0.1 M glycine buffer (pH 2.5) and further purified on a GPIIIa49-66 peptide-affinity column, as described.36
Platelet preparation
Gel-filtered murine and human platelets were isolated from EDTA-anticoagulated blood and labeled with anti-CD61-FITC (human) or anti-CD41b–FITC (murine) Ab as previously described.1
Platelet particle formation
Particle formation was assayed by FACScan (Becton Dickinson Immunocytometry Systems, Mountain View, CA) as previously described, employing Tyrode buffer (pH 7.4).1 Fluorescent-labeled intact platelets were monitored in the right upper quadrant, with the y-axis measuring forward scatter and the x-axis measuring fluorescence. A shift in fluorescent particles from right upper quadrant to left upper and lower quadrants reflected the percentage of platelet particle induction of 10 000 enumerated events.
Platelet annexin binding
Gel-filtered platelets were treated with anti–GPIIIa49–66 for 4 hours at 37°C and then labeled with annexin V–FITC in the presence of 2 mM Ca2+ at 4°C for 30 minutes, prior to assay.
Platelet oxidation
Gel-filtered platelets were loaded with 10 μM DCFH-DA for 30 minutes at 37°C as described previously1 and challenged with patient Ab, A23187, PMA, or 12(S)HETE. Oxidation was quantified by measuring the increase in mean fluorescence with a flow cytometer.
Platelet Ca2+ flux
Platelets were isolated from blood anticoagulated with 0.32% Na citrate, gel filtered, and loaded with 5 μM fura-2-AM (Molecular Probes) at 37°C for 30 minutes with gentle shaking. Excess probe was removed with Tyrode-HEPES buffer, added to a cuvette, and warmed at 37°C for 3 minutes. CaCl2 was then added to a final concentration of 2 mM to saturate intracellular Ca2+. Control IgG or patient IgG (25 μg/mL) or Ca2+ ionophore was then added and fluorescence monitored for 10 minutes in a Fluoromax-3 fluorometer (Jobin Yvon–Spex, Edison, NJ). The suspension was excited at 340 and 380 nm and fluorescence measured at 510 nm. Cytosolic Ca2+ levels were determined from the fluorescence ratios.37
Cytosol membrane translocation
Translocation of cytosol to platelet membrane was measured as previously described.3 Platelets (5 × 107/100 μL) were incubated in buffer containing control or patient IgG for 4 hours at 37°C and extracted in relaxation buffer, with membrane and cytosol fractions prepared as described,28 with minor modifications as described.3 Measurement of the cytosol marker lactic dehydrogenase (LDH) revealed that the membrane fractions contained minimal trapping of total LDH (9.5% ± 1.4%, n=8) with an absence of significant variation before or after translocation.
Activation of platelet Rac
Activation of Rac was determined with a kit supplied by Chemicon (Temecula, CA). This test uses a specific probe (PAK1 [p21 activated kinase]) to detect the active conformation of Rac-GTPase. Platelets are lysed with assay buffer and centrifuged at 12 000g at 4°C for 10 minutes. The supernatant is incubated on ice for 3 minutes and PAK1 beads are added to pull down GTP-Rac. The beads are centrifuged, washed with assay buffer, and boiled in an SDS sample buffer and the supernatant is resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). An immunoblot is then performed and PAK1-GTP-Rac detected with a Rac-specific monoclonal Ab, followed by a goat anti–mouse Ab conjugated to horseradish peroxidase and detected by chemiluminescence.
Platelet 12(S)-HETE production
Gel-purified platelets (108/100 μL) were incubated with control or patient Ab as described.3 The reaction was stopped by addition of 1 volume of ice-cold acetone (giving a total volume of 200 μL) and the samples were kept at −70°C until extracted. Lipids were extracted into ether and analyzed by reverse-phase high-performance liquid chromatography (HPLC) as previously described.38 The 12(S)-HETE was quantified by UV absorbance at 237 nm.
Phospholipase A2 assay
Gel-filtered platelets (108/mL) were incubated with [3H] arachidonic acid for 2 hours at 37°C in Ca2+/Mg2+-free PBS containing 5 μCi/mL (0.185 MBq). Platelets were extensively washed in Tyrode buffer containing 0.1% fatty acid–free human serum albumin (to wash out free radioactivity) and suspended in this buffer. Platelets (106) were treated with platelet agonist in the absence or presence of the PLA2 inhibitor bromophenacyl bromide (200 μM) or dexamethasone (3.2 μM) for various time intervals in a final volume of 150 μL. The reaction mixture at the end of each time point was centrifuged at 14 000g for 2 minutes at 4°C and an equal volume of absolute ethanol was added. Radioactivity was measured by liquid scintillation counting. Virtually all of the radioactivity was arachidonate in this assay, as verified by analysis of independent samples by HPLC.
Results
Comparison of platelet particle formation, size, and annexin binding with anti–GPIIIa49-66 Ab versus Ca2+ ionophore
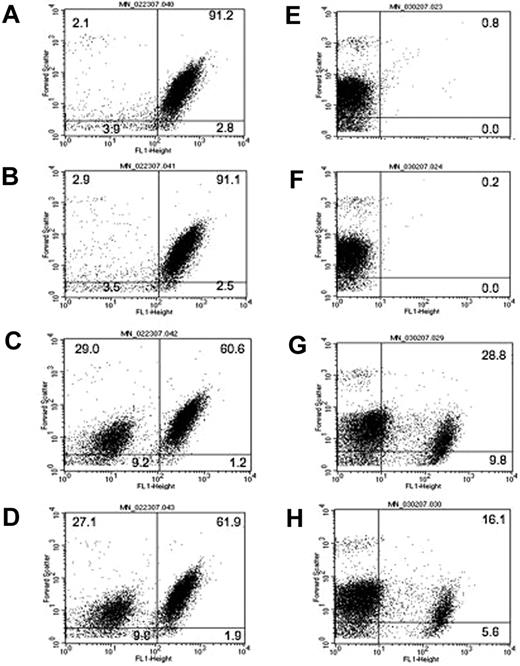
Figure 1 demonstrates the effect of anti–GPIIIa49-66 or Ca2+ ionophore on the percentage of particle formation, size distribution, and ability to bind annexin (measurement of phosphatidyl serine, surrogate for platelet hypercoagulability). Figure 1A-C represents the effect of anti–GPIIIa49-66 on platelet particle formation (a shift to the upper left [UL] and lower left [LL] quadrants) following incubation with buffer (A), control IgG (B), or patient IgG (C). Similar results were noted with Ca2+ ionophore (Figure 1D). An apparent continuum of platelet size was noted and displayed in the UL and LL quadrants. With the gates employed, particles in the UL were on average 2-fold smaller in size than the average intact platelet in the right upper (RU) quadrant, as measured by forward light scatter. Particles in the LL were 16 times smaller than intact platelets. These observations were compatible with Ab-induced break up of platelets into smaller and smaller particles rather than budding off of microparticles from the platelet surface. When these platelet particles are stained with annexin-FITC, a shift to the right of fluorescence represents annexin binding. Annexin bound to both larger and smaller platelet particles induced by anti–GPIIIa49-66 Ab (Figure 1E-G), indicating that they are likely to be procoagulant, confirming our previous report.1 Figure 1H demonstrates similar findings with the Ca2+ ionophore with respect to annexin binding.
Platelet particle formation, size, and annexin binding induced by anti–GPIIIa49-66 Ab or Ca2+ ionophore. (A) Studies with anti–GPIIIa49-66 Ab. Gel-filtered platelets were labeled with either CD61-FITC (left panels) or annexin-FITC (right panels) incubated with buffer (A,E), control IgG (B,F), or anti–GPIIIa49-66 (C,G) for 4 hours at 37°C and analyzed by flow cytometry (representative of 3 experiments). (B) Studies with Ca2+ ionophore. Similar studies were performed with Ca2+ ionophore as agonist (D,H). The numbers in the quadrants refer to the percentage of cells in the quadrant as determined by fluorescent labeling.
Platelet particle formation, size, and annexin binding induced by anti–GPIIIa49-66 Ab or Ca2+ ionophore. (A) Studies with anti–GPIIIa49-66 Ab. Gel-filtered platelets were labeled with either CD61-FITC (left panels) or annexin-FITC (right panels) incubated with buffer (A,E), control IgG (B,F), or anti–GPIIIa49-66 (C,G) for 4 hours at 37°C and analyzed by flow cytometry (representative of 3 experiments). (B) Studies with Ca2+ ionophore. Similar studies were performed with Ca2+ ionophore as agonist (D,H). The numbers in the quadrants refer to the percentage of cells in the quadrant as determined by fluorescent labeling.
Ca2+ ionophore– or PMA-induced platelet particle formation requires the generation of 12(S)-HETE and activation of platelet NADPH oxidase
We next sought to determine whether other activators of platelet particle formation could also result from ROS production. Therefore, we examined the mechanism(s) of platelet fragmentation induced by the Ca2+ ionophore A23187 and by the PKC activator PMA.
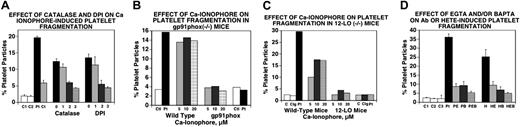
Since activation of 12-LO and NADPH oxidase are both critical to the induction of platelet particles induced by Ab, we investigated whether these pathways were also required to induce particle formation with the calcium ionophore A23187. Inhibitors of ROS such as catalase, an H2O2 scavenger, and diphenyleneiodonium (DPI), an NADPH inhibitor, effectively blocked platelet particle formation induced by A23187 (Figure 2A). In addition, platelets from mice devoid of either gp91phox, an essential component of the NADPH oxidase system (Figure 2B), or 12-LO, and thus unable to produce 12(S)-HETE, showed no A23187-inducible fragmentation (Figure 2C). Finally, we tested the requirement for an elevation of intracellular Ca2+ levels in both Ab- and 12(S)-HETE–induced particle oxidative platelet fragmentation by treating platelets with the extracellular Ca2+ chelator EGTA or the intracellular Ca2+ chelator BAPTA. Both agents inhibited particle formation induced by Ab (Figure 2D left) or 12(S)-HETE (Figure 2D right). We conclude from these results that both Ab- and 12(S)-HETE–induced particle oxidative fragmentation requires elevation of intracellular Ca2+, which is derived from a Ca2+ flux.
Requirement of Ca2+ flux for oxidative platelet particle formation induced by Ca2+ ionophore through activation of the NADPH oxidase and 12-LO pathways. (A) Catalase and DPI inhibit Ca2+ ionophore–induced platelet particle formation. C1 and C2 indicate control buffer and control IgG (25 μg/mL), respectively; Pt, patient anti–GPIIIa49-66 Ab (25 μg/mL); Ct, catalase (100 μg/mL). Lane 0 is A23187 alone. Lanes 1, 2, and 3 refer to doubling concentration of either catalase starting at 50 μg/mL in the presence of 10 μM A23187 or DPI starting at 20 μM (n=4). (B) Ca2+ ionophore–induced platelet particle formation requires the NADPH oxidase pathway. Ctl and Pt refer to control and patient Ab. Ca2+ ionophore concentrations are given for wild-type and NADPH oxidase–deficient (gp91phox−/−) platelets. (C) Ca2+ ionophore–induced platelet particle formation requires 12-LO (same designations as in panel B; Cig indicates control IgG). (D) Ab- or 12(S)-HETE–induced platelet particle formation is inhibited by Ca2+ chelator EGTA (extracellular) or BAPTA (intracellular). C1, C2, and C3 refer to control IgG without and with EGTA and BAPTA, respectively. Pt indicates patient Ab; PE, PB, and PEB, patient Ab in the presence of EGTA (100 μM), BAPTA (10 μM), and EGTA + BAPTA; H, 12(S)-HETE (0.1 μM); HE, HB, and HEB, 12(S)-HETE plus EGTA, BAPTA, and EGTA + BAPTA (n=4). SEM is given.
Requirement of Ca2+ flux for oxidative platelet particle formation induced by Ca2+ ionophore through activation of the NADPH oxidase and 12-LO pathways. (A) Catalase and DPI inhibit Ca2+ ionophore–induced platelet particle formation. C1 and C2 indicate control buffer and control IgG (25 μg/mL), respectively; Pt, patient anti–GPIIIa49-66 Ab (25 μg/mL); Ct, catalase (100 μg/mL). Lane 0 is A23187 alone. Lanes 1, 2, and 3 refer to doubling concentration of either catalase starting at 50 μg/mL in the presence of 10 μM A23187 or DPI starting at 20 μM (n=4). (B) Ca2+ ionophore–induced platelet particle formation requires the NADPH oxidase pathway. Ctl and Pt refer to control and patient Ab. Ca2+ ionophore concentrations are given for wild-type and NADPH oxidase–deficient (gp91phox−/−) platelets. (C) Ca2+ ionophore–induced platelet particle formation requires 12-LO (same designations as in panel B; Cig indicates control IgG). (D) Ab- or 12(S)-HETE–induced platelet particle formation is inhibited by Ca2+ chelator EGTA (extracellular) or BAPTA (intracellular). C1, C2, and C3 refer to control IgG without and with EGTA and BAPTA, respectively. Pt indicates patient Ab; PE, PB, and PEB, patient Ab in the presence of EGTA (100 μM), BAPTA (10 μM), and EGTA + BAPTA; H, 12(S)-HETE (0.1 μM); HE, HB, and HEB, 12(S)-HETE plus EGTA, BAPTA, and EGTA + BAPTA (n=4). SEM is given.
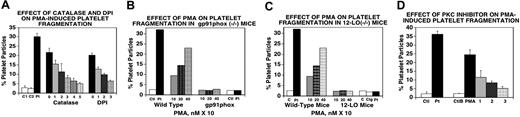
The role of PKC in particle formation was first suggested by our observation that the PKC inhibitor bisindolylmaleimide (BIM) was capable of inhibiting this reaction in platelets.3 Because PMA is a strong inducer of PKC and because we have observed that PMA induces platelet particle formation, we next explored the possibility that this PMA-induced fragmentation was an oxidative process requiring ROS. Both catalase and DPI inhibited PMA-induced particle formation (Figure 3A). As is the case with Ab-induced particle formation, PMA induction requires both a functional NADPH oxidase system and an active 12-LO. This is demonstrated by the inability of PMA to induce particles in platelets derived from mice devoid of gp91phox (gp91phox−/−; Figure 3B) or in platelets from 12-LO knockout mice (12-LO−/−; Figure 3C). The dose-dependent activity of the PKC inhibitor BIM is shown in Figure 3D. These data indicate that activation of PKC can lead to oxidative platelet particle formation.
Requirement of PKC for oxidative platelet particle formation induced by PMA (activates PKC). (A) Catalase and DPI inhibit PMA-induced (200 nM) platelet particle formation. C1 and C2 represent control buffer and control IgG. Pt is patient Ab. Catalase and DPI lanes are as in Figure 2A, with lane 0 as PMA alone (n=4). (B) PMA-induced platelet particle formation requires the NADPH oxidase pathway (same designations as in Figure 2B). (C) PMA-induced platelet particle formation requires 12-LO (same designations as in Figure 2C). (D) PMA-induced platelet particle formation (200 nM) is inhibited by the PKC inhibitor bisindolylmaleimide. Ctl and Ctlb are respective controls for patient IgG and PMA, respectively. Lanes, 1, 2, and 3 refer to doubling PKC inhibitor concentrations starting at 100 μM (n=4). SEM is given.
Requirement of PKC for oxidative platelet particle formation induced by PMA (activates PKC). (A) Catalase and DPI inhibit PMA-induced (200 nM) platelet particle formation. C1 and C2 represent control buffer and control IgG. Pt is patient Ab. Catalase and DPI lanes are as in Figure 2A, with lane 0 as PMA alone (n=4). (B) PMA-induced platelet particle formation requires the NADPH oxidase pathway (same designations as in Figure 2B). (C) PMA-induced platelet particle formation requires 12-LO (same designations as in Figure 2C). (D) PMA-induced platelet particle formation (200 nM) is inhibited by the PKC inhibitor bisindolylmaleimide. Ctl and Ctlb are respective controls for patient IgG and PMA, respectively. Lanes, 1, 2, and 3 refer to doubling PKC inhibitor concentrations starting at 100 μM (n=4). SEM is given.
Effect of dexamethasone on agonist-induced particle formation
We first asked if anti–GPIIIa49-66 Ab or Ca2+ ionophore, at levels that induced platelet particles, also caused an increase in intracellular Ca2+ by monitoring Ca2+ levels in gel-filtered platelets loaded with Fura-2. While control Ab had little effect on platelet calcium levels, we detected a rapid increase in intracellular Ca2+ within seconds of the addition of anti–GPIIIa49-66 Ab. Dex had no effect on Ab- or Ca2+ ionophore–induced Ca2+ flux (Figure 4).
Effect of Dex on anti–GPIIIa-49-66 Ab– or Ca2+ ionophore–induced intracellular Ca2+ flux. Control or patient IgG (25 μg/mL) was added to fura-2 AM–loaded gel-filtered platelets and fluorescence monitored for 20 minutes. Ca2+ flux was determined from fluorescence ratios following excitations at 340 and 380 nm and fluorescence detection at 510 nm. (A) Control. (B) Anti–GPIIIa49-66. (C) Ab + Dex. (D) Ca2+ ionophore. (E) Ca2+ ionophore + Dex (n=3).
Effect of Dex on anti–GPIIIa-49-66 Ab– or Ca2+ ionophore–induced intracellular Ca2+ flux. Control or patient IgG (25 μg/mL) was added to fura-2 AM–loaded gel-filtered platelets and fluorescence monitored for 20 minutes. Ca2+ flux was determined from fluorescence ratios following excitations at 340 and 380 nm and fluorescence detection at 510 nm. (A) Control. (B) Anti–GPIIIa49-66. (C) Ab + Dex. (D) Ca2+ ionophore. (E) Ca2+ ionophore + Dex (n=3).
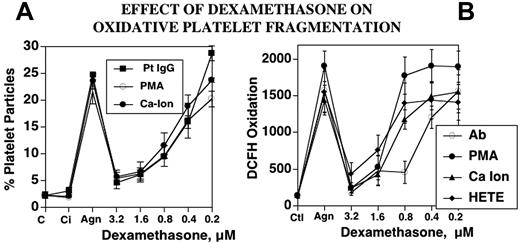
Dexamethasone is clinically useful in the treatment of both HIV-ITP and classic AITP, although the mechanism for platelet clearance differs in the 2 diseases. This clinical observation led us to ask whether steroids, specifically dexamethasone, could be operating by inhibiting the NADPH oxidase/12-LO pathways. This proved to be the case. Dexamethasone inhibits platelet particle formation induced by Ab, PMA, or A23187 (Figure 5A). All 3 agonists induced particle formation that was nearly 10-fold greater than baseline, whereas dexamethasone, at concentrations achieved therapeutically (1-3 μM), nearly completely blocked agonist-induced platelet particle formation. Furthermore, dexamethasone also blocked oxidation of dichlorodihydrodifluorescein diacetate (DCFH), an oxidative fluor that is an independent measure of the activation of platelet oxidation. In addition to the 3 agonists, 12(S)-HETE enhanced oxidation from 15- to 20-fold over baseline. The oxidation of DCFH induced by all of these agents was inhibited by dexamethasone (1-3 μM; Figure 5B). Of further interest was the observation that the glucocorticoid receptor antagonist RU 38486 (5-50 μM) had no effect on dexamethasone inhibition of platelet particle formation (n=2; data not shown). This would suggest that dexamethasone inhibition does not require glucocorticoid receptor ligation. Finally, the non–anti-inflammatory steroid corticosterone also had no effect, indicating specificity for anti-inflammatory dexamethasone (n=4; data not shown).
Effect of dexamethasone on oxidative platelet particle formation induced by Ab, PMA, Ca2+ ionophore, or 12(S)-HETE. (A) Platelet particle formation. C indicates control buffer; Ci, control plus dexamethasone in the absence of agonist; Agn, agonists Pt IgG (patient IgG), PMA, or Ca-Ion (Ca2+ ionophore) in the absence and presence of dexamethasone 15 minutes prior to and then 4 hours after incubation of agonists at 37°C (n=5). (B) Platelet oxidation of oxidative fluor, DCFH. HETE refers to 12(S)-HETE (n=5). SEM is given.
Effect of dexamethasone on oxidative platelet particle formation induced by Ab, PMA, Ca2+ ionophore, or 12(S)-HETE. (A) Platelet particle formation. C indicates control buffer; Ci, control plus dexamethasone in the absence of agonist; Agn, agonists Pt IgG (patient IgG), PMA, or Ca-Ion (Ca2+ ionophore) in the absence and presence of dexamethasone 15 minutes prior to and then 4 hours after incubation of agonists at 37°C (n=5). (B) Platelet oxidation of oxidative fluor, DCFH. HETE refers to 12(S)-HETE (n=5). SEM is given.
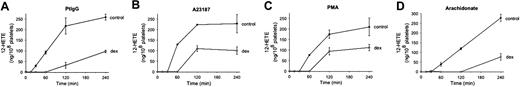
Dexamethasone inhibits 12-LO activity and 12(S)-HETE production induced by Ab, PMA, or A23187
Since we have previously shown that 12-LO is upstream of NADPH oxidase in the pathway to oxidative platelet fragmentation, we asked whether dexamethasone might inhibit particle formation by inhibiting 12(S)-HETE synthesis. Patient Ab (Figure 6A), the Ca2+ ionophore A23187 (Figure 6B), and PMA (Figure 6C) all activated platelet 12(S)-HETE production. The addition of dexamethasone (3.2 μM) significantly reduced the generation of this key lipid mediator. The synthesis of 12(S)-HETE requires the prior release of the 12-LO substrate arachidonic acid from membrane phospholipids by the action of a PLA2. In order to differentiate a direct effect of dexamethasone on the 12-LO from an indirect effect on the PLA2, we incubated platelets with arachidonic acid in the presence of dexamethasone. Dexamethasone significantly inhibited arachidonate-induced 12(S)-HETE synthesis (Figure 6D), demonstrating that the steroid inhibits platelet 12-LO.
Effect of dexamethasone on 12(S)-HETE production produced by patient IgG (Pt IgG), Ca2+ ionophore A23187, PMA, or arachidonic acid (AA). Gel-filtered platelets were incubated in the absence or presence of 3.2 μM dexamethasone (dex) for 240 minutes at 37°C. Platelets were preincubated with buffer or dexamethasone for 15 minutes, prior to addition of agonists (n=3). SEM is given.
Effect of dexamethasone on 12(S)-HETE production produced by patient IgG (Pt IgG), Ca2+ ionophore A23187, PMA, or arachidonic acid (AA). Gel-filtered platelets were incubated in the absence or presence of 3.2 μM dexamethasone (dex) for 240 minutes at 37°C. Platelets were preincubated with buffer or dexamethasone for 15 minutes, prior to addition of agonists (n=3). SEM is given.
Dexamethasone also inhibits PLA2 activity, resulting in reduced arachidonic acid release induced by anti–GPIIIa 49-66 patient Ab
The observation that dexamethasone inhibits 12-LO does not rule out the possibility that the steroid also effects PLA2. We therefore examined the possibility that dexamethasone could also inhibit this enzyme. In this case, platelet phospholipid pools were prelabeled with [3H] arachidonic acid production before they were exposed to patient Ab. Dexamethasone almost completely inhibited arachidonic acid release in this assay and was as effective as the nonspecific PLA2 inhibitor BPB (Figure 7).
Effect of dexamethasone or BPB on PLA2 activity activated by patient IgG. [H3] arachidonic acid was incorporated into platelet membrane phospholipid and incubated in the absence or presence of dexamethasone (as in Figure 5) in the presence of patient IgG, control IgG, or PLA2 inhibitor bromophenacyl bromide (BPB; 100 μM; n=3). SEM is given.
Effect of dexamethasone or BPB on PLA2 activity activated by patient IgG. [H3] arachidonic acid was incorporated into platelet membrane phospholipid and incubated in the absence or presence of dexamethasone (as in Figure 5) in the presence of patient IgG, control IgG, or PLA2 inhibitor bromophenacyl bromide (BPB; 100 μM; n=3). SEM is given.
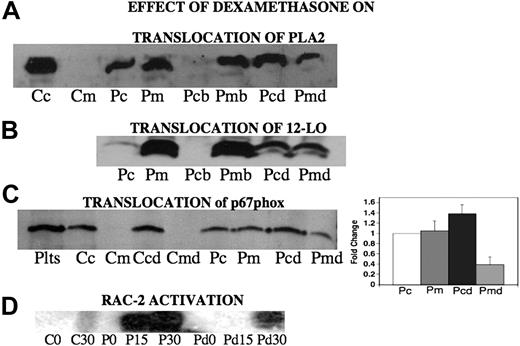
Dexamethasone inhibits the translocation of PLA2, 12-LO, and p67phox from cytosol to membrane
Translocation of p40phox, p47phox, p67phox, activated Rac-2,4 PKC,4 as well as cytosolic phospholipase A23 and other cytosol components is required for NADPH oxidase activation. We now report that dexamethasone alters the subcellular distribution of key enzymes in this pathway. Since PLA2 is required for the release of arachidonic acid, which is the substrate for 12-LO leading to 12(S)-HETE production, and both enzyme activities are inhibited by dexamethasone, we reasoned that dexamethasone might inhibit this translocation. When platelets are incubated with patient Ab for 4 hours, a substantial portion of the cPLA2 is translocated to the membrane (Figure 8A lanes 1-4). Addition of dexamethasone partially inhibits this translocation (Figure 8A lanes 5-8). A similar result was obtained when the translocation of 12-LO was measured in Ab-activated platelets after 4 hours (Figure 8B lanes 1,2). This translocation was partially reversed by dexamethasone treatment (Figure 8B lanes 3-6). Thus, dexamethasone partially reverses the translocation of both cPLA2 and 12-LO to the membrane. Since both PLA2 and 12-LO are required for NADPH oxidase activation in platelets,3 these data would predict that NADPH oxidase should be inactive following dexamethasone treatment. This was confirmed by studies showing that p67phox translocation to the platelet membrane (Figure 8C) as well as Rac-2 activation (required for NADPH oxidase activation) were reduced by this treatment (Figure 8D). Reduction of platelet membrane translocation is shown in Figure 8C (bar graph; n=5). The differences between Pm and Pmd as well as Pc and Pcd are significant by Student t test (P < .01 and P = .05, respectively).
Effect of dexamethasone on translocation of PLA2, 12-LO, and p67phox from cytosol to membrane and activation of Rac-2 following Ab-induced oxidative platelet particle formation. (A) PLA2 translocation. Cc and Cm refer to control cytosol and membrane after incubation of platelets for 4 hours at 37°C. Pc and Pm refer to cytosol and membrane after incubation with patient anti–GPIIIa49-66 (P) for 4 hours. The next set of experiments measure elution of membrane PLA2 with dexamethasone. Pcb and Pmb refer to previous Pm incubated an additional 4 hours with buffer alone. Pcd and Pmd refer to the same incubation plus dexamethasone (d). (B) 12-LO translocation. Pc and Pm refer to platelet cytosol and membrane respectively, following 4 hours of incubation with patient anti–GPIIIa49-66 (P). Pcb and Pmb refer to platelet cytosol and membrane fractions of Pm incubated for a further 4-hour incubation in the presence of buffer. Pcd and Pmd refer to the same incubation time in the presence of dexamethasone (n=2). (C, left panel) p67phox translocation. Plts refer to platelet extract. Cc and Cm refer to control cytosol and membrane fractions of platelets after incubation of platelets for 4 hours at 37°C. Ccd and Cmd refer to same incubation with dexamethasone. Pc and Pm are cytosol membrane fractions of platelets treated with patient Ab. Pcd and Pmd are membrane fractions of platelets treated with dexamethasone and patient Ab. (Right panel) Bar graph and SEM is given for n=5. (D) Activation of Rac-2. CO and C30 refer to control IgG at 0 and 30 minutes of incubation. PO, P15, and P30 refer to patient Ab at 0, 15, and 30 minutes. Pd0, Pd15, and Pd30 refer to patient Ab in the presence of dexamethasone (n=4).
Effect of dexamethasone on translocation of PLA2, 12-LO, and p67phox from cytosol to membrane and activation of Rac-2 following Ab-induced oxidative platelet particle formation. (A) PLA2 translocation. Cc and Cm refer to control cytosol and membrane after incubation of platelets for 4 hours at 37°C. Pc and Pm refer to cytosol and membrane after incubation with patient anti–GPIIIa49-66 (P) for 4 hours. The next set of experiments measure elution of membrane PLA2 with dexamethasone. Pcb and Pmb refer to previous Pm incubated an additional 4 hours with buffer alone. Pcd and Pmd refer to the same incubation plus dexamethasone (d). (B) 12-LO translocation. Pc and Pm refer to platelet cytosol and membrane respectively, following 4 hours of incubation with patient anti–GPIIIa49-66 (P). Pcb and Pmb refer to platelet cytosol and membrane fractions of Pm incubated for a further 4-hour incubation in the presence of buffer. Pcd and Pmd refer to the same incubation time in the presence of dexamethasone (n=2). (C, left panel) p67phox translocation. Plts refer to platelet extract. Cc and Cm refer to control cytosol and membrane fractions of platelets after incubation of platelets for 4 hours at 37°C. Ccd and Cmd refer to same incubation with dexamethasone. Pc and Pm are cytosol membrane fractions of platelets treated with patient Ab. Pcd and Pmd are membrane fractions of platelets treated with dexamethasone and patient Ab. (Right panel) Bar graph and SEM is given for n=5. (D) Activation of Rac-2. CO and C30 refer to control IgG at 0 and 30 minutes of incubation. PO, P15, and P30 refer to patient Ab at 0, 15, and 30 minutes. Pd0, Pd15, and Pd30 refer to patient Ab in the presence of dexamethasone (n=4).
Discussion
The purpose of this investigation was to determine whether other inducers of platelet particle formation operated through the elaboration of ROS and whether oxidative particle formation could be inhibited by glucocorticoids, since both HIV-ITP and classic AITP patients respond to glucocorticoids. Indeed both HIV-ITP and AITP patients have particle formation associated with their clinical thrombocytopenia,39–41 and platelet particles with potential prothrombotic properties could contribute significantly to the pathophysiology of these as well as other diseases.42 The therapeutic benefit of glucocorticoids should therefore be considered in prothrombotic situations associated with or initiated by platelet particles.
Our data clearly demonstrate that platelet particle formation is also initiated by the Ca2+ ionophore A2318743 and by PMA-mediated PKC activation.31 A23187-induced platelet particle formation, like Ab-induced particle formation, required Ca2+ flux, was inhibited by catalase or DPI, and did not occur in platelets from mice that were deficient in either NADPH oxidase (gp91phox−/−) or 12-LO (12-LO−/−). These observations suggest a role for Ca2+ flux and PKC activation in Ab-induced oxidative platelet particle formation. The role of intracellular Ca2+ flux in Ab-induced oxidative platelet particle formation is supported by the observation that Dex inhibits Ab-induced Ca2+ flux, and calcium chelators, such as EGTA or BAPTA, inhibit the reaction. Similarly, our data show that PMA-induced oxidative platelet particle formation is inhibited by EGTA/BAPTA. In a previous communication, we reported that Ab-induced oxidative platelet particle formation was inhibited by the PKC inhibitor bisindolylamide3 and we now show that the PMA effect, as expected, is also inhibited by the inhibitor.
Our data demonstrate for the first time that Ab-, Ca2+ ionophore–, or PMA-associated particle formation is induced by ROSs and inhibited by dexamethasone. It is of interest that dexamethasone also inhibits classic macrophage Fc-γ receptor–mediated phagocytosis, as is the case with classic AITP. It is therefore tempting to speculate that Fc-γ–mediated phagocytosis also requires LO activity, more specifically 5-LO activity, in macrophages. This is supported by the apparent ability of LTB4 to activate NADPH oxidase in human granulocytes.44 In addition, a recent study has demonstrated that leukotrienes enhance bactericidal activity of alveolar macrophages through activation of NADPH oxidase (generation of H2O2 and translocation of p47phox to the membrane).45 Indeed, numerous reports on the role of leukotrienes, particularly LTB4, in activation of phagocytosis and killing of invading organisms support this proposal.46–52
In part, dexamethasone inhibits macrophage-induced phagocytosis killing by inhibiting PLA2 activity, which is required to release arachidonic acid from membrane phospholipid. This appears to be secondary to the induction of lipocortin 1 (LC1), a member of the annexin family53 that inhibits PLA2 directly. However, this induction requires a minimum of 3 hours of incubation and the maximum effective dose does not occur until 24 hours.54,55 It is therefore unlikely that the inhibitory effect of dexamethasone on the Ab-induced particle formation is due to LC-1 induction, since inhibition of oxidative platelet fragmentation is complete and almost immediate (a dexamethasone preincubation of 15 minutes or less). In addition, protein synthesis in platelets is negligible.
The ability of 3 distinct agonists, Ab, A23187, and PMA, to induce platelet particle formation contributed to our identification of the mechanism of dexamethasone inhibition of this reaction. All 3 agonist-induced platelet particles as well as oxidation could be inhibited by dexamethasone in a concentration-dependent manner. 12(S)-HETE production was similarly significantly inhibited by dexamethasone. Of particular interest was the inhibition of arachidonic acid–induced 12(S)-HETE synthesis in dexamethasone-treated platelets. These data strongly suggest that dexamethasone has a direct effect on 12-LO. This assumption is further supported by our membrane translocation experiments, which indicate that dexamethasone both inhibits translocation of 12-LO to the membrane and reverses translocation, returning the enzyme to the cytosol. Similarly, inhibition of PLA2 activity and translocation of cPLA2 to the membrane was also noted with dexamethasone. Dexamethasone also altered the localization of the NADPH oxidase component p67phox, indicating that dexamethasone was destabilizing and/or reversing membrane translocation in platelets as well as in granulocytes. Inhibition of NADPH oxidase was predicted from our previous report, which indicated that 12-LO production of 12(S)-HETE was required for NADPH oxidase activation in platelets.3 For similar reasons, inhibition of Rac activation was predicted. Both proved to be the case. To our knowledge this is the first report of inhibition of 12-LO activation by dexamethasone, as well as inhibition of its membrane translocation in platelets. This suggests that the 12-LO pathway may be a therapeutic target and could be a possible alternative to the use of glucocorticoids in the treatment of HIV-1-ITP. Indeed, preliminary data reveal that the selective 12-LO inhibitor cinnamyl-3, 4-dihydroxy-α-cyanocinnanate (CDC) blocks HIV-1 Ab-induced thrombocytopenia and particle formation in mice (M.A.N. and S.R., unpublished data, January 2006).
In summary, we provide evidence that particle formation induced by 3 different agonists involves intracellular signaling associated with Ca2+ flux, PKC activation, and ROS formation. We also demonstrate that this reaction is inhibited by dexamethasone by its inhibition of PLA2 and 12-LO activity. The mechanism of PLA2 and 12-LO inhibition by dexamethasone could also be contributed to impairment of its membrane translocation, which is necessary for activation of membrane-bound NADPH oxidase.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants HL 13336 and DA 04315 (S.K.).
National Institutes of Health
Authorship
Contribution: M.A.N. performed the platelet studies. Y.G. performed the PLA2, 12-LO, and 12(S)-HETE studies. S.J.F. designed the lipid research. F.X. performed the Ca2+ Flux study. S.K. designed the platelet studies and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simon Karpatkin, New York University School of Medicine, 550 First Avenue, New York, NY 10016; e-mail: simon.karpatkin@med.nyu.edu.


![Figure 7. Effect of dexamethasone or BPB on PLA2 activity activated by patient IgG. [H3] arachidonic acid was incorporated into platelet membrane phospholipid and incubated in the absence or presence of dexamethasone (as in Figure 5) in the presence of patient IgG, control IgG, or PLA2 inhibitor bromophenacyl bromide (BPB; 100 μM; n=3). SEM is given.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2006-10-054064/7/m_zh80180707050007.jpeg?Expires=1769307638&Signature=iylig3frr5oiEY5NZ37VA2jkYUpQSKCJ~CEaynINIGaF0dxgRAqx5OHNbWelnyrciOqSzVrs-KrigWLUh~FXkEPKuTVlayZV-fwlt1LX4jLpoEVlV7AF9ja0VvMtpUOK5LziflKgDUhY6O-IyKlqnHpPvWDRzVeE3ena~uZsQJsP5CWKLedcjVSrpCPyp56VV-FBmzkljo7K-uSjdb-mPFtJLq-ubcDoKpDLEhcm0wH31TDYFuc9rRRIqlJyZCGzEp0LUxT7ZLW1zjL7~MKVMpZXXETtuJaFbgLPeLPfcQtHqbzsZOxp50~x~YhBKw5cO1usDbtq01zPFciDUR82tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal