Abstract
The molecular basis of the erythrocytosis group of red cell disorders is incompletely defined. Some cases are due to dysregulation of erythropoietin (Epo) synthesis. The hypoxia inducible transcription factor (HIF) tightly regulates Epo synthesis. HIF in turn is regulated through its α subunit, which under normoxic conditions is hydroxylated on specific prolines and targeted for degradation by the von Hippel Lindau (VHL) protein. Several mutations in VHL have been reported in erythrocytosis, but only 1 mutation in the HIF prolyl hydroxylase PHD2 (prolyl hydroxylase domain protein 2) has been described. Here, we report a novel PHD2 mutation, Arg371His, which causes decreased HIF binding, HIF hydroxylase, and HIF inhibitory activities. In the tertiary structure of PHD2, Arg371 lies close to the previously described Pro317Arg mutation site. These findings substantiate PHD2 as a critical enzyme controlling HIF and therefore Epo in humans, and furthermore suggest the location of an active site groove in PHD2 that binds HIF.
Introduction
Idiopathic erythrocytosis (IE) is a rare condition characterized by an increase in red cell mass, and is assumed to be caused by a heterogeneous group of unidentified genetic mechanisms.1 Erythrocytosis (also referred to in the literature as polycythemia) has been defined as primary or secondary. In primary erythrocytosis, a genetic defect in the erythroid progenitor cells leads to enhanced production of red blood cells, as exemplified by truncation of the erythropoietin receptor (EpoR)2,3 and JAK2 mutations.4–7 It is associated with subnormal Epo levels. In contrast, secondary erythrocytosis is driven by increased production of Epo, resulting in increased or inappropriately normal levels of Epo
An autosomal recessively inherited form of erythrocytosis, endemic in the Chuvash population in Russia, is caused by a homozygous mutation, Arg200Trp, in the von Hippel Lindau (VHL) protein. The mutation also exists in families of Asian and European ancestry.8–12 The VHL protein is part of an E3 ubiquitin ligase complex that targets hypoxia inducible transcription factor-α (HIF-α) for proteasomal degradation. This is dependent on hydroxylation of specific proline residues in the oxygen-dependent degradation domain (ODD) of HIF-α mediated by the prolyl hydroxylase domain (PHD; also known as HPH and EGLN) family of prolyl hydroxylases.13–15 Under hypoxic conditions, this modification is inhibited, thereby stabilizing HIF-α. Recently, we identified a heterozygous mutation in PHD2, Pro317Arg, in a family with erythrocytosis,16 thus confirming PHD2 as a major regulator of HIF-α in oxygen homeostasis. Consequently, PHD2 associated erythrocytosis has been designated as a separate disease entity by the Online Mendelian Inheritance in Man (OMIM; no. 609820).17 Moreover, this raises the possibility that other patients with IE may harbor mutations in the PHD2 gene, and we report 1 such patient here. The findings not only lend further support to a critical role for PHD2 in red blood cell control, but also point to a potential substrate-binding site in PHD2, which has been previously undefined.
Patients, materials, and methods
Patients
A total of 181 patients, who did not fulfil the polycythemia vera (PV) diagnostic criteria proposed by the British Committee for Standards in Haematology,18 and had a raised red cell mass, have been investigated. All patients provided informed written consent on entering the study, which had been approved by the Queen's University Research Ethics Committee, in accordance with the Declaration of Helsinki.
Mutation screening
Using polymerase chain reaction (PCR)–direct sequencing, PHD1 (exons 1-5), PHD2 (exons 1-4), and PHD3 (exons 1-3) were examined as described previously.16 A group of 200 normal control samples (Human Random Control DNA panels; ECACC [European Collection of Cell Cultures], Salisbury, United Kingdom) was screened for the G1112A base change by restriction digest with Tsp45I.
Plasmids and proteins
pcDNA3-FlagPHD2 Arg371His and pGEX-HIF-2α (aa516-549) were constructed by standard recombinant DNA methods. The sources of all other plasmids, as well as the HEK293 cells used in the reporter gene assays, have been described.19–22 GST-HIF-2α (516-549) and GST-HIF-1α (531-575) were purified as described previously.19 35S-labeled, in vitro–translated FlagPHD2 or FlagPHD2 Arg371His was prepared using either rabbit reticulocyte lysate (binding assays) or wheat germ (hydroxylase assays) TnT kits (Promega, Madison, WI). FeCl2 (50 μM) was included in the reaction mixture. 35S-labeled, in vitro–translated Flag-VHL was prepared using a rabbit reticulocyte lysate TnT Quick kit (Promega).
Assays
GST pulldown assays and prolyl hydroxylase and luciferase assays were performed essentially as previously described.19–22
Patient history
A young man at the age of 29 years was diagnosed with a raised hemoglobin (Hb; 188 g/L [18.8 g/dL]) and an elevated hematocrit (Hct; 0.56). His red cell mass was 139% of the predicted value. White cell counts (8.2 × 109/L) and platelet counts (190 × 109/L) were in the normal range. The Epo level was inappropriately normal at 12 mIU/mL (normal range of the assay was 5-25 mIU/mL). His spleen was normal on ultrasound. Both bone marrow aspirate and trephine had normal appearance. At 35 years of age, he had head computed tomography (CT) and magnetic resonance imaging (MRI) studies in which an acute sagittal sinus thrombosis was seen; no other abnormality was noted. He also had a normal chest X-ray and abdominal ultrasound. There is no history of wound healing problems. He has been managed for the past 9 years by regular venesection and remains well at the age of 38 years.
Results and discussion
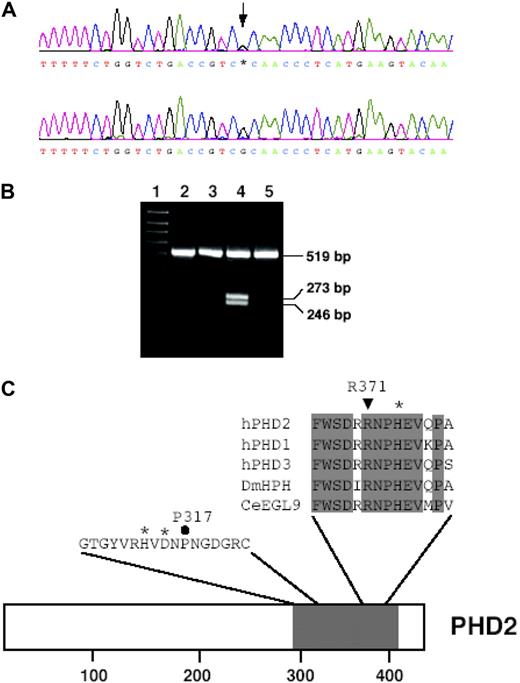
The EpoR,3 VHL,8–10 and PHD216 genes, as reflected in the current OMIM classification of erythrocytosis, were screened in all patients with IE. Consequently, a novel mutation, G1112A, in PHD2 exon 3 (Figure 1A) was detected in 1 patient with wild-type EpoR and VHL. In addition, screening the other HIF prolyl hydroxylases, PHD1 and PHD3, did not reveal any further defects. To eliminate the possibility that the base change was a single-nucleotide polymorphism, a group of 200 normal control samples was examined by restriction digest, as the presence of an A at base 1112 forms a new restriction site for the enzyme Tsp45I. The G1112A base change was absent in this group of controls (data not shown). Furthermore, screening the mother who had normal Hct (0.436) and Hb (135 g/L [13.5 g/dL]) levels did not detect this mutation (Figure 1B). Unfortunately, the father was not available for testing.
Identification of the 1112 G > A mutation in the PHD2 gene. (A) Detection of the G1112A mutation by PCR-direct sequencing. PCR was performed on total peripheral blood DNA using a set of primers to specifically amplify exon 3. Sequencing detected a heterozygous G-to-A change at base 1112 as indicated by an arrow (top panel) as compared with wild-type sequence (bottom panel). Shown are nucleotides 1093 to 1131. Bases are as follows: G, black; A, green; T, red; and C, blue. (B) Screening family members for G1112A mutation. The presence of A at base 1112 creates a restriction site for the enzyme Tsp45I to give 2 products of 246 bp and 273 bp from an exon 3 PCR product from the patient (lane 4). The mother of the patient (lane 3) was screened, and the absence of the 246 bp and 273 bp bands indicated she did not possess the G1112A mutation. Lane 1 contains a 100-bp DNA size marker; lane 2, nondigested exon 3 PCR product of 519 bp; and lane 5, Tsp45I-digested exon 3 PCR product from a control sample negative for the mutation as detected by sequencing. (C) Comparison of amino acid sequence from residues 366-379 (numbering according to hPHD2 nomenclature) in human HIF prolyl hydroxylases with those from D. melanogaster (DmHPH) and C. elegans (CeEGL9). Sequence shading indicates completely conserved residues. *Iron-chelating residue His-374. ▼ indicates Arg371 of human PHD2, which is predicted to be changed to His by the G1112A mutation. Also shown is hPHD2 sequence from residues 307-323. *Iron-chelating residues His313 and Asp315. ● indicates Pro317. The positions of these sequences in full-length hPHD2 is shown, with shading indicating prolyl hydroxylase domain. Numbers at bottom indicate residues.
Identification of the 1112 G > A mutation in the PHD2 gene. (A) Detection of the G1112A mutation by PCR-direct sequencing. PCR was performed on total peripheral blood DNA using a set of primers to specifically amplify exon 3. Sequencing detected a heterozygous G-to-A change at base 1112 as indicated by an arrow (top panel) as compared with wild-type sequence (bottom panel). Shown are nucleotides 1093 to 1131. Bases are as follows: G, black; A, green; T, red; and C, blue. (B) Screening family members for G1112A mutation. The presence of A at base 1112 creates a restriction site for the enzyme Tsp45I to give 2 products of 246 bp and 273 bp from an exon 3 PCR product from the patient (lane 4). The mother of the patient (lane 3) was screened, and the absence of the 246 bp and 273 bp bands indicated she did not possess the G1112A mutation. Lane 1 contains a 100-bp DNA size marker; lane 2, nondigested exon 3 PCR product of 519 bp; and lane 5, Tsp45I-digested exon 3 PCR product from a control sample negative for the mutation as detected by sequencing. (C) Comparison of amino acid sequence from residues 366-379 (numbering according to hPHD2 nomenclature) in human HIF prolyl hydroxylases with those from D. melanogaster (DmHPH) and C. elegans (CeEGL9). Sequence shading indicates completely conserved residues. *Iron-chelating residue His-374. ▼ indicates Arg371 of human PHD2, which is predicted to be changed to His by the G1112A mutation. Also shown is hPHD2 sequence from residues 307-323. *Iron-chelating residues His313 and Asp315. ● indicates Pro317. The positions of these sequences in full-length hPHD2 is shown, with shading indicating prolyl hydroxylase domain. Numbers at bottom indicate residues.
The G1112A mutation is predicted to result in loss of arginine at amino acid 371 and replacement with histidine. Residue 371 is 3 amino acids away from the His374 iron-chelating residue (Figure 1C). This parallels the previously reported PHD2 mutation, Pro317Arg, which was also located close to an iron-chelating residue, His313. Arg371 is conserved in all 3 PHD isoforms, as well as the single HIF prolyl hydroxylases from Drosophila melanogaster and Caenorhabditis elegans (Figure 1C).
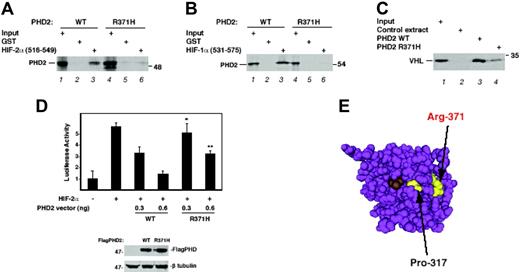
To investigate the effect that the Arg371His mutation may exert on PHD2 function, both wild-type and mutant proteins were prepared by in vitro translation and examined by in vitro binding and enzymatic assays. We found the mutant bound substantially more weakly than wild-type to HIF-2α (aa516-549), which contains the primary hydroxylacceptor prolyl residue, Pro531 (Figure 2A lanes 3 and 6). Similar assays showed that, compared with wild-type PHD2, the mutant also had a significantly decreased ability to bind HIF-1α (531-575), which contains the primary hydroxylation site, Pro564 (Figure 2B). We assayed prolyl hydroxylase activity toward HIF-2α (516-549) and observed that mutated PHD2 displays significantly less HIF hydroxylase activity than does wild-type (Figure 2C lanes 3 and 4). Furthermore, in transfection assays, Arg371His PHD2 was less effective than wild-type PHD2 in suppression of HIF-induced activation of a hypoxia response element reporter gene (Figure 2D). Taken together, the functional data all point toward a significant loss of PHD2 activity in these assays arising from the mutation.
Functional characterization of the Arg371His PHD2 mutant. (A) Association of HIF-2α with Arg371His PHD2. 35S-labeled, in vitro–translated wild-type or Arg371His FlagPHD2 was incubated with 1 μg of either GST or GST–HIF-2α (516-549) immobilized on GSH-agarose. The resins were washed and eluted, and the eluates were subjected to SDS-PAGE and autoradiography. Input represents 10% of the total. The relative recovery of wild-type PHD2 from 3 replicates is 100 ± 24 AU (arbitrary units ± SD), whereas that of Arg371His PHD2 is 2.4 ± 4 AU (P < .005). (B) Association of HIF-1α with Arg371His PHD2. Binding assays with GST–HIF-1α (531-575) were performed as described in panel A. Input represents 5% of total. The relative recovery of wild-type PHD2 from 3 replicates is 100 ± 0.1 AU, whereas that of Arg371His PHD2 is 0.8 ± 0.0001 AU (P < .005). (C) Hydroxylase activity of Arg371His PHD2. Equal quantities (as determined by phosphorimager analysis) of in vitro–translated wild-type or Arg371His FlagPHD2, or mock in vitro translation reaction, was incubated with 0.75 μg of GST–HIF-2α (516-549) for 1 hour in the presence of 2-oxoglutarate, ascorbic acid, and FeCl2. The GST–HIF-2α (516-549) was isolated using GSH-agarose, washed, and then the degree of HIF hydroxylation was assessed by subsequent incubation with 35S-labeled, in vitro–translated VHL. Input represents 5% of the total. Under the conditions of the assay, the recovery of 35S-labeled, in vitro–translated VHL using wild-type PHD2 from 3 independent experiments is 100 ± 38 AU, while that using Arg371His PHD2 is 12 ± 7.6 AU (P < .05). (D) HIF-inhibitory activity of Arg371His PHD2. HEK293 cells were cotransfected with 150 ng of (eHRE)3-Luc, 150 ng of pRL-TK, 300 ng of either pcDNA3 or pSV-Sport-HA-hHIF-2α, and either 0, 0.3, or 0.6 ng of pcDNA3-FlagPHD2 (wild-type or P317R). The total DNA dose was normalized with pcDNA3. At 48 hours after transfection, the cells were harvested and assayed for luciferase activity. Activities were normalized to that of the Renilla luciferase internal transfection control. Shown are results performed in triplicate ± SD. *P < .05; **P < .01 when comparing results of wild-type and mutant PHD2 at the same dose. In separate experiments, HEK293 cells were transfected with 2 μg of wild-type or Arg371His pcDNA3-FlagPHD2; 48 hours later, extracts (15 μg) were analyzed by Western blotting with anti-Flag (M2; Sigma, St Louis, MO) or anti–β tubulin (H-235; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. The position of PHD2, β-tubulin, and a molecular weight marker are as indicated. (E) 3D structure of PHD2. The structure was generated using Cn3D from Protein Data Bank coordinates (2G1M) deposited by McDonough et al.23 Arg371 and Pro317 are highlighted in yellow. Compound A (a 2-oxoglutarate competitive inhibitor) is shown in brown.
Functional characterization of the Arg371His PHD2 mutant. (A) Association of HIF-2α with Arg371His PHD2. 35S-labeled, in vitro–translated wild-type or Arg371His FlagPHD2 was incubated with 1 μg of either GST or GST–HIF-2α (516-549) immobilized on GSH-agarose. The resins were washed and eluted, and the eluates were subjected to SDS-PAGE and autoradiography. Input represents 10% of the total. The relative recovery of wild-type PHD2 from 3 replicates is 100 ± 24 AU (arbitrary units ± SD), whereas that of Arg371His PHD2 is 2.4 ± 4 AU (P < .005). (B) Association of HIF-1α with Arg371His PHD2. Binding assays with GST–HIF-1α (531-575) were performed as described in panel A. Input represents 5% of total. The relative recovery of wild-type PHD2 from 3 replicates is 100 ± 0.1 AU, whereas that of Arg371His PHD2 is 0.8 ± 0.0001 AU (P < .005). (C) Hydroxylase activity of Arg371His PHD2. Equal quantities (as determined by phosphorimager analysis) of in vitro–translated wild-type or Arg371His FlagPHD2, or mock in vitro translation reaction, was incubated with 0.75 μg of GST–HIF-2α (516-549) for 1 hour in the presence of 2-oxoglutarate, ascorbic acid, and FeCl2. The GST–HIF-2α (516-549) was isolated using GSH-agarose, washed, and then the degree of HIF hydroxylation was assessed by subsequent incubation with 35S-labeled, in vitro–translated VHL. Input represents 5% of the total. Under the conditions of the assay, the recovery of 35S-labeled, in vitro–translated VHL using wild-type PHD2 from 3 independent experiments is 100 ± 38 AU, while that using Arg371His PHD2 is 12 ± 7.6 AU (P < .05). (D) HIF-inhibitory activity of Arg371His PHD2. HEK293 cells were cotransfected with 150 ng of (eHRE)3-Luc, 150 ng of pRL-TK, 300 ng of either pcDNA3 or pSV-Sport-HA-hHIF-2α, and either 0, 0.3, or 0.6 ng of pcDNA3-FlagPHD2 (wild-type or P317R). The total DNA dose was normalized with pcDNA3. At 48 hours after transfection, the cells were harvested and assayed for luciferase activity. Activities were normalized to that of the Renilla luciferase internal transfection control. Shown are results performed in triplicate ± SD. *P < .05; **P < .01 when comparing results of wild-type and mutant PHD2 at the same dose. In separate experiments, HEK293 cells were transfected with 2 μg of wild-type or Arg371His pcDNA3-FlagPHD2; 48 hours later, extracts (15 μg) were analyzed by Western blotting with anti-Flag (M2; Sigma, St Louis, MO) or anti–β tubulin (H-235; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. The position of PHD2, β-tubulin, and a molecular weight marker are as indicated. (E) 3D structure of PHD2. The structure was generated using Cn3D from Protein Data Bank coordinates (2G1M) deposited by McDonough et al.23 Arg371 and Pro317 are highlighted in yellow. Compound A (a 2-oxoglutarate competitive inhibitor) is shown in brown.
The present findings provide further support for a central role for PHD2 in the control of red cell mass in humans. The patient in the present study shares certain features with the affected members of the family previously studied.16 First, the erythrocytosis is modest. Second, the Epo levels were normal, although as before, this should be regarded as inappropriately normal given the elevated hematocrit. Third, the patient is heterozygous for the PHD2 mutation, suggesting that partial loss of total cellular PHD2 activity is sufficient to induce this phenotype.
From the recently resolved 3D structure of PHD2,23 Arg371 is in the vicinity of Pro317 (Figure 2E). This therefore suggests that in PHD2, both Arg371 and Pro317 may contribute to a HIF-α substrate binding groove. The primary site of hydroxylation in HIF-α is rich in acidic residues both N- and C-terminal to the hydroxylacceptor proline. Therefore, it will also be of interest to determine if Arg371 contacts such a residue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grant R01 C090261 (to F.S.L.).
National Institutes of Health
Authorship
Contribution: M.J.P., P.W.F., T.R.J.L., and F.S.L. designed research; M.J.P. and P.W.F. performed research; M.J.P., P.W.F., P.A.B., T.R.J.L., M.F.M., and F.S.L. analyzed data; P.A.B. and M.F.M. clinically assessed the patients; and M.J.P., P.W.F., T.R.J.L., M.F.M., and F.S.L. wrote the paper. M.J.P. and P.W.F. contributed equally to the manuscript and should be considered joint first authors.
Conflict-of-interest disclosure: F.S.L. receives research grant support from GlaxoSmithKline. All other authors declare no competing financial interests.
Correspondence: Melanie J. Percy, Department of Haematology, Floor C, Tower Block, Belfast City Hospital, Lisburn Road, Belfast BT9 7AB, Northern Ireland; e-mail: melanie.percy@belfasttrust.hscni.net.