Abstract
The role of Bim in synergistic interactions between UCN-01 and MEK1/2 inhibitors in human multiple myeloma cells was investigated. Exposure of U266 or RPMI8226 cells to UCN-01 resulted in ERK1/2 activation-associated BimEL phosphorylation/down-regulation, events abrogated by MEK1/2 inhibitors. Enforced activation of ERK1/2 by transfection with constitutively active MEK1 diminished the capacity of PD98059 but not PD184352 to block UCN-01–mediated BimEL phosphorylation and to potentiate apoptosis. Cotreatment with MEK1/2 inhibitors increased the association of BimEL with both Bcl-2 and Bcl-xL in UCN-01–treated cells, leading to Bax/Bak conformational change and Bax mitochondrial translocation. Down-regulation of BimEL by shRNA substantially diminished UCN-01/MEK inhibitor-mediated Bax/Bak activation and apoptosis. Furthermore, transfection of cells with S65A Bim, a mutant resistant to UCN-01–mediated phosphorylation, significantly sensitized cells to UCN-01 lethality. Conversely, ectopic expression of either Bcl-2 or Bcl-xL did not alter UCN-01/MEK1/2 inhibitor-mediated modifications in BimEL phosphorylation but largely prevented cell death. Finally, IL-6 or IGF-1 failed to prevent MEK1/2 inhibitors from blocking UCN-01–induced BimEL phosphorylation/degradation or cell death. Collectively, these findings argue that UCN-01–mediated ERK1/2 activation leads to BimEL phosphorylation/inactivation, resulting in cytoprotection, and that interference with these events by MEK1/2 inhibitors plays a critical role in synergistic induction of apoptosis by these agents.
Introduction
The decision of a cell to undergo apoptosis or to survive following environmental stresses (eg, growth factor deprivation or exposure to cytotoxic agents) is largely determined by proapoptotic and antiapoptotic proteins of the Bcl-2 family, which contain 1 to 4 Bcl-2 homology domains (BH1 to BH4). Multidomain members either mediate (eg, Bax and Bak) or prevent (eg, Bcl-2, Bcl-xL, Mcl-1) apoptosis, while BH3-only members are exclusively proapoptotic.1 The BH3-only proteins can be further subdivided into “activators” (eg, tBid or Bim) and “sensitizers” (eg, Bad, Noxa, Bik, Hrk).1,2 Among “activator” BH3-only proteins, Bid is primarily involved in the receptor-mediated extrinsic death pathway in that it requires cleavage by activated caspase-8 to yield a “truncated” (active) form (tBid).3 In contrast, Bim is a critical Bcl-2 family member involved in activation of the intrinsic apoptotic imatinib mesylate pathway triggered by growth factor deprivation as well as other noxious stimuli including various chemotherapeutic agents (eg, paclitaxel, Gleevec STI571, glucocorticoids).4,5
Bim consists of at least 3 isoforms that result from alternative splicing: BimEL, BimL, and BimS.4 Bim is widely expressed in diverse tissues including hematopoietic cells, while BimEL is the most abundant isoform.6 Bim expression and function are regulated at both the transcriptional and posttranslational levels.7 The transcriptional regulation of Bim expression involves the PI3K-PKB-FOXO, JNK-AP1, and MEK1/2/ERK1/2 (extracellular signal-regulating kinse1/2) pathways,8–10 among others. For example, following withdrawal of cytokines or survival factors, expression of Bim is rapidly induced due to inactivation of PKB or ERK1/2.11 Moreover, Bim (particularly BimL and BimEL) is regulated by posttranslational mechanisms involving phosphorylation. In viable cells, BimL and BimEL are bound to dynein light chain 1 (DLC1) and sequestered with microtubules and distant from other Bcl-2 family members such as Bcl-2/Bcl-xL and Bax.12 In response to stress (eg, exposure to UV light), activated JNK phosphorylates BimL at Thr56 within the DLC1-binding motif (and at either Ser44 or Ser58), leading to release of Bim from the microtubule-associated dynein motor complex, resulting in cell death.13 JNK can also phosphorylate BimEL at Thr116, Ser104, or Ser118,4 although evidence that JNK-mediated phosphorylation of BimEL disassociates BimEL-DLC1 is lacking. However, posttranslational regulation of BimEL is primarily mediated by MEK1/2/ERK1/2 signals.4 Specifically, ERK1/2 directly binds to and phosphorylates BimEL primarily at Ser69 (Ser65 in rat and mouse BimEL) and possibly at Ser59 and Ser104 as well, resulting in its ubiquitination and proteasomal degradation.14,15 In addition, phosphorylation at Ser65 is critical in that mutation of Ser65 (eg, Ser65Ala) completely abolishes ERK1/2-mediated BimEL phosphorylation.14 Moreover, MEK1/2 inhibitors (eg, U0126 and PD184352) substantially diminish BimEL phosphorylation and induce BimEL accumulation in various cell types.16,17 Aside from phosphorylating BimEL and enhancing its elimination, ERK1/2-mediated BimEL phosphorylation may also diminish its capacity to directly activate Bax/Bak.18 It remains uncertain whether ERK1/2 also phosphorylates BimL. In addition, JNK may also be responsible for BimEL phosphorylation at Ser65 and enhancement of its proapoptotic activity, although this phenomenon may be restricted to certain cell types such as neurons.19 More recently, it has been found that Akt phosphorylates BimEL at Ser87 following IL-3 stimulation in IL-3–depedent Ba/F3 cells and that mutation of Ser87 dramatically increases the apoptotic potency of BimEL.20
The mechanisms by which alterations in survival signaling pathways are transduced into death signals remain to be fully elucidated. UCN-01 is a PKC, cyclin-dependent kinase, PDK1, and Chk1 inhibitor that is currently undergoing clinical evaluation in various malignancies, including those of hematopoietic origin.21,22 Previously, our group demonstrated that exposure of human multiple myeloma (MM) and leukemia cells to UCN-01 resulted in marked activation of the MEK1/2 (mitogen-activated protein kinase kinase1/2)/ERK1/2 cascade and that interruption of this pathway (eg, by MEK1/2 inhibitors) dramatically potentiated UCN-01-induced apoptosis in these cells.23,24 Subsequently, we demonstrated that other agents acting upstream of MEK1/2/ERK1/2 (ie, farnesyltransferase inhibitors and HMG CoA-reductase inhibitors) exhibited similar effects.25–27 These agents all shared the ability to prevent or attenuate UCN-01–induced ERK1/2 activation. However, the mechanism by which ERK1/2 inactivation triggers apoptosis in this setting has not been defined. In view of evidence implicating Bim (BimEL in particular) in ERK1/2-mediated antagonism of apoptosis and ERK1/2 activation in cytoprotective responses to UCN-01, we attempted to define the role of Bim in the activity of the MEK1/2 inhibitor/UCN-01 regimen in human MM cells. Our results indicate that perturbations in BimEL phosphorylation play a significant functional role in mediating apoptosis in MM cells coexposed to these agents.
Materials and methods
Cells and reagents
The human MM cell line U266 was purchased from ATCC (Rockville, MD) and maintained as described earlier.23 RPMI8226 cells were kindly provided by Dr Alan Lichtenstein (UCLA, Los Angeles, CA) and cultured as reported previously.28 All experiments were performed using logarithmically growing cells (4 × 105 to 6 × 105 mL). UCN-01 was provided by the Cancer Treatment and Evaluation Program, National Center Institute (NCI), and prepared as described previously.23 The selective MEK inhibitors PD184352, U0126 and PD98059 were purchased from Upstate Biotechnology (Lake Placid, NY) and Calbiochem (San Diego, CA), respectively. Reagents were dissolved in sterile DMSO and stored frozen under light protection and stored at −20°C. In all experiments, final concentrations of DMSO did not exceed 0.1%. Recombinant human IL-6 and IGF-I were purchased from Sigma (St Louis, MO) and R&D Systems (Minneapolis, MN), rehydrated in PBS and 10 mM acetic acid (containing 0.1% BSA), respectively, aliquoted, and stored at −80°C.
Plasmids and transfections
pSR-Bim and pSR-con constructs encoding short hairpin RNA (shRNA) for Bim or scrambled shRNA as control were constructed by inserting the target sequence for human Bim (GenBank AF032457, nucleotides 37 to 56; GACCGAGAAGGTAGACAATT) or the nonspecific sequence (AATTCTCCGAACGTGTCACGT) into pSUPER.retro.puro (Oligoengine, Seattle, WA).5,18 Wild-type (wt) and mutant S65A Bim cDNAs were constructed and cloned into pCDNA3 with hemagglutinin (HA)–tag as reported earlier.18 cDNAs encoding mouse full-length Bcl-2 or HA-tagged constitutive-active (CA) MEK1 (activating mutations of serine 218 and 222 to aspartic acid),28 both cloned into pUSE vectors, were obtained from Upstate Biotechnology. Bcl-xL constructs in pSFFV vectors were obtained as described previously.29 Transfections were performed using an Amaxa Nucleofector device and Cell Line Specific Nucleofector Kits (Amaxa, Cologne, Germany) as per the manufacturer's instructions: Kit V/program G-015 for RPMI8226 and Kit C/program X-005 for U266. For transient transfections, cells were incubated for 24 hours and then treated with UCN-01 with or without MEK1/2 inhibitors. For stable transfections, cells were continuously cultured under selection with G418 (750 μg/mL for RPMI8226 or 400 μg/mL for U266) or puromycin (2 μg/mL for both RPMI8226 and U266).
Western blot analysis
Western blot samples were prepared from whole-cell pellets using Triton X-100 buffer as described previously.28 The amount of total protein was quantified using Coomassie Protein Assay Reagent (Pierce, Rockford, IL). Equal amounts of protein (20 μg per condition) were separated on precast sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Invitrogen, Carlsbad, CA) and electrotransferred onto nitrocellulose membranes. For detection of phosphorylated proteins, no SDS was included in the transfer buffer, and Tris-buffered saline (TBS) was used throughout. Blots were probed with primary antibodies as follows. Blots were reprobed with antiactin (Sigma) or antitubulin antibody (Oncogene, San Diego, CA) to ensure equal loading and transfer of proteins. Primary antibodies included anti-Bim (Calbiochem), anti–phospho-p44/42 (Thr202/Tyr204) MAPK (ERK1/2) and anti-p44/42 MAPK (Cell Signaling, Beverly, MA), anti-MEK1 (BD Transduction Laboratories, Lexington, KY), anti–phospho-MEK1/2 (Cell Signaling), anti–human Bcl-2 oncoprotein (DAKO, Carpinteria, CA), anti–Bcl-xL (Cell Signaling), anti-Bax and anti-Bak (Santa Cruz Biotechnology, Santa Cruz, CA), anti–Mcl-1 (BD PharMingen, San Diego, CA), anti-PARP (poly-adenosine diphosphate-ribose polymerase; Biomol, Plymouth Meeting, PA), anti-HA (Santa Cruz Biotechnology), anti-HA.11 (Covance, Princeton, NJ), anti–caspase-3 (BD Transduction Laboratories), anti–cleaved caspase-3 (17 kDa) (Cell Signaling), and anti–caspase-9 (BD PharMingen).
Subcellular fractionation
A total of 2 × 106 cells were lysed in digitonin lysis buffer.30 Lysates were centrifuged, and the supernatant (S-100 cytosolic fraction) was collected and added to an equal volume of 2× sample buffer. The pellets (organelle/membrane fractions) were washed once in cold PBS and lysed in 1× sample buffer. The S-100 and pellet samples were quantified, separated by SDS-PAGE, and subjected to immunoblot. For analysis of release of mitochondrial proapoptotic factors, anti–cytochrome c (BD PharMingen) and anti-smac/DIABLO (Upstate Biotechnology) were used as primary antibodies. Anti-Bax antibody (Santa Cruz Biotechnology) was employed to evaluate redistribution of Bax.
Coimmunoprecipitation
The interaction between Bim and Bcl-2 or Bcl-xL was evaluated by coimmunoprecipitation analysis. For these studies, CHAPS buffer (150 mM NaCl, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 7.4], protease inhibitors, and 1% CHAPS) was employed to avoid artifactual associations reported with other buffers.31 Briefly, cells were lysed in CHAPS buffer, and 200 μg protein per condition was incubated with 1 μg anti–Bcl-2 (DAKO), anti–Bcl-xL (Cell Signaling), or anti-Bim (Santa Cruz Biotechnology) overnight at 4°C. A total of 20 μL per condition of Dynabeads (Dynal, Oslo, Norway) was then added and incubated for an additional 4 hours. After washing, the beadbound protein was eluted by vortexing and boiling in 20 μL of 1× sample buffer. The samples were separated by SDS-PAGE and subjected to Western blot analysis. Anti-Bim (Calbiochem) or anti–Bcl-2 (DAKO) were used as primary antibodies.
Analysis of Bak and Bax conformational change
Cells were lysed in 1% CHAPS buffer, and 200 μg protein was immunoprecipited using anti-Bax (6A7; Sigma) or anti-Bak (Ab-1; Calbiochem), which only recognizes Bax or Bak that have undergone conformation change, and Dynabeads. Immunoprecipitated protein was then subjected to Western blot analysis by using anti-Bax and anti-Bak as primary antibodies.
Assessment of apoptosis
The extent of apoptosis was evaluated by annexin V–fluorescein isothiocyanate (FITC) staining and flow cytometry as described previously.23 Briefly, 1 × 106 cells were stained with annexin V–FITC (BD PharMingen) and 5 μg/mL propidium iodide (PI; Sigma) in 1× binding buffer for 15 minutes at room temperature in the dark. Samples were then analyzed by flow cytometry within 1 hour to determine the percentage of cells displaying annexin V positivity. In some cases, cell death was also assessed by 7-AAD staining (0.5 μg/mL 7-AAD at 37°C for 30 minutes) and flow cytometry.
Statistical analysis
For flow cytometric analyses of annexin V/PI and 7-AAD, values represent the means (± SD) for at least 3 separate experiments performed in triplicate experiments. The significance of differences between experimental variables was determined using the Student t test.
Results
PD184352 blocks UCN-01–mediated BimEL phosphorylation and down-regulation in human MM cells, accompanied by caspase activation and PARP cleavage
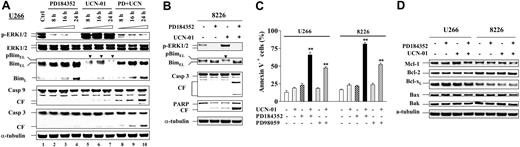
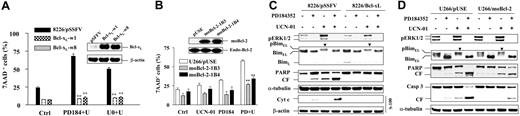
As previously reported,23 exposure of U266 cells to UCN-01 (100 nM; 8 to 24 hours) resulted in a marked increase in phosphorylation of ERK1/2, and this was blocked by coadministration of 5 μM PD184352 (Figure 1A). UCN-01 treatment also induced phosphorylation of BimEL, manifested by slowly migrating species (Figure 1A, lanes 5-7, arrows), and a clear reduction in BimEL levels, presumably reflecting degradation of the phosphorylated species.16 Significantly, coadministration of PD184352 dramatically blocked the phosphorylation and down-regulation of BimEL induced by UCN-01 (Figure 1A, lanes 8-10). Parallel studies in RPMI8226 cells revealed similar phenomenon (Figure 1B). These events were accompanied by enhanced cleavage/activation of caspases and degradation of PARP (Figure 1A,B) as well as a dramatic increase in apoptosis (Figure 1C).
PD184352 blocks UCN-01–mediated BimEL phosphorylation and down-regulation in human MM cells. (A) U266 cells were exposed to 100 nM UCN-01 ± 5 μM PD184352, and at the indicated intervals the cells were harvested and subjected to Western blot analysis to monitor phosphorylation of ERK, expression of Bim, as well as cleavage of caspase-9 and -3. (B) RPMI8226 cells were treated with 150 nM UCN-01 plus or minus 5 μM PD184352 for 24 hours, after which Western blot analysis was performed to assess ERK activation, BimEL phosphorylation, and cleavage of caspase-3 and PARP. (C) U266 and RPMI8226 were exposed to UCN-01 (U266, 100 nM; RPMI8226, 150 nM) plus or minus PD184352 (5 μM) or PD98059 (50 μM) for 24 hours (RPMI8226) or 40 hours (U266), after which the percentage of apoptotic cells (annexin V+) was determined by flow cytometry as described in “Materials and methods.” Results represent the means (± SD) for 3 separate experiments performed in triplicate. **Significantly greater than the value for cells treated with UCN-01 alone (P < .001). (D) U266 and RPMI8226 cells were treated as described for panel C, after which cells were lysed and subjected to Western blot analysis to examine expression of other Bcl-2 family members using the indicated primary antibodies. For panels A, B and D, each lane was loaded with 20 μg protein; the blots were subsequently stripped and reprobed with antitubulin antibody to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (arrows). CF indicates cleavage fragment. Two additional studies yielded equivalent results.
PD184352 blocks UCN-01–mediated BimEL phosphorylation and down-regulation in human MM cells. (A) U266 cells were exposed to 100 nM UCN-01 ± 5 μM PD184352, and at the indicated intervals the cells were harvested and subjected to Western blot analysis to monitor phosphorylation of ERK, expression of Bim, as well as cleavage of caspase-9 and -3. (B) RPMI8226 cells were treated with 150 nM UCN-01 plus or minus 5 μM PD184352 for 24 hours, after which Western blot analysis was performed to assess ERK activation, BimEL phosphorylation, and cleavage of caspase-3 and PARP. (C) U266 and RPMI8226 were exposed to UCN-01 (U266, 100 nM; RPMI8226, 150 nM) plus or minus PD184352 (5 μM) or PD98059 (50 μM) for 24 hours (RPMI8226) or 40 hours (U266), after which the percentage of apoptotic cells (annexin V+) was determined by flow cytometry as described in “Materials and methods.” Results represent the means (± SD) for 3 separate experiments performed in triplicate. **Significantly greater than the value for cells treated with UCN-01 alone (P < .001). (D) U266 and RPMI8226 cells were treated as described for panel C, after which cells were lysed and subjected to Western blot analysis to examine expression of other Bcl-2 family members using the indicated primary antibodies. For panels A, B and D, each lane was loaded with 20 μg protein; the blots were subsequently stripped and reprobed with antitubulin antibody to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (arrows). CF indicates cleavage fragment. Two additional studies yielded equivalent results.
Effects of the regimen on other Bcl-2 family members were also examined in both U266 and RPMI8226 cells. In both cell lines, administration of UCN-01 and PD184352 either alone or in combination had little effect on levels of Bcl-2, Bcl-xL, Mcl-1, Bax, or Bak (Figure 1D). Together, these findings indicate that MEK1/2 inhibition opposes UCN-01–mediated BimEL phosphorylation and down-regulation, events associated with synergistic induction of apoptosis.
Constitutive activation of MEK1 promotes BimEL down-regulation and diminishes the capacity of PD98059, but not PD184352, to block UCN-01–mediated BimEL phosphorylation/down-regulation
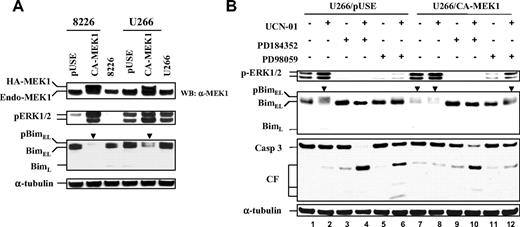
To evaluate the functional significance of ERK1/2 activation, U226 and RPMI8266 cells expressing CA-MEK1 were employed. As noted in previous studies,28 these cells exhibited increased basal ERK1/2 activation compared with empty-vector controls (pUSE; Figure 2A). Notably, enforced ERK activation by ectopic expression of CA-MEK1 was associated with phosphorylation (Figure 2A, arrows) and marked down-regulation of BimEL in both U266 and RPMI8226 cells (Figure 2A), a phenomenon consistent with other reports.4
Ectopic expression of constitutively activate MEK1 promotes BimEL phosphorylation/degradation as well as diminishes the ability of PD98059 but not PD184352 to block UCN-01–induced BimEL phosphorylation. (A) RPMI8226 and U266 cells were stably transfected with a HA-tagged CA-MEK1 construct or its empty vector (pUSE). Western blot analysis was performed to confirm expression of HA-CA-MEK1 as well as to monitor phosphorylation/expression of ERK1/2 and Bim. (B) U266/pUSE and U266/CA-MEK1 cells were exposed to 100 nM UCN-01 plus or minus 50 μM PD98059 or 5 μM PD184352 for 40 hours, respectively, after which cells were lysed and subjected to Western blot analysis to assess ERK1/2 activation, Bim phosphorylation, and caspase-3 cleavage. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). CF indicates cleavage fragment. The results of representative experiments are shown; 2 additional studies yielded equivalent results.
Ectopic expression of constitutively activate MEK1 promotes BimEL phosphorylation/degradation as well as diminishes the ability of PD98059 but not PD184352 to block UCN-01–induced BimEL phosphorylation. (A) RPMI8226 and U266 cells were stably transfected with a HA-tagged CA-MEK1 construct or its empty vector (pUSE). Western blot analysis was performed to confirm expression of HA-CA-MEK1 as well as to monitor phosphorylation/expression of ERK1/2 and Bim. (B) U266/pUSE and U266/CA-MEK1 cells were exposed to 100 nM UCN-01 plus or minus 50 μM PD98059 or 5 μM PD184352 for 40 hours, respectively, after which cells were lysed and subjected to Western blot analysis to assess ERK1/2 activation, Bim phosphorylation, and caspase-3 cleavage. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). CF indicates cleavage fragment. The results of representative experiments are shown; 2 additional studies yielded equivalent results.
Earlier studies have shown that the MEK1/2 inhibitor PD184352 but not PD98059 inhibits CA-MEK1–induced ERK1/2 activation, presumably due to different targets in the Raf/MEK/ERK signaling cascade.32,33 To further define the functional role of MEK1/2/ERK1/2 signaling, the abilities of PD184352 and PD98059 to block UCN-01–induced BimEL phosphorylation in MM cells expressing CA-MEK1 were compared. As reported previously,28 PD98059 only partially reduced levels of phosphorylated ERK1/2 in U266/CA-MEK1 cells compared with those in U266/pUSE empty-vector controls (Figure 2B, lane 12 versus lane 6). In contrast, PD184352 almost completely blocked UCN-01–induced ERK1/2 phosphorylation in both U266/pUSE and U266/CA-MEK1 cells (Figure 2B, lanes 4 and 10). As observed in parental U266 cells, exposure to UCN-01 (100 nM; 40 hours) induced BimEL phosphorylation (Figure 2B, lane 2, arrow) and down-regulation in U266/pUSE cells, while these effects were largely blocked by both PD184352 and PD98059 (Figure 2B, lanes 4 and 6). However, in U266/CA-MEK1 cells, only coadministration of PD184352 diminished BimEL phosphorylation and down-regulation in UCN-01–treated cells (Figure 2B, lane 10 versus lane 8), whereas PD98059 was significantly less effective in blocking BimEL phosphorylation/down-regulation (arrows) compared with effects in U266/pUSE cells (Figure 2B, lane 12 versus lane 6). Consistent with these findings, U266/CA-MEK1 cells were substantially resistant to UCN-01/PD98059–mediated caspase-3 cleavage/activation (Figure 2B) and apoptosis (data not shown), consistent with our previous report,28 but displayed equivalent sensitivity to the UCN-01/PD184352 regimen compared with U266/pUSE cells (Figure 2B) and parental cells (data not shown). Collectively, these findings support the notion that ERK1/2 activation protects cells from UCN-01 lethality through a mechanism involving BimEL phosphorylation and down-regulation.
Coadministration of PD184352 with UCN-01 increases the association of BimEL with Bcl-2 and Bcl-xL and potentiates Bax and Bak conformational change accompanied by Bax mitochondrial translocation
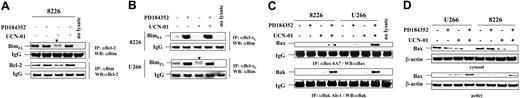
Interactions between Bim and the antiapoptotic proteins Bcl-2 and Bcl-xL, as well as resulting effects on activation of the multidomain proapoptotic members Bax and Bak, were then examined. To this end, coimmunoprecipitation/Western blot analysis was employed. Notably, as shown in Figure 3A,B, UCN-01 diminished the association of BimEL with both Bcl-2 and Bcl-xL in both RPMI8226 and U266 cells, while coadministration of PD184352 clearly restored these associations. Interestingly, interactions between BimEL and Bcl-2 or Bcl-xL did not appear to be modified by BimEL phosphorylation status in that the phosphorylated form of BimEL, reflected by the slowly migrating bands in UCN-01–treated cells (arrows), also coimmunopreciptated with Bcl-2 and Bcl-xL (Figures 3A and 3B). Such findings suggest that enhanced BimEL association with Bcl-2/Bcl-xL stems primarily from inhibition of UCN-01–mediated BimEL degradation by MEK1/2 inhibitors. In addition, treatment with PD184352 alone or in combination with UCN-01 resulted in a roughly equivalent increase in association of BimEL with both Bcl-2 and Bcl-xL (Figure 3A,B), while PD184352 itself, at least at the concentrations used in this study, did not induce pronounced apoptosis (Figure 1C). These results raise the possibility that while accumulated BimEL binds to and disables the antiapoptotic functions of Bcl-2/Bcl-xL in cells treated with MEK1/2 inhibitors, other UCN-01–related death signals (eg, “unscheduled” cdc2 activation)24,28 cooperate with Bim to induce apoptosis by this regimen.
UCN-01 diminished the association of BimEL with Bcl-2 and Bcl-xL, which is restored by coadministration of PD184352. (A) RPMI8226 cells were exposed to 150 nM UCN-01 plus or minus 5 μM PD184352 for 24 hours, after which cells were lysed in CHAPS buffer and immunoprecipitation (IP) performed using either anti–Bcl-2 or anti-Bim antibody as described in “Materials and methods.” Immunopreciptates were then subjected to Western blot analysis (WB) using anti-Bim or anti–Bcl-2 antibody, respectively. (B) RPMI8226 cells (top panels) were treated as described for panel A, and U266 cells (bottom panels) were exposed to 100 nM UCN-01 ± 5 μM PD184352 for 40 hours, after which the association between Bcl-xL and Bim was evaluated by IP/WB analysis using anti-Bcl-xL (for IP) and anti-Bim (for WB), respectively. (C) RPMI8226 and U266 cells were treated as described for panels A and B, after which IP/WB was performed to examine the conformational change of Bax and Bak using anti-Bax 6A7 or anti-Bak Ab-1 (for IP), and anti-Bax or anti-Bak (for WB), respectively. For IP/WB analysis in panels A-C, 200 μg protein per condition was employed for IP. IgG levels are shown to ensure equal loading of IP antibodies and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). The results of representative experiments are shown; 2 additional studies yielded equivalent results. (D) Alternatively, cells were lysed in digitonin buffer, and subcellular fractions were prepared as described in “Materials and methods,” after which Western blot analysis was performed to monitor localization of Bax in either cytosol and organellar membrane (pellet) fractions. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antiactin antibody to ensure equal loading and transfer. Two additional studies yielded equivalent results.
UCN-01 diminished the association of BimEL with Bcl-2 and Bcl-xL, which is restored by coadministration of PD184352. (A) RPMI8226 cells were exposed to 150 nM UCN-01 plus or minus 5 μM PD184352 for 24 hours, after which cells were lysed in CHAPS buffer and immunoprecipitation (IP) performed using either anti–Bcl-2 or anti-Bim antibody as described in “Materials and methods.” Immunopreciptates were then subjected to Western blot analysis (WB) using anti-Bim or anti–Bcl-2 antibody, respectively. (B) RPMI8226 cells (top panels) were treated as described for panel A, and U266 cells (bottom panels) were exposed to 100 nM UCN-01 ± 5 μM PD184352 for 40 hours, after which the association between Bcl-xL and Bim was evaluated by IP/WB analysis using anti-Bcl-xL (for IP) and anti-Bim (for WB), respectively. (C) RPMI8226 and U266 cells were treated as described for panels A and B, after which IP/WB was performed to examine the conformational change of Bax and Bak using anti-Bax 6A7 or anti-Bak Ab-1 (for IP), and anti-Bax or anti-Bak (for WB), respectively. For IP/WB analysis in panels A-C, 200 μg protein per condition was employed for IP. IgG levels are shown to ensure equal loading of IP antibodies and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). The results of representative experiments are shown; 2 additional studies yielded equivalent results. (D) Alternatively, cells were lysed in digitonin buffer, and subcellular fractions were prepared as described in “Materials and methods,” after which Western blot analysis was performed to monitor localization of Bax in either cytosol and organellar membrane (pellet) fractions. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antiactin antibody to ensure equal loading and transfer. Two additional studies yielded equivalent results.
Activation of Bax and Bak (ie, conformational change in Bax and Bak and, in the case of Bax, mitochondrial translocation)34 was then evaluated in MM cells exposed to PD184352 with or without UCN-01. Conformational status of Bax and Bak was monitored using antibodies that selectively recognize conformationally changed proteins.35 Notably, individual drug treatment was without effect, but combined treatment resulted in a pronounced increase in the expression of conformationally changed Bak and Bax in RPMI8226 and U266 cells (Figure 3C). Exposure of cells to UCN-01 with or without PD184352 failed to modify total Bax or Bak levels in either cell line (Figure 1D). In addition, combined (but not individual) drug exposure resulted in a marked relocalization of Bax from cytosol to organellar membrane (Figure 3D). Together, these findings are consistent with the notion that administration of MEK1/2 inhibitors results in marked BimEL accumulation and an increase in the association of BimEL with both Bcl-2 and Bcl-xL, events that lead to a dramatic increase in Bax/Bak conformational change and Bax mitochondrial translocation in cells exposed to UCN-01.
BimEL knockdown by shRNA protects cells from UCN-01/MEK1/2 inhibitor-mediated lethality
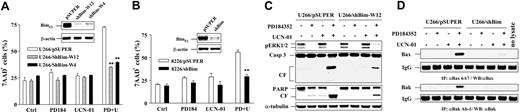
To assess the functional role of perturbations in Bim on the response of MM cells to the UCN-01/MEK1/2 inhibitor regimen, shRNA strategies targeting Bim were employed. When U266 and RPMI8226 cells were stably transfected with shRNA directed against Bim, Western blot analysis revealed clear reductions in expression of BimEL in both cell lines (Figure 4A,B, insets). Cells were exposed to PD184352 with or without UCN-01 for 24 hours (RPMI8226) or 40 hours (U266), after which cell death was assessed by 7-AAD uptake. As shown in Figure 4A-B, both U266 and RPMI8226 displaying shRNA-mediated BimEL down-regulation were markedly resistant to UCN-01/PD184352 lethality (both P < .001), events confirmed by monitoring cleavage of caspase-3 and PARP (Figure 4C). Furthermore, knockdown of BimEL by shRNA did not affect ERK1/2 phosphorylation status following exposure of PD184352 with or without UCN-01 in either U266 (Figure 4C) or RPMI8226 cells (data not shown), indicating that ERK1/2 inactivation acts upstream of BimEL in this setting. Similar results were obtained in both cell lines when BimEL was knocked down by transient transfection with shBim constructs (data not shown). Finally, conformational change of Bax and Bax following PD184352/UCN-01 exposure was markedly diminished in U266/shBim cells compared with controls (Figure 4D). Together, these findings argue strongly that Bim plays a critical functional role in potentiation of Bax/Bak activation and apoptosis induced by UCN-01/MEK1/2 inhibitor regimen.
BimEL knockdown by shRNA prevents conformational change of Bax/Bak and apoptosis in cells coexposed to PD184352/UCN-01. (A) U226 cells were stably transfected with constructs encoding shRNA against human Bim (shBim) or scrambled shRNA (pSUPER) as control, respectively, after which stable clones (1 for pSUPER control and 2 for shBim, designed W4 and W12) were obtained by selection with puromycin. Western blot analysis was performed to monitor down-regulation of Bim expression in both shBim clones (inset). Cells were then exposed to 100 nM UCN-01 plus or minus 5 μM PD184352 for 40 hours, after which the percentage of dead cells (7-AAD+) was determined by 7-AAD staining and flow cytometry. (B) Parallel studies were performed in RPMI8226. 8226/pSUPER and 8226/shBim cells were treated with 150 nM UCN-01 plus or minus 5 μM PD184352 for 24 hours, and the content of dead cells was determined. For panels A and B, the results represent the means (± SD) for 3 separate experiments performed in triplicate. **Significantly lower than the value for pSUPER control cells with same treatment (P < .001 for both panels). (C) U266/shBim and U266/pSUPER cells were treated as described for panel A, after which cells were lysed and subjected to Western blot analysis to assess phospho-ERK1/2 expression, caspase-3 cleavage, and PARP degradation. CF indicates cleavage fragment. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer. Two additional studies yielded equivalent results. (D) Alternatively, cells were lysed and subjected to immunoprecipitation (IP) with Bax 6A7 or Bak Ab-1 and then WB using anti-Bax or anti-Bak to monitor the conformational change of Bax and Bak as described for Figure 3C; 200 μg protein per condition was employed for IP. IgG levels are shown to ensure equal loading and transfer. The results of representative experiments are shown; 2 additional studies yielded equivalent results.
BimEL knockdown by shRNA prevents conformational change of Bax/Bak and apoptosis in cells coexposed to PD184352/UCN-01. (A) U226 cells were stably transfected with constructs encoding shRNA against human Bim (shBim) or scrambled shRNA (pSUPER) as control, respectively, after which stable clones (1 for pSUPER control and 2 for shBim, designed W4 and W12) were obtained by selection with puromycin. Western blot analysis was performed to monitor down-regulation of Bim expression in both shBim clones (inset). Cells were then exposed to 100 nM UCN-01 plus or minus 5 μM PD184352 for 40 hours, after which the percentage of dead cells (7-AAD+) was determined by 7-AAD staining and flow cytometry. (B) Parallel studies were performed in RPMI8226. 8226/pSUPER and 8226/shBim cells were treated with 150 nM UCN-01 plus or minus 5 μM PD184352 for 24 hours, and the content of dead cells was determined. For panels A and B, the results represent the means (± SD) for 3 separate experiments performed in triplicate. **Significantly lower than the value for pSUPER control cells with same treatment (P < .001 for both panels). (C) U266/shBim and U266/pSUPER cells were treated as described for panel A, after which cells were lysed and subjected to Western blot analysis to assess phospho-ERK1/2 expression, caspase-3 cleavage, and PARP degradation. CF indicates cleavage fragment. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antitubulin antibodies to ensure equal loading and transfer. Two additional studies yielded equivalent results. (D) Alternatively, cells were lysed and subjected to immunoprecipitation (IP) with Bax 6A7 or Bak Ab-1 and then WB using anti-Bax or anti-Bak to monitor the conformational change of Bax and Bak as described for Figure 3C; 200 μg protein per condition was employed for IP. IgG levels are shown to ensure equal loading and transfer. The results of representative experiments are shown; 2 additional studies yielded equivalent results.
Mutation of Bim phosphorylation site serine 65 sensitizes MM cells to UCN-01
The functional significance of BimEL phosphorylation in the response of human MM cells to UCN-01 was then examined more rigorously. To this end, U266 cells were stably transfected with wild-type (wt) or an S65A mutant (serine substituted by alanine) Bim construct (Figure 5A). These cells exhibited equivalent basal levels of phospho-ERK1/2 compared with empty-vector controls. Similar to endogenous BimEL in parental cells (Figure 1A), wt BimEL was susceptible to phosphorylation and degradation following UCN-01 exposure (Figure 5B). In marked contrast, S65A-mutated BimEL was largely resistant to UCN-01–mediated BimEL phosphorylation or down-regulation, although UCN-01 induced roughly comparable ERK1/2 activation in both wt and S65A cells (Figure 5B). As shown in Figure 5C, cells transfected with wt Bim exhibited equivalent sensitivity to UCN-01 as empty-vector controls (P > .05) and parental cells (data not shown), presumably due to degradation of the exogenous wt BimEL by UCN-01. Notably, cells transfected with S65A mutants were significantly more sensitive to UCN-01 lethality than cells transfected with wt Bim (P < .05 or .01 for 100 and 150 nM UCN-01, respectively). Collectively, these findings support the notion that prevention of BimEL phosphorylation/down-regulation induced by UCN-01 plays a significant functional role in potentiation of UCN-01 lethality by MEK1/2 inhibitors.
Mutation of serine 65 on Bim confers resistance to UCN-01–mediated phosphorylation/degradation and sensitizes MM cells to UCN-01 treatment. (A) U266 cells were stably transfected with HA-tagged mouse wt and serine 65 mutant (S65A) Bim constructs or its empty vector (pCDNA3). The stable clones (1 for empty-vector control and wt Bim each and 2 for S65A mutants designated c19 and c32) were obtained by selection with G418. WB was performed to monitor expression of HA-tagged target proteins as well as phospho-ERK expression in these cells. (B) U266/wt Bim and U266/S65A Bim cells were exposed to 100 nM and 150 nM UCN-01 for 36 hours, after which ERK1/2 phosphorylation and HA-tagged Bim expression were monitored by WB. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). Two additional studies yielded equivalent results. (C) Cells were treated as described for panel B, after which the percentage of apoptotic cells (annexin V+) was determined by annexin V–FITC staining and flow cytometry. Results represent the means (± SD) for 3 separate experiments performed in triplicate.*Significantly greater than the value for wt Bim-transfected cells with the same treatment (*P < .05 and **P < .01).
Mutation of serine 65 on Bim confers resistance to UCN-01–mediated phosphorylation/degradation and sensitizes MM cells to UCN-01 treatment. (A) U266 cells were stably transfected with HA-tagged mouse wt and serine 65 mutant (S65A) Bim constructs or its empty vector (pCDNA3). The stable clones (1 for empty-vector control and wt Bim each and 2 for S65A mutants designated c19 and c32) were obtained by selection with G418. WB was performed to monitor expression of HA-tagged target proteins as well as phospho-ERK expression in these cells. (B) U266/wt Bim and U266/S65A Bim cells were exposed to 100 nM and 150 nM UCN-01 for 36 hours, after which ERK1/2 phosphorylation and HA-tagged Bim expression were monitored by WB. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). Two additional studies yielded equivalent results. (C) Cells were treated as described for panel B, after which the percentage of apoptotic cells (annexin V+) was determined by annexin V–FITC staining and flow cytometry. Results represent the means (± SD) for 3 separate experiments performed in triplicate.*Significantly greater than the value for wt Bim-transfected cells with the same treatment (*P < .05 and **P < .01).
Ectopic expression of Bcl-xL or Bcl-2 blocks PD184352/UCN-01–induced apoptosis independently of effects on BimEL phosphorylation
To gain insights into the relationship between the protective effects of Bcl-xL/Bcl-2 and phosphorylation of BimEL, RPMI8226 cells and U266 cells were stably transfected with full-length human Bcl-xL or murine Bcl-2, respectively (Figure 6A,B, insets). As shown in Figure 6A,B, ectopic expression of either Bcl-xL or Bcl-2 dramatically protected cells from PD184352/UCN-01 and/or U0126/UCN-01 lethality compared with empty-vector controls (P < .001 in both cases), consistent with previous observations in human leukemia cells.36 However, ectopically expressed Bcl-xL and Bcl-2 failed to modify the ability of PD184352 to block UCN-01–mediated ERK1/2 and BimEL phosphorylation (Figure 6C,D), although they almost completely blocked cytochrome c release, caspase-3 activation, and PARP degradation in cells coexposed to PD184352 and UCN-01. These findings suggest that Bcl-xL and Bcl-2 act independently of BimEL phosphorylation/degradation to prevent PD184352/UCN-01–induced mitochondrial injury and apoptosis.
Ectopic expression of Bcl-xL or Bcl-2 blocks PD184352/UCN-01–induced apoptosis without affecting phosphorylation status of BimEL. (A) RPMI8226 cells were stably transfected with human wild-type Bcl-xL construct or its empty vector (pSSFV). The stable clones (1 for empty-vector control and 2 for Bcl-xL designated w1 and w8) were obtained by selection with G418, and expression of Bcl-xL was monitored by Western blot analysis (inset). 8226/Bcl-xL and 8226/pSFFV cells were exposed to 150 nM UCN-01 and 5 μM PD184352 or 20 μM U0126 for 24 hours, after which the percentage of dead cells (7-AAD+) was determined by 7-AAD staining and flow cytometry. (B) U266 cells were stably transfected with wild-type mouse Bcl-2 (moBcl-2) and its empty vector (pUSE). The stable clones (1 for pUSE and 2 for moBcl-2 designated 1B3 and 1B4) were obtained by selection with G814, and Western blot analysis was performed to monitor expression of either mouse Bcl-2 or endogenous Bcl-2 using antibodies against mouse and human Bcl-2 (inset), respectively. These cells were exposed to 100 nM UCN-01 plus or minus 5 μM PD184352 for 40 hours, and the percentage of dead cells (7-AAD+) was determined by flow cytometry. Results represent the means (± SD) for 3 separate experiments performed in triplicate. **Significantly lower than the value for empty-vector controls with the same treatment (P < .001). (C,D) 8226/Bcl-xL (clone w1), U266/Bcl-2 (clone 1B4), and their empty-vector control cells were treated as described for panels A and B, after which WB was performed to assess expression of phospho-ERK1/2, Bim, cleavage of caspase-3, and PARP degradation. Phosphorylated forms of BimEL are manifested by slowly migrating species (arrows). CF indicates cleavage fragments. Alternatively, for 8226/Bcl-xL and 8226/pSFFV cells in Figure 6C, cytosolic S-100 fractions were prepared and subjected to WB to monitor cytochrome c expression as described in “Materials and methods.” Each lane was loaded with 20 μg protein, and blots were stripped and reprobed with antiactin or antitubulin antibodies to ensure equal loading and transfer. Two additional studies yielded equivalent results.
Ectopic expression of Bcl-xL or Bcl-2 blocks PD184352/UCN-01–induced apoptosis without affecting phosphorylation status of BimEL. (A) RPMI8226 cells were stably transfected with human wild-type Bcl-xL construct or its empty vector (pSSFV). The stable clones (1 for empty-vector control and 2 for Bcl-xL designated w1 and w8) were obtained by selection with G418, and expression of Bcl-xL was monitored by Western blot analysis (inset). 8226/Bcl-xL and 8226/pSFFV cells were exposed to 150 nM UCN-01 and 5 μM PD184352 or 20 μM U0126 for 24 hours, after which the percentage of dead cells (7-AAD+) was determined by 7-AAD staining and flow cytometry. (B) U266 cells were stably transfected with wild-type mouse Bcl-2 (moBcl-2) and its empty vector (pUSE). The stable clones (1 for pUSE and 2 for moBcl-2 designated 1B3 and 1B4) were obtained by selection with G814, and Western blot analysis was performed to monitor expression of either mouse Bcl-2 or endogenous Bcl-2 using antibodies against mouse and human Bcl-2 (inset), respectively. These cells were exposed to 100 nM UCN-01 plus or minus 5 μM PD184352 for 40 hours, and the percentage of dead cells (7-AAD+) was determined by flow cytometry. Results represent the means (± SD) for 3 separate experiments performed in triplicate. **Significantly lower than the value for empty-vector controls with the same treatment (P < .001). (C,D) 8226/Bcl-xL (clone w1), U266/Bcl-2 (clone 1B4), and their empty-vector control cells were treated as described for panels A and B, after which WB was performed to assess expression of phospho-ERK1/2, Bim, cleavage of caspase-3, and PARP degradation. Phosphorylated forms of BimEL are manifested by slowly migrating species (arrows). CF indicates cleavage fragments. Alternatively, for 8226/Bcl-xL and 8226/pSFFV cells in Figure 6C, cytosolic S-100 fractions were prepared and subjected to WB to monitor cytochrome c expression as described in “Materials and methods.” Each lane was loaded with 20 μg protein, and blots were stripped and reprobed with antiactin or antitubulin antibodies to ensure equal loading and transfer. Two additional studies yielded equivalent results.
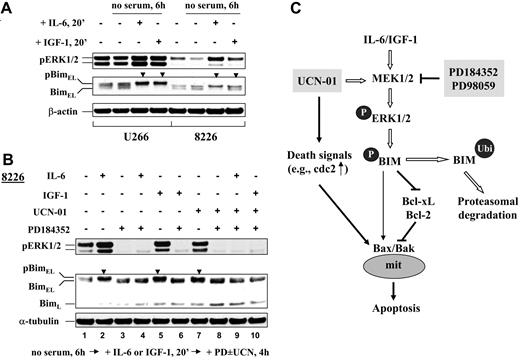
The UCN-01/PD184352 regimen blocks ERK1/2 activation and BimEL phosphorylation in MM cells exposed to IGF-1 and IL-6
We previously reported that IL-6 and IGF-1, factors critical to the survival of MM cells and that confer resistance to conventional chemotherapeutic agents by activating survival signal pathways, including MEK1/2/ERK1/2,37 failed to protect human MM cellsfrom the UCN-01/MEK1/2 inhibitor regimen.23 However, the mechanism underlying this phenomenon is currently unclear. Because ERK1/2 activation represents a major upstream signal for BimEL phosphorylation induced by survival factors,4 the effects of IL-6 and IGF-1 on BimEL phosphorylation were examined in U266 and RPMI8226 cells exposed to the UCN-01/PD184352 regimen. As shown in Figure 7A, both IGF-1 and IL-6 (20-minute exposure) induced ERK1/2 activation, consistent with previous reports,23 as well as BimEL phosphorylation (Figure 7A, arrows), reflected by the appearance of a slowly migrating band (Figure 7A, arrows). Longer IL-6 or IGF-1 exposure intervals (eg, 18 hours) also induced BimEL degradation (data not shown). Parallel studies were then performed in RPMI8226 cells exposed to PD184352 with or without UCN-01 in the presence or absence of IL-6 or IGF-1. As shown in Figure 7B, administration of PD184352 blocked ERK1/2 activation and BimEL phosphorylation induced by IL-6 (Figure 7B, lane 4 versus lane 2) and IGF-1 (Figure 7B, lane 6 versus lane 5). Moreover, both IL-6 and IGF-1 failed to prevent PD184352 from inhibiting ERK1/2 activation or BimEL phosphorylation in UCN-01–treated cells (Fiugre 7B, lanes 8-10); nor did they block apoptosis induced by the PD184352/UCN-01 regimen as reported previously.23 Together, these findings raise the possibility that continuing interference with BimEL phosphorylation may contribute to the inability of IL-6 or IGF-1 to oppose UCN-01/PD184352 lethality.
IL-6 and IGF-1 fail to prevent PD184352 from blocking UCN-01–mediated BimEL phosphorylation. (A) U266 and RPMI8226 cells were cultured in serum-free medium for 6 hours followed by exposure to IL-6 (100 ng/mL) or IGF-1 (400 ng/mL) for 20 minutes, after which cells were lysed and subjected to Western blot analysis to assess expression of phospho-ERK1/2 and Bim. (B) RPMI8226 cells were starved and preincubated (in some cases) with IL-6 or IGF-1 as described for panel A and then exposed to 150 nM UCN-01 plus or minus 5 μM PD184352 for 4 hours, after which cells were lysed and subjected to Western blot analysis to monitor ERK1/2 phosphorylation and Bim expression. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antiactin or antitubulin antibodies to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). Two additional studies yielded equivalent results. (C) Hypothetical model for Bim involvement in UCN-01 and MEK1/2 inhibitor interactions in MM cells. UCN-01 triggers death signals (eg, “unscheduled” activation of p34cdc2 through inhibition of Chk1), which leads to apoptosis. However, the putative proapoptotic actions of UCN-01 may be opposed by a compensatory activation of ERK1/2, which leads to phosphorylation and proteasomal degradation of the proapoptotic BH3 protein Bim. These events can also be stimulated by several MM survival factors, including IL-6 and IGF-1. Blocking ERK1/2 activation by pharmacologic MEK1/2 inhibitors (eg, PD184352 and PD98059) leads to accumulation of Bim, which binds to and disables Bcl-xL/Bcl-2, thus rendering MM cells more vulnerable to UCN-01 lethality, even in the presence of IL-6 or IGF-1. The possibility that increased expression of Bim may directly induce activation of the multidomain proapoptotic proteins Bax/Bak cannot be excluded.
IL-6 and IGF-1 fail to prevent PD184352 from blocking UCN-01–mediated BimEL phosphorylation. (A) U266 and RPMI8226 cells were cultured in serum-free medium for 6 hours followed by exposure to IL-6 (100 ng/mL) or IGF-1 (400 ng/mL) for 20 minutes, after which cells were lysed and subjected to Western blot analysis to assess expression of phospho-ERK1/2 and Bim. (B) RPMI8226 cells were starved and preincubated (in some cases) with IL-6 or IGF-1 as described for panel A and then exposed to 150 nM UCN-01 plus or minus 5 μM PD184352 for 4 hours, after which cells were lysed and subjected to Western blot analysis to monitor ERK1/2 phosphorylation and Bim expression. Each lane was loaded with 20 μg protein; blots were stripped and reprobed with antiactin or antitubulin antibodies to ensure equal loading and transfer. Phosphorylated forms of BimEL are manifested by slowly migrating species (▼). Two additional studies yielded equivalent results. (C) Hypothetical model for Bim involvement in UCN-01 and MEK1/2 inhibitor interactions in MM cells. UCN-01 triggers death signals (eg, “unscheduled” activation of p34cdc2 through inhibition of Chk1), which leads to apoptosis. However, the putative proapoptotic actions of UCN-01 may be opposed by a compensatory activation of ERK1/2, which leads to phosphorylation and proteasomal degradation of the proapoptotic BH3 protein Bim. These events can also be stimulated by several MM survival factors, including IL-6 and IGF-1. Blocking ERK1/2 activation by pharmacologic MEK1/2 inhibitors (eg, PD184352 and PD98059) leads to accumulation of Bim, which binds to and disables Bcl-xL/Bcl-2, thus rendering MM cells more vulnerable to UCN-01 lethality, even in the presence of IL-6 or IGF-1. The possibility that increased expression of Bim may directly induce activation of the multidomain proapoptotic proteins Bax/Bak cannot be excluded.
Discussion
While previous studies have shown that in malignant hematopoietic cells exposure to UCN-01 results in the pronounced activation of the MEK1/2/ERK1/2 pathway and that pharmacologic interruption of the latter process (eg, by MEK1/2 inhibitors, farnesyltransferase inhibitors, and HMG CoA-reductase inhibitors) dramatically sensitizes cells to UCN-01 lethality,23,26,27 the mechanism(s) underlying this phenomenon has not yet been defined. Among numerous possibilities, the BH3-only proapoptotic protein Bim represents a plausible candidate linking ERK1/2 inactivation to the apoptotic machinery in this setting. Bim expression is regulated at both transcriptional and posttranslational levels, depending upon the stimuli and signaling pathways involved.7 In the latter case, multiple kinases have been implicated in posttranslational regulation (phosphorylation in particular) of Bim, including Akt,20 JNK,13 and ERK1/2.16 More specifically, ERK1/2 represents a major kinase for BimEL phosphorylation on multiple sites, culminating in its ubiquitination and proteasomal degradation.14,16 The present results indicate for the first time that UCN-01 induces BimEL phosphorylation and degradation, events largely abrogated by pharmacologic MEK1/2 inhibitors (eg, PD184352 and PD98059). Furthermore, constitutively active MEK1 reduced the ability of PD98059, a MEK1/2 inhibitor that primarily inhibits the Raf/MEK1 interaction,32,33 from blocking UCN-01–mediated ERK1/2 activation and BimEL phosphorylation/degradation. In contrast, CA-MEK1 was ineffective in overcoming the effects of PD184352, which inhibits both the Raf/MEK1 interaction and MEK1 activity directly.38,39 In addition, UCN-01 failed to induce BimEL phosphorylation of a protein mutated at serine 65, a critical phosphorylation site for ERK1/2,14 despite equivalent ERK1/2 activation. In contrast, neither BimEL knockdown by shRNA nor ectopic expression of wt or S65A Bim affected UCN-01–induced ERK1/2 activation. Taken together, these findings argue that UCN-01–induced BimEL phosphorylation/degradation proceeds through ERK1/2 activation.
The bulk of evidence indicates that ERK1/2-mediated posttranslational phosphorylation of Bim (specifically BimEL) plays a significantly functional role in apoptosis induced by the UCN-01/MEK1/2 inhibitor regimen. First, CA-MEK1 significantly diminished apoptosis induced by UCN-01 in combination with PD98059, consistent with diminished ERK1/2 activation and BimEL phosphorylation noted above. In marked contrast, CA-MEK1 failed to protect cells from PD184352/UCN-01 lethality, presumably due to ability of this MEK1/2 inhibitor to inhibit completely the activity of CA-MEK1. Importantly, down-regulation of BimEL by shRNA significantly diminished caspase-3 cleavage/activation, PARP degradation, and apoptosis in PD184352/UCN-01–treated cells. Notably, ectopic expression of S65A BimEL, a mutant nonphosphorylatable by ERK1/2,14,18 significantly sensitized MM cells to UCN-01 lethality, presumably due to the inability of UCN-01 to induce BimEL phosphorylation/down-regulation in these cells. Collectively, these findings argue that MEK1/2 inhibitor-mediated interference with UCN-01–induced BimEL phosphorylation/down-regulation, stemming from ERK1/2 inactivation, plays a significant functional role in the lethality of this regimen. In addition to inactivation of BimEL, activation of ERK1/2 has been shown to antagonize apoptosis by multiple mechanisms, including inactivation of procaspase-940 or the “sensitizer” Bcl-2 family protein Bad.41 Consequently, the possibility that other mechanism(s) may also contribute to synergistic interactions between UCN-01 and MEK1/2 inhibitors cannot be excluded.
The mode of action of Bim in promoting apoptosis is complex and, in all likelihood, multifactorial. For example, Bim is generally viewed, like Bid and Puma, as an “activator” BH3-only protein that directly activate Bax/Bak,42,43 whereas it can bind to and antagonize the ability of antiapoptotic proteins (eg, Bcl-2 and Bcl-xL) to prevent Bax/Bak activation.44,45 Currently, the mechanism by which proapoptotic BH3-only molecules induce apoptosis is controversial, and a number of competing models exist.2 While there is general agreement that “sensitizer” BH3-only molecules (eg, Bad) act primarily by interfering with the function of antiapoptotic family members (ie, Bcl-2 and Bcl-xL),1 the mechanism by which “activator” BH3-only proteins (eg, Bid, Bim, and Puma) exert their effects remains the subject of dispute. For example, Bim could theoretically bind to and thereby directly activate Bax and probably Bak1,18 or, alternatively, act indirectly via preventing antiapoptotic Bcl-2 family members from inhibiting Bax/Bak.42,45 In a recent study,44 Willis et al demonstrated that induction of apoptosis in various cell types occurred in the absence of discernible interactions between the “activator” BH3-only proteins (Bim, Bid, and Puma) and the proapoptotic multidomain proteins (Bax and Bak). Moreover, Bax/Bak activation and apoptosis were unimpaired in cells lacking these BH3-only proteins. Such findings are most compatible with a model in which BH3-only proteins like Bim act primarily by blocking the function of antiapoptotic family members such as Bcl-2 and Bcl-xL rather than activating Bax/Bak directly. In this context, it is noteworthy that MEK1/2 inhibitors, which blocked BimEL phosphorylation and induced its accumulation, led to an increase in the associations between BimEL and Bcl-2 or Bcl-xL. Notably, increased binding between BimEL and Bcl-2 or Bcl-xL was associated with conformational changes in Bax/Bak as well as Bax translocation to organellar membrane in cells coexposed to UCN-01. In addition, ectopic expression of Bcl-2 or Bcl-xL substantially reduced PD184352/UCN-01–mediated lethality without affecting phosphorylation status or protein levels of BimEL in cells exposed to these agents individually or in combination. It is possible that in such cells, the large excess of ectopically expressed Bcl-xL or Bcl-2 overcomes the binding capacity of accumulated BimEL. Collectively, these observations are consistent with a model in which Bim, or its specific isoform BimEL, interacts with antiapoptotic proteins (eg, Bcl-2/Bcl-xL) and, in so doing, releases its inhibition of Bax/Bak.44 However, this evidence does not exclude the possibility that prevention of phosphorylation of BimEL (ie, by MEK1/2 inhibitors) allows this protein to interact directly with and activate Bax.18 Whatever the primary mechanism(s) of BimEL action, the observation that knockdown of BimEL by shRNA diminished conformational change of Bax and Bak in PD184352/UCN-01–treated cells argues strongly that Bim is critical for activation of these multidomain proapoptotic proteins.
Previous studies have shown that coadministration of MEK1/2 inhibitors and UCN-01 overcomes the antiapoptotic effects of prosurvival cytokines such as IL-6 and IGF-1 in human MM cells.23 In addition, the antiapoptotic effects of these factors have been related to ERK1/2 activation, among other signaling pathways.37 Notably, exposure to IL-6 or IGF-1 induced ERK1/2 activation and BimEL phosphorylation, an effect blocked by MEK1/2 inhibitors. It is therefore tempting to speculate that phosphorylating/inactivating BimEL through ERK1/2 activation represents one of the prosurvival actions of IL-6 and IGF-1 in human MM cells. However, MEK1/2 inhibitors were fully capable of preventing UCN-01–induced ERK1/2 activation and BimEL phosphorylation in the presence of IL-6 and IGF-1, potentially accounting for the ability of MEK1/2 inhibitors to circumvent IL-6– or IGF-1–mediated antiapoptotic effects in UCN-01–treated MM cells. In view of evidence that MEK1/2 inhibitors potentiate the lethal effects of several other novel agents in addition to UCN-01, including tyrosine kinase inhibitors46 and small molecule Bcl-2 antagonists,47 it is possible that activation of BimEL may represent a common mechanism underlying interactions between these agents. These findings also raise the possibility that MEK1/2 inhibitors may oppose cytokine- or stromal cell–mediated resistance to more conventional antimyeloma agents37 mediated by the MEK1/2/ERK1/2 pathway. Taken together, these findings argue that while in malignant hematopoietic cells, UCN-01 exerts certain proapoptotic actions (eg, “inappropriate” activation of cdc2 through Chk1 inhibition24 and/or inhibition of PDK148 ), other actions such as MEK1/2/ERK1/2 activation and phosphorylation/degradation of BimEL, serve to attenuate lethality. Thus, by blocking this cytoprotective arm, MEK1/2 inhibitors may shift the balance in favor of the proapoptotic actions of UCN-01.
The mechanism by which UCN-01 activates MEK1/2/ERK1/2 remains to be fully elucidated, although recent studies indicate that in human MM cells exposure to UCN-01 induces activation of Ras, an upstream effector of MEK1/2/ERK1/2.26 The implication of the present findings is that in human MM cells, phosphorylation/degradation of BimEL mediated by ERK1/2 may represent a prosurvival mechanism by which such cells escape the lethal consequences of cell-cycle checkpoint dysregulation and/or “inappropriate” activation of cdc2 (eg, by UCN-01). A model providing a framework for understanding the present findings is illustrated in Figure 7C. UCN-01 triggers death signals (eg, “inappropriate” p34cdc2 activation through inhibition of Chk1). The compensatory activation of ERK1/2, which lies downstream of IL-6 and IGF-1, may neutralize these death signals by phosphorylating the BH3-only protein Bim, a critical mediator of the apoptotic signaling machinery. Phosphorylation of Bim leads to its ubiquitination and proteasomal degradation, thereby permitting cells to survive UCN-01 exposure. Conversely, coadministration of MEK1/2 inhibitors (eg, PD184352 and PD98059) blocks Bim phosphorylation/degradation by abrogating ERK1/2 activation in UCN-01–treated cells, resulting in Bim accumulation. As a consequence, increased Bim binds to Bcl-xL and Bcl-2 and thus disables their antiapoptotic function (ie, prevention of Bax/Bak activation). These events lower the threshold for UCN-01–associated death signals, rendering cells significantly more sensitive to this agent. Such a model could also explain the observation that IL-6 and IGF-1 fail to confer resistance to this combination regimen. Thus, BimEL phosphorylation/expression status might represent a determinant of the activity of such strategies. If validated, this concept could have implications for the development of novel antimyeloma regimens involving the combined administration of clinically relevant agents targeting the Ras/Raf/MEK/ERK pathway (eg, MEK1/2 inhibitors, farnesyltransferase inhibitors, or HMG CoA-reductase inhibitors) and cell-cycle checkpoint inhibitors (eg, UCN-01). It could also have implications for attempts to understand mechanisms underlying synergistic interactions between MEK1/2 inhibitors and other targeted agents in MM and possibly other hematologic malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA93738, CA100866, CA88906, and CA72955 from the National Institutes of Health (NIH), a Translational Research Award from the Leukemia and Lymphoma Society of America (6045-03), an award from the Department of Defense (DAMD-17-03-1-0209), an award from the V Foundation., and the Universal Professorship (P.D.).
National Institutes of Health
Authorship
Contribution: X-Y.P. designed and performed the research, analyzed the data, and wrote the paper; Y.D. and S.G. designed the research, analyzed the data, and wrote the paper; S.T. performed the research; and J.L., H.H., and P.D. helped to design the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University/Massey Cancer Center, MCV Station Box 230, Richmond VA, 23298; e-mail: sgrant@mcvh-vcu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal