Mutations in the kinase domain (KD) of BCR-ABL are the most prevalent mechanism of acquired imatinib resistance in patients with chronic myeloid leukemia (CML). Here we examine predisposing factors underlying acquisition of KD mutations, evidence for acquisition of mutations before and during therapy, and whether the detection of a KD mutation universally implies resistance. We also provide a perspective on how the second-line Abl inhibitors dasatinib and nilotinib are faring in the treatment of imatinib-resistant CML, especially in relation to specific KD mutations. We discuss the growing importance of the multi-inhibitor–resistant 315T>I mutant and the therapeutic potential that a 315T>I inhibitor would have. Last, we assess the potential of Abl kinase inhibitor combinations to induce stable responses even in advanced CML and interpret the emerging data in the context of CML pathogenesis.
Introduction
Patients with chronic myeloid leukemia (CML) in chronic phase (CP) who receive imatinib from diagnosis have a very good prognosis. In a recent update of the IRIS study (International Randomized Study of IFN-α plus Ara-C vs STI571), the projected overall survival rate of these patients was 89% by 60 months and freedom from progression to accelerated phase (AP) or blast crisis (BC) was 93%.1 Patients achieving a 3-log reduction of BCR-ABL mRNA at 18 months had a projected overall survival rate of 100% at 60 months and a negligible risk of progression to AP/BC. Overall, the failure rate at 60 months for patients receiving imatinib in this study was 17%. Of note, failures peaked in the second year, reaching less than 1% in the fifth year. Whether the downward trend in failure rate will continue until the risk of relapse is effectively zero or whether a small risk of relapse will persist remains to be seen. Despite the extremely high response rate to imatinib and low rate of relapse, residual leukemia usually remains detectable by quantitative reverse-transcription polymerase chain reaction.2,–4 If imatinib is discontinued, recurrence is the rule. Thus, if one defines cure as the elimination of all leukemia cells, then imatinib is probably not capable of curing patients with CML, despite the remarkable improvement in therapeutic outcomes.
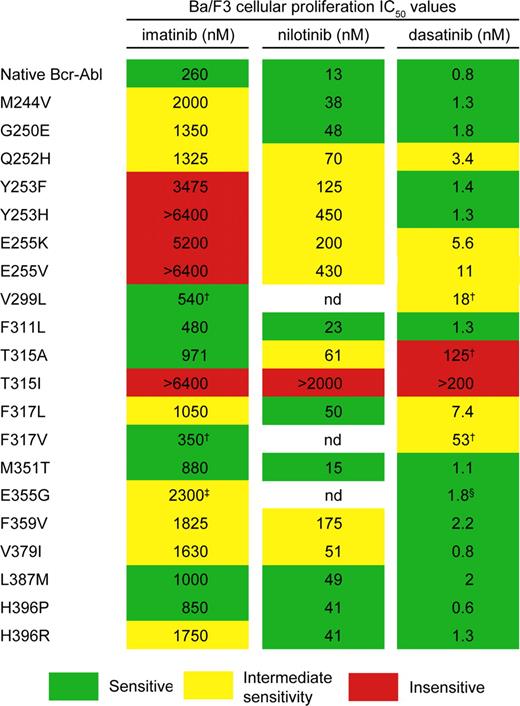
Compared with CP, imatinib responses are much less durable in patients with AP or BC,5,–7 but the second-line Abl inhibitors dasatinib8 and nilotinib9 are emerging as effective salvage therapies for imatinib failure.10,11 The best-studied and clinically dominant mechanism of imatinib resistance involves acquired point mutations within the kinase domain (KD) of BCR-ABL, most of which have been validated in vitro and profiled for resistance to imatinib, nilotinib, and dasatinib (Figure 1).12 Although beyond the scope of this article, 2 important aspects of CML research are the role of Bcr-Abl–independent mechanisms in resistance to these inhibitors and observations that the presence of a BCR-ABL mutant allele does not always explain clinical resistance to imatinib.13,14 Here we review BCR-ABL mutations in the context of CML pathogenesis and speculate on future prospects in achieving and maintaining responses on the road to a cure for CML.
Sensitivity of Bcr-Abl kinase domain mutants to Abl kinase inhibitors. Imatinib: sensitive (≤1000 nM), intermediate (≤3000 nM), insensitive (>3000 nM). Nilotinib: sensitive (≤50 nM), intermediate (≤500 nM), insensitive (>500 nM). Dasatinib: sensitive (≤3 nM), intermediate (≤60 nM), insensitive (>60 nM). aThe IC50 value is the concentration of inhibitor resulting in a 50% reduction in cell viability.8,12,21,29 †IC50 values are from Burgess et al, 2005.21 ‡IC50 value is from Shah et al, 2002.29 §IC50 value was estimated from Shah et al, 2004.8
Sensitivity of Bcr-Abl kinase domain mutants to Abl kinase inhibitors. Imatinib: sensitive (≤1000 nM), intermediate (≤3000 nM), insensitive (>3000 nM). Nilotinib: sensitive (≤50 nM), intermediate (≤500 nM), insensitive (>500 nM). Dasatinib: sensitive (≤3 nM), intermediate (≤60 nM), insensitive (>60 nM). aThe IC50 value is the concentration of inhibitor resulting in a 50% reduction in cell viability.8,12,21,29 †IC50 values are from Burgess et al, 2005.21 ‡IC50 value is from Shah et al, 2002.29 §IC50 value was estimated from Shah et al, 2004.8
How do point mutations cause resistance to imatinib, nilotinib, and dasatinib?
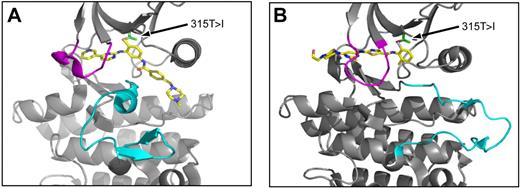
Crystallographic analysis has revealed that imatinib binds to and stabilizes an inactive, DFG (aspartate-phenylalanine-glycine)-out conformation of Abl in which the activation loop is in a “closed” substrate-mimicking position and there is extensive distortion of the ATP binding loop (Figure 2A).15 Predictably, mutations involving contact residues such as T315 confer resistance by impeding inhibitor access or eliminating critical hydrogen bonds. A second group of mutations, including those within the ATP binding loop, confer resistance by preventing Abl from adopting the specific conformation required for high-affinity imatinib binding. Last, mutations in regulatory motifs such as the activation loop may stabilize an active conformation that is inaccessible to imatinib. Nilotinib, which was derived from the imatinib scaffold, exhibits a similar binding mode, although potency is increased by approximately 30-fold because of an improved topologic fit to the enzyme.9 The enhancement in affinity translates into improved inhibitory activity against most of the common KD mutations that confer imatinib resistance.16,–18 Dasatinib is a dual Src/Abl inhibitor with more than 300-fold increased potency compared with imatinib.8 X-ray structural analysis revealed that dasatinib binds to the catalytically active state of the enzyme (Figure 2B). Although dasatinib has been widely reported to be capable of binding to both the inactive and active states of Abl, a recent study suggests that it may be excluded from the inactive conformation.19,20 The majority of mutations that confer resistance to dasatinib are predicted to involve drug contact residues, which is the basis for the activity of dasatinib against most imatinib-resistant Bcr-Abl mutants.16,21,22
Structures of imatinib and dasatinib bound to Abl kinase. (A) View of imatinib (gold) in complex with the Abl kinase domain, in which the activation loop (cyan) is in the “closed” position and the ATP binding loop (magenta) is distorted downward relative to the ATP-bound state. (B) View of dasatinib (gold) in complex with the Abl kinase domain. The activation loop (cyan) is in the “open” position and the ATP binding loop is shown in magenta. In both panels, an arrow denotes the position of Abl residue threonine-315 (side chain in green). For both inhibitors, oxygen (red) and nitrogen (blue) atoms are highlighted.
Structures of imatinib and dasatinib bound to Abl kinase. (A) View of imatinib (gold) in complex with the Abl kinase domain, in which the activation loop (cyan) is in the “closed” position and the ATP binding loop (magenta) is distorted downward relative to the ATP-bound state. (B) View of dasatinib (gold) in complex with the Abl kinase domain. The activation loop (cyan) is in the “open” position and the ATP binding loop is shown in magenta. In both panels, an arrow denotes the position of Abl residue threonine-315 (side chain in green). For both inhibitors, oxygen (red) and nitrogen (blue) atoms are highlighted.
Ascribing clinical resistance to a KD mutation requires biochemical confirmation (cell proliferation assays and purified kinase assays) that the mutation confers imatinib resistance in vitro,8,12,23 even if the mutant clone represents 100% of BCR-ABL alleles. Some rare mutations may be bystanders that comigrated with the resistant phenotype, whereas recurrent mutations that confer a minor degree of resistance (311F>L23 ) may be “propped-up” by mechanisms such as increased Bcr-Abl expression or drug efflux.24
When are KD mutations acquired?
Several observations suggest that KD mutations may be part of the natural disease evolution, irrespective of imatinib therapy. The frequency of KD mutations is higher in AP/BC compared with CP and increases with disease duration.25,26 Allele-specific polymerase chain reaction–based analysis of pretherapeutic samples revealed the same mutation type as detected at relapse, consistent with selection of resistant clones during therapy.27 A similar analysis of pre-imatinib samples from unselected CML patients detected KD mutants at low levels in patients with AP/BC but not CP, and presence of mutations was correlated with clonal cytogenetic evolution. Contrary to expectations, however, even the completely resistant 315T>I mutant was not consistently selected during subsequent imatinib therapy.28 A possible explanation is that only KD mutant clones originating in a leukemic stem cell are able to sustain malignant hematopoiesis, whereas mutants generated in more differentiated progenitor cells are passively phased-out over time. As a practical consequence, high-sensitivity mutation screening of CML patients before therapy may produce misleading results and is not indicated. The situation is different if the mutant clone is dominant before imatinib treatment; none of these rare patients responded to imatinib.28,29 In addition, once a patient has experienced relapse with a KD mutation, the mutant clone has proven its self-renewal capacity. Consistent with this, KD mutations were detected in long-term culture-initiating cells from patients with imatinib resistance.30
Which factors are driving the types of mutations seen in patients with resistance?
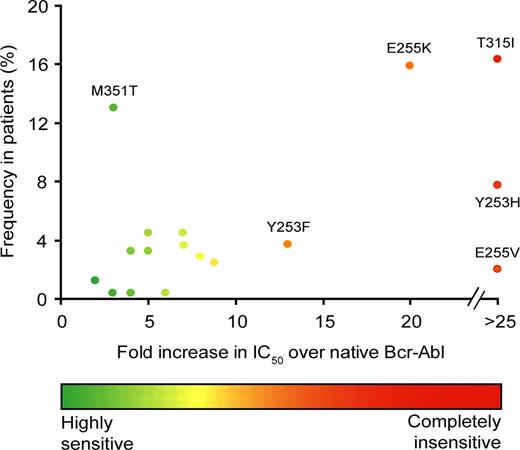
Analyzing the level of imatinib resistance imparted by a specific mutation as it relates to frequency of clinical occurrence demonstrates that mutants with a higher degree of resistance are preferentially selected (Figure 3). However, there are outliers. Most notably, the weakly resistant 351M>T mutant occurs more frequently than some highly resistant mutants such as 255E>V. Thus, either the likelihood of acquiring particular mutations must differ or mutations must cause biologic effects other than drug resistance that favor or disfavor their survival. There is evidence for both. Nucleotide exchange analysis shows a bias for transitions (76.4%) over transversions (23.6%), with C←→ T transitions accounting for approximately 40% of reported mutations.3 Second, KD mutants may be gain-of-function or loss-of-function, which could influence their chances of survival.31,32 For example, compared with native Bcr-Abl, the 253Y>F mutant has increased kinase activity and transformation potency, particularly in conditions of low serum.31 Under nonselective conditions the growth differential versus native Bcr-Abl may not be sufficient to allow the mutant clone to predominate. However, the selection pressure exercised by imatinib may amplify the differences and facilitate outgrowth of gain-of-function mutants. The enhanced transformation potency of several ATP binding loop mutants may also explain their association with poor prognosis in 2 retrospective studies.25,33
Correlation between imatinib resistance level and frequency of mutations isolated from patients. The fold-increase in IC50 value for inhibition of cell proliferation by imatinib compared with cells expressing native Bcr-Abl is plotted on the abscissa versus the clinical frequency in patients (%). As described in Hughes et al,3 the results are compiled from 20 studies. To allow for better comparison, only mutants whose IC50 was determined in the same laboratory were included in the analysis. Unlabeled points represent M244V, G250E, Q252H, F311L, F317L, E355G, F359V, V379I, L387M, H396P, and H396R.
Correlation between imatinib resistance level and frequency of mutations isolated from patients. The fold-increase in IC50 value for inhibition of cell proliferation by imatinib compared with cells expressing native Bcr-Abl is plotted on the abscissa versus the clinical frequency in patients (%). As described in Hughes et al,3 the results are compiled from 20 studies. To allow for better comparison, only mutants whose IC50 was determined in the same laboratory were included in the analysis. Unlabeled points represent M244V, G250E, Q252H, F311L, F317L, E355G, F359V, V379I, L387M, H396P, and H396R.
Does Bcr-Abl induce self-mutagenesis and can KD mutation risk be minimized?
There is increasing evidence supporting a role for Bcr-Abl kinase activity in the induction of genomic instability by a variety of mechanisms, including generation of reactive oxygen species.34 Clonal cytogenetic evolution, KD mutation rate, and advanced disease phase are correlated, consistent with the risk of mutation acquisition being a function of exposure time to Bcr-Abl kinase activity.25,28 Once imatinib treatment is started, the mutation rate should decline sharply, because of the reduction of the number of cells at risk. In addition, a direct correlation between Bcr-Abl–induced reactive oxygen species and KD mutations was recently shown in vitro and in a murine model, suggesting that inhibition of Bcr-Abl in CML cells may further reduce mutation risk by inhibiting reactive oxygen species production.35 Rapid reduction of disease burden combined with maximal inhibition of Bcr-Abl kinase activity should therefore minimize the mutation risk on therapy, providing a rationale for up-front therapy with higher doses of imatinib,36 more potent Abl inhibitors, or Abl kinase inhibitor combinations. In practice, these considerations must be balanced against the proven safety profile of imatinib and the excellent prognosis of patients with CML in CP treated with standard-dose imatinib (400 mg daily).
Can mutations conferring resistance to nilotinib and dasatinib be predicted in vitro?
It took less than 5 years from the identification of KD mutations in Bcr-Abl as the leading cause of imatinib resistance to the regulatory approval of dasatinib as a rational countermeasure. Despite this impressive timeframe, the ideal approach would be to predict resistance mutations a priori. Several strategies have passed the litmus test of retrospectively “predicting” the spectrum of mutations in patients with imatinib resistance, and these methods are now being applied to nilotinib and dasatinib. A compilation of the results from 3 independent in vitro studies for nilotinib (Figure 4) shows that mutations at positions Y253, E255, and T315 are consistently identified, whereas there is some variability with respect to novel sites.16,–18 The observed variability in mutation patterns among these 3 studies, which occurred predominantly at low nilotinib concentrations, may be attributable to methodologic differences.16,–18,37 Similar studies performed with dasatinib identified a small set of mutations that was essentially limited to contact points, including V299, T315, and F317 (Figure 4).21 These results were subsequently confirmed in a cell-based assay in which Ba/F3 cells expressing native Bcr-Abl were chemically mutagenized before exposure to inhibitors (Figure 4).16 Application of this technique to dual combinations of Abl kinase inhibitors revealed that only the 315T>I mutation conferred high-level cross-resistance to all 3 clinical Abl kinase inhibitors (Figure 4), leading to the suggestion that inclusion of a 315T>I inhibitor will be essential to prevent resistance to Abl kinase inhibitor therapy for CML.
In vitro mutagenesis survey for resistance to Abl kinase inhibitors. The compiled results compare the outcomes of methodologically different screens, which probably accounts for the variation between reported resistance profiles. For an informative review and comparison of in vitro mutagenesis techniques, see von Bubnoff et al, 2005.37 †von Bubnoff et al recovered the 349S>L mutation only as a double mutant with 252Q>H. ‡Burgess et al recovered 252Q>H and 315T>1 in [10 μM imatinib + 25 nM dasatinib] and only 315T>I in [10 mM imatinib + 50 nM dasatinib].
In vitro mutagenesis survey for resistance to Abl kinase inhibitors. The compiled results compare the outcomes of methodologically different screens, which probably accounts for the variation between reported resistance profiles. For an informative review and comparison of in vitro mutagenesis techniques, see von Bubnoff et al, 2005.37 †von Bubnoff et al recovered the 349S>L mutation only as a double mutant with 252Q>H. ‡Burgess et al recovered 252Q>H and 315T>1 in [10 μM imatinib + 25 nM dasatinib] and only 315T>I in [10 mM imatinib + 50 nM dasatinib].
Few novel vulnerable sites were identified in the screens of dasatinib16,21 and nilotinib16,–18 (Figure 4), and further biochemical testing is required to validate their resistance profiles. Thus, although additional resistance mutations are theoretically possible, most may not be viable because of kinase inactivation. For example, all 3 inhibitors interact with the amide moiety of M318, suggesting that mutating this residue would impair drug binding,15,20 and yet no such mutants have been recovered in vitro or from relapsed CML patients. M318 plays a key role in ATP binding, and mutations of this residue may be incompatible with sustained kinase activity. Mutants with reduced kinase activity compared with native Bcr-Abl, such as 315T>I and 351M>T, apparently strike a compromise between biologic activity and drug resistance.31 Importantly, the spectrum of possible cross-resistant mutations is finite. For example, imatinib, the inhibitor with the broadest spectrum of resistance mutations, is nonetheless effective against 299V>L, 315T>A, and 317F>L/V, 3 mutants with intermediate- to high-level resistance to dasatinib (Figure 1).21
How are dasatinib and nilotinib doing so far and what is the influence of KD mutations?
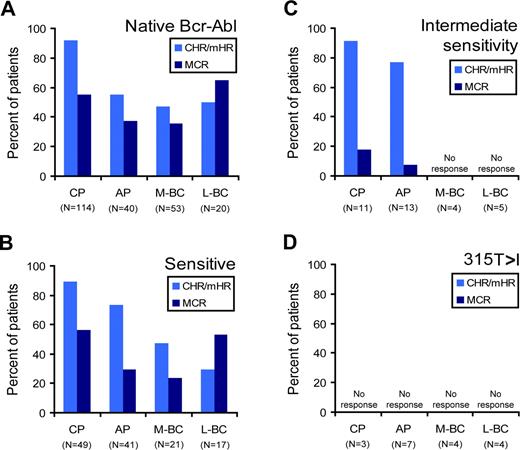
The results of 2 phase 1 trials using dasatinib or nilotinib in the treatment of imatinib-resistant or imatinib-intolerant Philadelphia (Ph)-positive leukemia were reported in mid 2006.10,11 Several phase 2 trials of dasatinib involving patients with CML in various phases have since been published.38,–40 Remarkably, the rate of hematologic response to dasatinib and nilotinib is more than 90% for chronic-phase patients with imatinib failure. In AP/BC, 20% to 40% of patients are refractory to dasatinib and 30% to 60% of patients are refractory to nilotinib.10,11 Overall, no correlation was observed between the presence of a KD mutation and response in either study, with the exception of 315T>I invariably predicting primary resistance.10,11 However, a closer look at responses to dasatinib observed in the phase 1 and phase 2 studies reveals that that even a moderate degree of resistance imparts lower response rates compared with sensitive mutants (Figure 5).11,38,–40 Specifically, patients harboring native Bcr-Abl and patients with a dasatinib-sensitive mutation (as classified in Figure 1) had comparable response rates, whereas patients with mutations that confer intermediate sensitivity to dasatinib in vitro exhibited lower rates of major cytogenetic response. Although preliminary, this analysis strongly suggests that levels of response to dasatinib will depend on the type of Bcr-Abl KD mutation. With respect to nilotinib, a similar analysis could not be performed because the mutational status of individual patients was not revealed.10 Preliminary data from phase 2 studies demonstrated that cytogenetic responses to nilotinib were more frequent in patients harboring KD mutations with low IC50 (the concentration of inhibitor resulting in a 50% reduction in cell viability), whereas several patients with relatively resistant mutations progressed.41 Thus, as greater in vitro sensitivity may be associated with better clinical responses, even mutations that do not confer absolute resistance may prove to be clinically relevant. If this preliminary observation holds, rational decisions on drug and dose may require consideration of mutation type.
Clinical responses to dasatinib according to mutation type. The percentage of patients with imatinib-resistant or imatinib-intolerant CML achieving a complete or minor hematologic (CHR/mHR; light blue bars) or major cytogenetic response (dark blue bars) is analyzed according to dasatinib sensitivity (using classifications listed in Figure 1). The 4 panels represent patients with (A) no Bcr-Abl mutation or (B-D) baseline Bcr-Abl mutations predicted to confer (B) dasatinib sensitivity, (C) intermediate dasatinib sensitivity, or (D) dasatinib insensitivity. The results were compiled from four studies and are segregated by disease stage (M-BC, myeloid blast crisis; L-BC, lymphoid blast crisis).11,38,–40 The number of patients (no.) in each category is indicated. Note that this analysis does not take the duration of response into account. Dasatinib response data is categorized by mutation type: native Bcr-Abl, sensitive 244M>V, 250G>E, 253Y>H, 311F>L, 351M>T, 359F>V, 379V>I, 387L>M, 396H>R, intermediate sensitivity (252Q>H, 255E>K, 255E>V, 317F>L), or 315T>I.
Clinical responses to dasatinib according to mutation type. The percentage of patients with imatinib-resistant or imatinib-intolerant CML achieving a complete or minor hematologic (CHR/mHR; light blue bars) or major cytogenetic response (dark blue bars) is analyzed according to dasatinib sensitivity (using classifications listed in Figure 1). The 4 panels represent patients with (A) no Bcr-Abl mutation or (B-D) baseline Bcr-Abl mutations predicted to confer (B) dasatinib sensitivity, (C) intermediate dasatinib sensitivity, or (D) dasatinib insensitivity. The results were compiled from four studies and are segregated by disease stage (M-BC, myeloid blast crisis; L-BC, lymphoid blast crisis).11,38,–40 The number of patients (no.) in each category is indicated. Note that this analysis does not take the duration of response into account. Dasatinib response data is categorized by mutation type: native Bcr-Abl, sensitive 244M>V, 250G>E, 253Y>H, 311F>L, 351M>T, 359F>V, 379V>I, 387L>M, 396H>R, intermediate sensitivity (252Q>H, 255E>K, 255E>V, 317F>L), or 315T>I.
Where do we stand on clinical development of a 315T>I inhibitor?
Developing a 315T>I inhibitor is challenging, because most ATP-competitive inhibitors directly interact with threonine-315 via hydrogen bonding and with the specificity-imparting hydrophobic pocket for which this residue serves as a “gatekeeper” (Figure 2).9,15,20
Several 315T>I inhibitors are currently undergoing development (Table 1). MK-0457 (previously VX-680), a compound initially developed as an Aurora kinase inhibitor, has shown activity in patients with a variety of hematologic malignancies, including 3 of 3 CML patients with a 315T>I mutation.42 The clinical effects of this agent may not be related to inhibition of the 315T>I mutant, however, because the IC50 for inhibition of Bcr-Abl kinase in cells is approximately 10 μM, whereas responses have occurred at lower plasma levels. In vitro inhibition of Ba/F3 cells occurs at nanomolar concentrations irrespective of Bcr-Abl expression.42,–44 Several other ATP-competitive inhibitors with activity against Bcr-Abl315T>I, including SGX70393,45 a nanomolar 315T>I inhibitor that is nontoxic to parental cells, are undergoing development.45,–47 Another compound, ON012380, acts as a substrate-competitive Abl inhibitor.48 None of these compounds except MK-0457 has entered clinical trials, but such studies may be on the horizon.
Abl kinase inhibitors with anti-T315>I activity
| Compound . | Ba/F3 cellular proliferation IC50 (nM) . | Purified enzyme IC50 (nM) . | Stage of development . | Reference . | |||
|---|---|---|---|---|---|---|---|
| Native Bcr-Abl . | Bcr-AblT315I . | Parental . | Native Abl . | AblT315I . | |||
| MK-0457 | 100-200 | 100-200 | 100-200 | 10-2200* | 30-3700* | Phase 2 clinical trials | 42,–44 |
| ON012380 | 10 | 7.5 | NR | NR | 1.38 | Preclinical | 48 |
| SGX70393 | 12 | 7.3 | >3000† | 14.8† | 4.2† | Preclinical | 45 |
| TG101223 | NR | 500 | >5000 | NR | 50 | Preclinical | 47 |
| AP24534 | 2 | 14 | NR | 3 | 31 | Preclinical | 46 |
| Compound . | Ba/F3 cellular proliferation IC50 (nM) . | Purified enzyme IC50 (nM) . | Stage of development . | Reference . | |||
|---|---|---|---|---|---|---|---|
| Native Bcr-Abl . | Bcr-AblT315I . | Parental . | Native Abl . | AblT315I . | |||
| MK-0457 | 100-200 | 100-200 | 100-200 | 10-2200* | 30-3700* | Phase 2 clinical trials | 42,–44 |
| ON012380 | 10 | 7.5 | NR | NR | 1.38 | Preclinical | 48 |
| SGX70393 | 12 | 7.3 | >3000† | 14.8† | 4.2† | Preclinical | 45 |
| TG101223 | NR | 500 | >5000 | NR | 50 | Preclinical | 47 |
| AP24534 | 2 | 14 | NR | 3 | 31 | Preclinical | 46 |
The IC50 value is the concentration of inhibitor resulting in a 50% reduction in cell viability.8,12,21,29
NR indicates not reported.
Lower values were obtained using full-length Abl in the presence of 10 μM ATP43 ; higher values were obtained with the isolated kinase domains in the presence of 2.2 mM ATP.44
T.O., C.A.E., M.W.D., unpublished observations, 2006.
An effective inhibitor of the 315T>I mutant would open the possibility of using combined Abl kinase inhibitor therapy to completely prevent resistance caused by KD mutations. This raises the question of whether there are currently unrecognized combinations of mutations capable of conferring broad resistance to Abl kinase inhibitor combinations including an anti-315T>I agent. Although mutants of this type have not been observed in vitro, the clinical incidence of such composite mutations, whose emergence may be facilitated by sequential therapy with different Abl inhibitors,49 is currently unknown. Given that even single mutations in the Abl KD can alter substrate specificity,31,32 it is conceivable that combinations of multiple mutations would generate a kinase that is biologically distinct from native Bcr-Abl. Whether this hypothetical novel kinase would maintain the ability to transform hematopoietic cells is a matter of speculation.
What do relapse patterns tell us about CML pathogenesis and what are the prospects of curing CML with Abl kinase inhibitors?
The Bcr-Abl tyrosine kinase and CML are inextricably linked, a fact that has been used to considerable advantage in the clinical treatment of CML. The correlation between in vitro sensitivity and response rates supports a disease model in which responses are mediated by inhibition of Bcr-Abl and not other targets. Truly Bcr-Abl–independent primary resistance may be limited to the minority of patients who do not to achieve any response despite harboring native Bcr-Abl or a highly sensitive mutant. Apparently, these leukemias are somehow able to instantly activate a Bcr-Abl–independent mechanism of growth and survival on, or perhaps even before, challenge with an Abl kinase inhibitor. Bcr-Abl inhibition by new inhibitors, particularly dasatinib, is more pronounced compared with imatinib, providing a more stringent test for Bcr-Abl independence. Thus, primary resistance to dasatinib in a patient without 315T>I or a mutation conferring intermediate resistance (such as 255E>V) is strong evidence for Bcr-Abl independence. Similar conclusions can be drawn from the analysis of patients who received dasatinib after failing nilotinib.50 Although 13 of 14 patients harboring a KD mutation other than 315T>I responded, no responses were observed in 6 of 6 patients without KD mutations. Thus, failing nilotinib without a KD mutation implies Bcr-Abl independence and hence another Abl kinase inhibitor is unlikely to be effective for these patients, at least as a single agent. A better understanding of the factors responsible for leukemia cell survival in such patients will be required to develop targeted therapies that may lead to durable responses. Unfortunately, we do not yet know much about the identities of these targets. For now, treatment will have to rely on nonspecific cytotoxic drugs or allogeneic stem cell transplantation.
A striking preliminary finding is that acquired resistance to dasatinib is almost invariably associated with a small set of KD mutations,22,51 all of which were predicted by in vitro assays (Figure 4). Thus, once the leukemia is fully “committed” to Bcr-Abl, activating a Bcr-Abl–independent transformation program must be difficult, leaving KD mutations as the only escape route. This situation is very different from acute myeloid leukemia with acquired resistance to FLT3 inhibitors, in which resistance-conferring point mutations in FLT3 are rare.52 Why is it so easy for AML cells to achieve resistance by resorting to a FLT3-independent transformation mechanism but so difficult for CML cells to replace Bcr-Abl? The answer is probably that, unlike FLT3, Bcr-Abl is required both for initiation and maintenance of CML. Furthermore, the signaling output downstream of Bcr-Abl must be quite specific, such that it cannot readily be replaced by an alternative kinase in the relevant target cell. From a therapeutic standpoint this is good news, because it suggests that a proportion of patients with advanced CML may achieve prolonged responses with drug combinations that include a 315T>I inhibitor, an agent that is urgently needed. Evidently, at this point, one can only speculate whether such responses would be durable or whether the leukemia cells would eventually find a way to replace Bcr-Abl. Fortunately, the high success rate of imatinib therapy suggests that the majority of patients with newly diagnosed CP will probably not require multi-inhibitor therapy.
Importantly, the absence of KD mutations in many cases of imatinib resistance does not necessarily imply a Bcr-Abl–independent mechanism of resistance. In CP patients, in whom KD mutations are least common, hematologic responses to nilotinib and dasatinib exceed 90%, regardless of mutation status, indicating that resistance remains Bcr-Abl–dependent.10,11 Resistance in AP/BC is not only more consistently associated with KD mutations but also with an increased prevalence of highly resistant mutants,11,26 suggesting that essentially unimpaired kinase activity is required to support advanced CML. In addition, gain-of-function KD mutants may confer a more aggressive phenotype irrespective of their imatinib sensitivity.31,32
Closing thoughts
Only the future can tell whether any Bcr-Abl kinase–directed therapy is capable of turning effective disease suppression into disease eradication. Currently available evidence suggests that Bcr-Abl kinase inhibitor therapy is not curative even in excellent responders. Because neither imatinib nor the novel Abl kinase inhibitors appear to be capable of abrogating Bcr-Abl activity in vitro in primitive (lin−/CD34+/CD38−) BCR-ABL–positive cells, we have to assume that critical cells remain exposed to the mutagenic effects of reactive oxygen species.53,–55 How many cells capable of sustaining leukemic hematopoiesis remain in a patient with major molecular response (MMR) and how big is the risk that any one of these cells acquires a critical mutation? We do not know. Fortunately, the low relapse rate in these patients suggests that this risk may be quite small.1
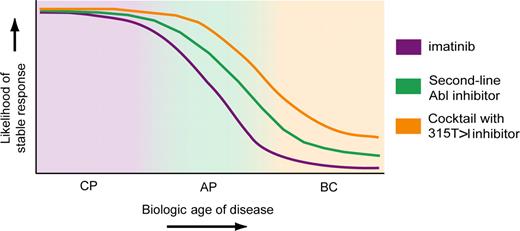
Most cases of CML diagnosed and treated in, CP are extremely well-controlled with imatinib monotherapy, and primary resistance is very uncommon. Given that treatment failure rates are declining with time, which factors could justify using a more potent Abl kinase inhibitor or combined Abl kinase inhibitor therapy up-front? If we assume that disease eradication is not feasible, then the second best aim is to minimize the risk of acquired drug resistance. There is evidence (although not from randomized studies) that high-dose imatinib (800 mg daily) compared with standard dose (400 mg daily) produces superior progression-free survival.36 This advantage is realized within the first 24 to 36 months of therapy, after which both curves approach a plateau. Thus, more aggressive therapy up-front is predicted to prevent early failure in a subset of patients destined to not respond to standard-dose imatinib. Nilotinib or dasatinib should reduce this subset even further, for example, by covering all those patients with pre-existing mutants that are sensitive to the novel inhibitors. Rational, noncross-resistant combinations that include a 315T>I inhibitor may extend the coverage to virtually all patients whose leukemia is still 100% Bcr-Abl kinase–dependent. This is the natural limitation of sustained responses to Bcr-Abl kinase–directed therapy. One would predict that the risk of harboring Bcr-Abl–independent leukemic cell clones increases with the “biologic age” of the disease, a virtual measure of chronologic duration and innate disease features that increase predisposition to additional mutations (Figure 6). Similarly, with increasing biologic age the proportion of patients requiring multi-inhibitor therapy will increase from almost zero to almost 100%. This implies that aggressive multi-inhibitor treatment is clearly justifiable in BC but would be overtreatment in a large proportion of patients with CP. Thus, one of the big challenges is to more precisely define the biologic age of individual leukemias. Surprisingly, even in the molecular age, a complete blood count and the old-fashioned Sokal risk score based on simple clinical data remain the mainstay of prognostication, although microarray data are maturing.56 For now, however, the best option is to use benchmarks of response to identify failure and suboptimal response as early as possible and adjust the therapeutic strategy accordingly.57
Speculative model of future clinical Abl kinase inhibitor treatment options for CML. Imatinib responses are well-established (purple trace). Durable responses are the rule in early chronic phase but the exception in blast crisis.1,6,7 Responses to second-line inhibitors (dasatinib11,38,39,50 and nilotinib10,41 ) are based on limited preliminary evidence (green trace). We speculate that a slightly larger proportion of patients with AP/BC may achieve a sustained response, but this remains to be determined. Abl kinase inhibitor cocktails that include a 315T>I inhibitor (orange trace) and cover all kinase domain mutants have not been used in the clinic. The hypothetical curve shown represents what may be achievable with Bcr-Abl kinase–directed therapy. Patients who have had relapse with partially or fully Bcr-Abl–independent disease are the most enigmatic population in terms of forecasting responses. This speculative model is most applicable to patients with Bcr-Abl–dependent disease.
Speculative model of future clinical Abl kinase inhibitor treatment options for CML. Imatinib responses are well-established (purple trace). Durable responses are the rule in early chronic phase but the exception in blast crisis.1,6,7 Responses to second-line inhibitors (dasatinib11,38,39,50 and nilotinib10,41 ) are based on limited preliminary evidence (green trace). We speculate that a slightly larger proportion of patients with AP/BC may achieve a sustained response, but this remains to be determined. Abl kinase inhibitor cocktails that include a 315T>I inhibitor (orange trace) and cover all kinase domain mutants have not been used in the clinic. The hypothetical curve shown represents what may be achievable with Bcr-Abl kinase–directed therapy. Patients who have had relapse with partially or fully Bcr-Abl–independent disease are the most enigmatic population in terms of forecasting responses. This speculative model is most applicable to patients with Bcr-Abl–dependent disease.
Once disease progression is documented before or during imatinib therapy, the probability of maintaining durable response decreases rapidly (Figure 6). Although dasatinib and nilotinib are encouragingly effective, it is too early to tell to what degree the picture will be different from the early imatinib studies, in which responses in AP/BC were not usually durable. The good news is that those patients who experience relapse usually harbor Bcr-Abl mutations. Thus, we can speculate that rational noncross-resistant inhibitor combinations may induce durable responses in some patients with AP/BC, a question that awaits clinical testing. Although eradicating CML may require a fundamental breakthrough, the development of a 315T>I inhibitor and its use in rational drug combinations could make long-term remissions an achievable goal at least for some patients with advanced CML.
Acknowledgments
The authors thank Daniel W. Sherbenou for assistance with preparation of Figure 2.
Authorship
Contribution: M.W.N.D. and T.O. wrote the paper. C.A.E. generated all tables and figures and helped with the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael W.N. Deininger, Oregon Health & Science University Cancer Institute, L592, 3181 SW Sam Jackson Park Road, Portland, OR 97239; e-mail:deininge@ohsu.edu.

![Figure 4. In vitro mutagenesis survey for resistance to Abl kinase inhibitors. The compiled results compare the outcomes of methodologically different screens, which probably accounts for the variation between reported resistance profiles. For an informative review and comparison of in vitro mutagenesis techniques, see von Bubnoff et al, 2005.37 †von Bubnoff et al recovered the 349S>L mutation only as a double mutant with 252Q>H. ‡Burgess et al recovered 252Q>H and 315T>1 in [10 μM imatinib + 25 nM dasatinib] and only 315T>I in [10 mM imatinib + 50 nM dasatinib].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-03-066936/2/m_zh80180706540004.jpeg?Expires=1768244909&Signature=BsI9E9s8DH486nRHir1AtdYw6MoZKhRFS8JLzuDVOy8I8BFJJJNM~M78dP-PDZk8P186acIdvQylPEAfLefPezR6TXTVx3FfgFKXPjtmzWEMKxQteMIafrUEdX0IXMX1UIIcvuoum2GUCR5BEt8q~Xf8dHW~GYr9zUH8L7s~ZRThy3McQAClQ9u0Ce5F5CgRXZHQot2EX9lfAqqw5MmQjBHO3T0JmUHBm5QluXAd0QjbyKVaDkFCpK-oRdzqqmJ3wa-FNaGmbUp9l7HF6AGI~gdcKagvhZSqtAyrbgZnYzThom5gAa~VBOLeX2QQ8AkarJ-hJKK0lbwJBmMlmwoZAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)