Abstract
Galectins are emerging as a family of proteins that play an important role in several steps of tumorigenesis. Evidence is accumulating that galectins are expressed by the tumor endothelium, where they contribute to different steps of tumor progression such as immune escape and metastasis. Recent studies have identified an important role for galectins in tumor angiogenesis. Moreover, it has been shown that galectins in the endothelium can be targeted for therapeutic applications. This opens a window of opportunity for the development of tumor-type independent treatment strategies. This review focuses on the expression of galectins in the tumor endothelium, their contribution to tumor progression, and their application in tumor-type independent cancer therapy.
Introduction
Cancer cells induce the growth of new blood vessels to grow beyond the size of a few cubic millimeters. This neovascularization, or angiogenesis, ensures proper blood supply and facilitates tumor metastasis (Figure 1). The dependence on neovascularization has made tumor angiogenesis an attractive target for therapeutic applications.1
Tumor angiogenesis. Tumor angiogenesis is a multistep process that occurs in almost all tumors and that is mediated by endothelial cells (ECs). When a cell has acquired genetic alterations that allow unlimited growth and escape from apoptosis, a small tumor is formed (A). As soon as the tumor volume has reached a few cubic millimeters, oxygen and nutrient supply are insufficient, and the tumor cells undergo an angiogenesis switch. This results in the production and release of growth factors into the surrounding tissue. The secreted growth factors bind to receptors on ECs in nearby vessels. Pericytes that stabilize the vessel detach, and vessel dilation occurs (B). In addition, the activated ECs start to produce proteases (not shown) that degrade the basal membrane and the extracellular matrix (C). Subsequently, the ECs start to migrate (D) and proliferate (E) into the growth factor gradient, forming new vascular structures. Finally, matrix proteins are deposited, and the new vessel is stabilized by pericytes to form a functional and mature blood vessel. The tumor cells can continue to grow, and metastasis formation is facilitated since the tumor cells now have easy access to the circulation (F).
Tumor angiogenesis. Tumor angiogenesis is a multistep process that occurs in almost all tumors and that is mediated by endothelial cells (ECs). When a cell has acquired genetic alterations that allow unlimited growth and escape from apoptosis, a small tumor is formed (A). As soon as the tumor volume has reached a few cubic millimeters, oxygen and nutrient supply are insufficient, and the tumor cells undergo an angiogenesis switch. This results in the production and release of growth factors into the surrounding tissue. The secreted growth factors bind to receptors on ECs in nearby vessels. Pericytes that stabilize the vessel detach, and vessel dilation occurs (B). In addition, the activated ECs start to produce proteases (not shown) that degrade the basal membrane and the extracellular matrix (C). Subsequently, the ECs start to migrate (D) and proliferate (E) into the growth factor gradient, forming new vascular structures. Finally, matrix proteins are deposited, and the new vessel is stabilized by pericytes to form a functional and mature blood vessel. The tumor cells can continue to grow, and metastasis formation is facilitated since the tumor cells now have easy access to the circulation (F).
It is thought that angiogenesis in different tumor types follows similar steps, allowing tumor-independent treatment strategies.2 Furthermore, the cells that play a pivotal role in angiogenesis (ie, the endothelial cells [ECs]) are particularly suitable targets for treatment. They line the luminal side of vessels and are in direct contact with blood, making them easily accessible. Furthermore, disrupting the tumor vasculature by killing the ECs results in massive loss of tumor cells.3 Finally, ECs are expected to be less susceptible to development of drug-induced resistance.
Effective EC-targeted therapy depends on the availability of molecules that can distinguish the tumor ECs from ECs in normal tissues.4 Currently, a limited number of such molecules has been identified, including integrins α5β1, αvβ3, and αvβ5; oncofetal fibronectin; ROBO4; CD36; and CD13.5,6 We recently described galectin-1 as a protein that is overexpressed on activated tumor ECs in vivo. We showed that tumor vessels hardly develop in mutant galectin-1 knockout mice.7 Evidence is accumulating that other galectin family members are also expressed by the endothelium, and that they contribute to different steps of tumor progression such as immune escape and metastasis. Moreover, it has been shown that galectins in the endothelium can be targeted for therapeutic applications.7-10 This opens opportunities for the development of tumor-type independent treatment strategies and justifies a review on the role of galectins in the tumor endothelium.
The galectin protein family
Galectins are a family of carbohydrate-binding proteins with a high affinity for β-galactosides on the cell surface and extracellular glycoproteins as well as on glycolipids. So far, 15 mammalian galectins have been identified that all share a conserved carbohydrate recognition domain (CRD) of approximately 130 amino acids.11 Based on their structure and the number of CRDs, galectins can be subdivided into 3 groups (Table 1): (1) prototype galectins, consisting of a single CRD (galectins-1, -2, -5, -7, -10, -11, -13, and -14); (2) tandem repeat galectins, consisting of 2 different but homologous CRDs, connected by a linker region (galectins-4, -6, -8, -9, and -12); and (3) chimera galectins, which have a tail of short tandem repeats fused to a single CRD (galectin-3). They can be secreted by nonclassical pathways12 and—depending on the cell type or differentiation state—they are found in the nucleus and the cytoplasm, on the cell surface, and in the extracellular matrix.13,14
The galectin family
| Name . | Prototype* . | Tandemrepeat . | Chimera† . | Splicevariants‡ . | Humangene . | ENSEMBLE accession number . |
|---|---|---|---|---|---|---|
| galectin-1 | • | — | — | — | • | ENSG00000100097 |
| galectin-2 | • | — | — | — | • | ENSG00000100079 |
| galectin-3 | — | — | • | — | • | ENSG00000131981 |
| galectin-4 | — | • | — | — | • | ENSG00000171747 |
| galectin-5 | • | — | — | — | — | — |
| galectin-6 | — | • | — | — | — | — |
| galectin-7 | • | — | — | — | • | ENSG00000178934 |
| galectin-8 | — | • | — | • | • | ENSG00000116977 |
| galectin-9 | — | • | — | • | • | ENSG00000168961 |
| galectin-10 | • | — | — | — | • | ENSG00000105205 |
| galectin-11 | • | — | — | — | — | — |
| galectin-12 | — | • | — | — | • | ENSG00000133317 |
| galectin-13 | • | — | — | — | • | ENSG00000105198 |
| galectin-14 | • | — | — | • | • | ENSG00000006659 |
| galectin-15 | • | — | — | — | — | — |
| Name . | Prototype* . | Tandemrepeat . | Chimera† . | Splicevariants‡ . | Humangene . | ENSEMBLE accession number . |
|---|---|---|---|---|---|---|
| galectin-1 | • | — | — | — | • | ENSG00000100097 |
| galectin-2 | • | — | — | — | • | ENSG00000100079 |
| galectin-3 | — | — | • | — | • | ENSG00000131981 |
| galectin-4 | — | • | — | — | • | ENSG00000171747 |
| galectin-5 | • | — | — | — | — | — |
| galectin-6 | — | • | — | — | — | — |
| galectin-7 | • | — | — | — | • | ENSG00000178934 |
| galectin-8 | — | • | — | • | • | ENSG00000116977 |
| galectin-9 | — | • | — | • | • | ENSG00000168961 |
| galectin-10 | • | — | — | — | • | ENSG00000105205 |
| galectin-11 | • | — | — | — | — | — |
| galectin-12 | — | • | — | — | • | ENSG00000133317 |
| galectin-13 | • | — | — | — | • | ENSG00000105198 |
| galectin-14 | • | — | — | • | • | ENSG00000006659 |
| galectin-15 | • | — | — | — | — | — |
— indicates not applicable.
*Several prototype galectins have been shown to form homodimers (gal-1, -2, and -13) in case of the tandem repeat galectins.
†The only chimera galectin known so far (ie, galectin-3) contains a proline- and glycine-rich C-terminal tail that can mediate oligomerization of the protein.
‡Splicing has been shown to occur in the linker region between the 2 carbohydrate recognition domains (CRDs). For galectin-8 an additional splice variant has been described that results in truncation of the C-terminal CRD.
Members of the galectin family are found in vertebrates, invertebrates, and protists. Galectin-related sequences have even been found in plants and viruses. This degree of conservation suggests an important role in basic cellular mechanisms.11,15 Indeed, galectins have been shown to be involved in cell interactions, proliferation, migration, apoptosis, and mRNA splicing (Figure 2).13,16-18 Galectins are also associated with different pathologies, and most importantly with cancer.19 Recent reviews have summarized the current knowledge on the expression of different galectins (eg, galectin-1, galectin-3,20 galectin-4,21 and galectin-8)8 in a variety of tumor types. They all conclude that galectins in tumor cells might provide targets for novel anticancer therapies. This is not surprising, since many functions of galectins, notably control of cell interactions/adhesion, effects on cell proliferation/apoptosis, and immunosuppressive properties, have been proposed to account for their role in tumor progression.19
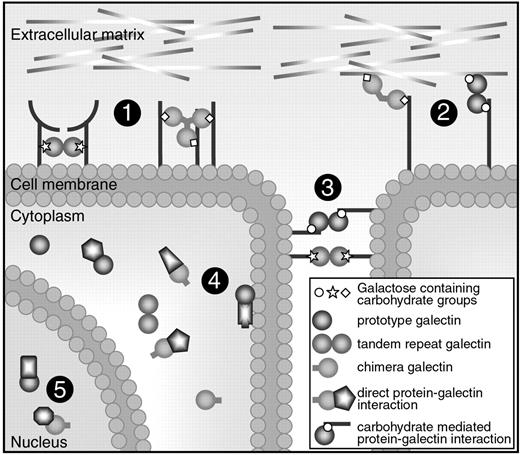
Cellular location and functions of galectins. Galectins can be found in the extracellular space, in the cell membrane, in the cytoplasm, and even in the nucleus. They can bind to proteins via a carbohydrate interaction or via direct protein-protein interactions. Most galectins have multiple functions and they can mediate (1) receptor cross-linking or lattice formation, (2) cell-extracellular matrix interactions, (3) cell-to-cell interactions, (4) intracellular signaling, and (5) posttranscriptional splicing.
Cellular location and functions of galectins. Galectins can be found in the extracellular space, in the cell membrane, in the cytoplasm, and even in the nucleus. They can bind to proteins via a carbohydrate interaction or via direct protein-protein interactions. Most galectins have multiple functions and they can mediate (1) receptor cross-linking or lattice formation, (2) cell-extracellular matrix interactions, (3) cell-to-cell interactions, (4) intracellular signaling, and (5) posttranscriptional splicing.
Galectin expression in the endothelium
The contribution of endothelial galectins to tumor progression has received relatively little attention. This is surprising because ECs have been found to express several galectins, and the endothelium is recognized as a good target for tumor-type independent therapy.
Galectin-1
Galectin-1 is the first galectin described in literature (for a recent review, see Camby et al22 ). It exists as a noncovalent homodimer of 2 14.5-kDa CRDs. It can be found intracellularly, extracellularly, and at the cell surface of different cell types. Each dimer can bind 2 sugar residues to mediate intramolecular and intermolecular interactions. Furthermore, galectin-1 can be involved in carbohydrate-independent protein-protein interactions, which further contributes to the variety of its biological functions.23,24 Several galectin-1-binding partners have been identified, including integrins such as α7β1 and α5β1, extracellular matrix components such as fibronectin and laminin, cytosolic proteins (oncogenic RAS), and several T-cell surface receptors, such as CD2/CD3/CD4/CD7/CD43/CD45.22
Galectin-1 expression has been reported in cultured ECs of different origins, including mouse liver, brain, and lung; rat lung; bovine aorta; and human aorta, umbilical vein, and pulmonary artery.9,25-29 Moreover, activation of ECs in vitro results in a rapid increase of galectin-1 expression. For example, treatment of cultured aortic and umbilical vein ECs with low-density lipoprotein (LDL), lipopolysaccharide (LPS), or tumor-conditioned medium resulted in increased galectin-1 expression at the extracellular side of the cell.26,30 Addition of interferon-γ or tumor-conditioned medium induced galectin-1 expression in ECs16,31 and increased galectin-1 secretion into the matrix.30 We found that galectin-1 is an early marker of human umbilical vein EC (HUVEC) activation in vitro.7 Increased galectin-1 expression also appears to correlate with EC activation in vivo. Galectin-1 is expressed in endothelial venules of activated lymphoid tissue, but not of resting lymph nodes.26 Elevated galectin-1 protein expression was also observed in the ECs of human colon carcinoma and Ewing sarcoma,7 in head and neck or lung cancer,25 and in prostate carcinoma.31
Galectin-3
Galectin-3 is also an extensively studied galectin family member (reviewed by Dumic et al32 ) that is expressed by ECs. The 30-kDa protein consists of a C-terminal CRD and a proline rich N-terminal domain containing several homologous repeats. Binding of the N-terminal domain can result in the formation of oligomers.33-35 The protein can be found both intracellularly and extracellularly, and numerous galectin-3-binding partners have been identified, including extracellular matrix components such as laminin, fibronectin, vitronectin, and elastin; cell adhesion molecules like N-CAM, lysosome-associated membrane protein (LAMP1)/LAMP2, and integrin α3β1; and cytosolic molecules such as Bcl-2, K-Ras, and annexin VII.32 Galectin-3 can be involved in different biological functions, including cell adhesion and activation, apoptosis, immune regulation, and gene transcription regulation.
Galectin-3 expression has been observed in the cultured ECs of mouse liver, brain, and lung; rat lung; bovine aorta; and human umbilical vein and dermal microvasculature.25,29,36 EC expression of galectin-3 in vivo has been reported in head and neck and in lung cancer tissues.25,37 In a murine tumor model, galectin-3 expression was reported to be approximately 30-fold up-regulated in liver carcinoma-derived ECs compared with normal liver-derived ECs.38
The relationship between the galectin-3 expression level and activation status of ECs is still somewhat unclear. Glinskii et al showed that the interaction between ECs and tumor cells expressing specific carbohydrate structures increases galectin-3 expression in ECs.39,40 Recently, it was shown that IL-8 treatment of a hybridoma of HUVECs and epithelioma cells resulted in increased galectin-3 protein levels in the cytoplasm and the cell membrane.41
Galectin-9
Galectin-9, a more recently discovered galectin family member, is also expressed in ECs. It is a tandem repeat galectin that was first cloned from the tumor tissue of patients with Hodgkin disease.42 The protein consists of 2 CRDs that are connected by a linker of about 30 amino acids. Originally, galectin-9 was identified as an eosinophil-specific chemoattractant.43 Recent studies have shown that it can also function as an urate channel.44 Known binding partners include type IV collagen45 and the Epstein-Barr virus latent membrane protein-1.46 Recently, galectin-9 was identified as a receptor for the T helper type 1 (TH1)-specific cell-surface molecule Tim-3, which is involved in the regulation of the TH1 response.47
In HUVECs, 3 constitutively expressed galectin-9 isoforms have been identified in which either exon 5 or exon 6 are alternatively spliced.48 Stimulation with interferon-γ increases galectin-9 expression in the membrane of HUVECs.49 EC expression of galectin-9 has been studied mainly in the context of viral infections. Infection of HUVECs with dengue virus induces galectin-9 mRNA expression.50 In addition, treatment of HUVECs with a synthetic mimetic of double-stranded RNA as a model for viral infection increases mRNA and protein expression of galectin-9.51
Endothelial galectins in tumor progression
As described here, there is clear evidence that ECs express galectin family members and that their expression can be altered during EC activation. The latter observation suggests that galectins contribute to different functions of activated tumor ECs, and that they might be involved in the steps of tumor progression (ie, immune modulation, metastasis, and angiogenesis).
Tumor immune modulation
Infiltration of tissues by immune cells is a multistep process, and ECs play an important role in several of these steps.52 The adhesion and rolling of leukocytes along the vessel wall, followed by transendothelial migration of activated leukocytes, are steps in which the endothelium is involved (K. Castermans, A.W.G., Tumor blood vessels, a difficult hurdle for infiltrating leukocytes; manuscript accepted, July 2007). ECs express adhesion molecules that influence leukocyte infiltration and activation (eg, E-selectin, P-selectin, ICAM, and different integrins). While inflammatory cytokines induce the expression of many adhesion molecules, several proangiogenesis factors released by tumor cells counteract the proinflammatory phenotype of ECs by turning off the “immunogenic switch.” This phenomenon is also referred to as EC anergy, and enables tumors to escape from immunosurveillance.53-55 In the last decade, it has become evident that tumor cells can exploit the expression of galectins to manipulate the immune system (for reviews, see Rabinovich et al56 and Liu et al19 ). Inhibition of galectin-1 expression in murine B16 melanoma cells stimulated a potent tumor-specific T1-type response in mice and a reduction in tumor progression.57 Observations in human cancers in vivo and human tumor cell lines in vitro also suggest a relation between galectin expression and immune evasion.58-61 There are also reports that indicate a link between ECs, galectins, and immune regulation. For example, galectin-3 is considered to be a proinflammatory protein since it promotes adhesion of neutrophils to ECs,62 acts as a chemoattractant for monocytes and macrophages, and facilitates phagocytosis.32 The interaction of neutrophils with the endothelium stimulates expression of galectin-3 in neutrophils and ECs.41 Galectin-9 has been shown to be involved in the adhesion of eosinophils to the endothelium.43,49 Interferon-γ-induced endothelial expression of galectin-9 is accompanied by enhanced adhesion of human eosinophilic leukemia-1 cells.49,51 Recently, binding of galectin-9 to Tim-3 was found to induce the aggregation and death of TH1 cells in vitro. Administration of galectin-9 in vivo resulted in suppression of TH1 autoimmunity, indicating that this interaction could represent a means to inhibit the inflammatory response. It was suggested that interfering with the signaling induced by the interaction might provide new opportunities for pharmacologic interventions.63 However, whether and how galectin-9 expression by ECs might regulate a Tim-3 mediated TH1 response needs further investigation.
Another role of galectins in the immunoresponse involves regulation of leukocyte survival and function. To infiltrate a tumor tissue, immune cells first have to pass the endothelium, which makes it an obvious site for immunomodulation. Indeed, the presentation of galectin-1 by activated ECs triggers apoptosis of activated T cells.16,64,65 Galectin-3 and galectin-9 are also able to induce T-cell apoptosis.66-68 Furthermore, galectin-3 can inhibit T-cell activation by complexing with the T-cell receptor.69 Thus, up-regulation of galectin expression by ECs may provide a mechanism to hamper the immune response. Unfortunately, strong evidence showing that these endothelial galectins effectively modulate the tumor immune response in vivo is still lacking.
It has also been suggested that EC expression of galectin-1 might mediate an apoptosis independent anti-inflammatory effect on leukocytes. Increased EC expression of galectin-1 was found to decrease transendothelial migration of leukocytes independent of the proapoptotic activity.30 However, in a teratocarcinoma tumor model in galectin-1-null mice, there was no statistically significant change in the number of CD45+ or CD8+ cells that were found in the tumors of null mice compared with those of wild-type animals.7 Since the teratocarcinoma cells express only low levels of galectin-1, the comparable infiltration could not be explained by an effect of the tumor cells. Further research is needed to determine whether and how endothelial galectin-1 influences the in vivo immune response.
As described, endothelial expression of galectins might influence processes of the immune response, including leukocyte apoptosis, adhesion, and migration. However, in contrast to our knowledge regarding the immunomodulatory role of galectins expressed by tumor cells, the amount of evidence directly linking endothelial galectins to immune escape is still scarce. The limited data available at present indicate that the possible immunomodulating effects of endothelial galectins depend on multiple aspects, like the expression level and localization, the type and activation status of the interacting cells, and the specific step in the immunogenic cascade. Whether targeting endothelial galectins generates sufficient immunomodulatory effects in vivo still needs to be resolved. One of the major challenges for future research will be to distinguish the contribution of endothelial galectins from that of the galectins expressed by the tumor cells. This would be facilitated by the development of animal models that allow conditional and endothelial-specific knockdown of galectin expression. In combination with tumor cells that display different levels of galectin expression, such models could be used to determine whether regulating the endothelial expression of galectins could be used to modulate the antitumor immune response, even in the absence of galectin expression by the tumor cells.
Tumor metastasis formation
The involvement of galectins in both homotypic and heterotypic cell adhesion (reviewed by Elola et al70 ) has been suggested to facilitate tumor metastasis. Galectin expression by tumor cells can play a role in different steps of metastasis such as cell detachment from the primary tumor site, diapedesis into vessels, and cell adhesion to ECs at distant sites.19,70,71 For example, high expression of galectin-3 by metastatic MDA-MB-435 breast carcinoma cells increased their adhesion to monolayers of ECs.72 At these heterotypic adhesion sites, homotypic cell aggregation was also observed, not only for breast carcinoma cells but also for DU-145 prostate carcinoma cells,40 which also express high levels of galectin-3. Moreover, clustering of galectin-3 occurred at the cell-contact sites40,73 suggesting that expression of this galectin can play a role in metastasis formation (for review, see Takenaka et al71 ). However, metastasis formation is a complex process, and the expression of galectins by tumor cells can have different effects that likely depend on factors like the tumor cell type, the specific step in the metastatic process, and the level of expression. This complicates the study of their function and could account for the sometimes-conflicting data when correlating galectin expression in tumors and tumor progression.
As expected from their multifunctional properties, the contribution of galectins to metastasis is not restricted to the adhering cell (tumor cell) but also involves the cell that is adhered to (EC). Lotan et al observed that the percentage of metastatic large-cell lymphoma cells that attached to either cultured liver or lung ECs reflected the organ preference for metastasis.25 Similar observations were reported when the adhesion of several different tumor cell lines to human bone marrow ECs and HUVECs was studied.74 Tumor cells preferentially adhered to bone marrow ECs. Moreover, the adhesion of PC3 prostate carcinoma cells was inhibited after preincubation of the ECs with anti–galectin-3 antibody.74 In a study by Clausse et al, culturing ECs in PC-3–conditioned medium induced galectin-1 expression, which resulted in increased attachment of PC-3 cells. This increased attachment was inhibited by pretreating the ECs with an anti–galectin-1 antibody.31 Apparently, the preference of tumor cells to bind to specific ECs involves the expression of galectins by the endothelium. The observation that the expression of endothelial galectins can be influenced by heterotypic cell-cell contact or by molecules secreted by tumor cells suggests an involvement of endothelial galectins in organ-specific tumor metastasis.
A possible mechanism by which endothelial galectins appear to contribute to metastasis involves binding to carbohydrate ligands presented by tumor cells. It was suggested 20 years ago that increased levels of beta1,6-branched N-linked oligosaccharides increased the metastatic potential of tumor cells.75 Thus, altered glycosylation and exposure of glycoproteins by tumor cells might facilitate their adhesion to endothelial galectins. For example, Kuźbicki et al as well as others observed increased expression and membrane translocation of LAMP1 in metastatic melanoma cells.37,76,77 LAMP1 contains high levels of carbohydrate moieties that bind galectin-3.77,78 Thus, elevated expression of galectin-3 by activated ECs could facilitate the binding of those tumor cells that expose LAMP1. In line with this, it was observed that preincubation of frozen lung tumor sections with lactose partially inhibited the adhesion of melanoma cells.37 Sarafian et al used lactose to inhibit galectin-3 binding to metastasizing LAMP1-expressing melanoma cells.77 Although the inhibitory effects of lactose could be caused by the inhibition of other galectin-binding glycoproteins, the observation that the metastatic tumor cells displayed increased translocation of LAMP1 strongly suggest a causal relationship.
Another galectin-binding carbohydrate that has been described to facilitate adhesion of cancer cells to the endothelium is the Thomsen-Friendenreich glycoantigen (TFag; recently reviewed by Yu79 ). High expression of TFag is associated with most human cancers, and at least 2 surface molecules are known to present TFag (ie, CD44v6 and the transmembrane mucin MUC1). It was shown that interactions between ECs and TFag-expressing tumor cells induces endothelial expression of galectin-3.39,40,72 In addition, it was found that endothelial adhesion of tumor cells that display TFag on MUC1 is facilitated by circulating galectin-3.80 Furthermore, blocking either TFag or galectin-3 could prevent the in vivo formation of breast and prostate carcinoma metastasis.10,73,81 All these data suggest that specific glycosylations on tumor cells provide ligands for galectin-mediated metastasis.
However, galectins are not the only molecules that can mediate adhesion between tumor cells and ECs. Saitoh et al compared the composition of Asn-linked oligosaccharides on LAMP1 and LAMP2 after immunoprecipitation from different colon carcinoma cells. They observed an increased expression of both proteins in highly metastatic cells that correlated with an increase in sialyl Lewis X carbohydrates.82 Further research indicated that sialyl Lewix X mediated the adhesion of the metastatic cells to activated ECs via E-selectin.83 Integrins have also been associated with heterotypic cell adhesion and tumor metastasis.84 Conclusively, the tumor ECs express a panel of molecules, including galectins, that can facilitate tumor metastasis. Unraveling the interplay between all these molecules and their ligands provides a challenge for future research. Furthermore, targeting the metastatic molecules that reside on the activated endothelium (eg, galectins) opens the possibility to develop treatment strategies that prevent or delay metastasis formation in patients with cancer, independent of the tumor type.
Tumor angiogenesis
Upon activation by growth factors produced by the tumor, ECs in nearby vessels embark on a cascade of events that results in the formation and growth of a mature tumor vasculature. This involves remodeling of the extracellular matrix, proliferation and migration of the ECs, formation of tubelike structures, and, eventually, maturation of the newly formed vessels.2 Given their multifunctionality, galectins are likely to play a role in several of these steps. Exogenously added galectin-3 was shown to induce in vitro tube formation in a carbohydrate-dependent manner.85 Tube formation and EC migration is also stimulated by the extracellular domain of NG2 proteoglycan, of which the proangiogenesis potential is thought to be mediated by galectin-3.36 We observed that knockdown of galectin-1 expression in cultured ECs inhibited cell proliferation and migration.7
A strong genetic argument linking galectins to tumor angiogenesis was recently obtained from experiments in vivo. When tumor cells were injected in wild-type and galectin-1-null mice, tumor growth was severely hampered in the absence of galectin-1. This growth delay was accompanied by a 4-fold decrease in microvessel density.7 The observation that the angiogenesis-independent onset of tumor growth was similar in wild-type and null mice, together with an unaltered tumor immune response, strongly suggested that the observed effects were mainly caused by decreased tumor angiogenesis.7 Future research using different galectin-null mice in combination with different tumor models will gain further insights in the exact role of galectins in tumor angiogenesis.
Knocking-down galectin-1 expression during zebrafish development resulted in abnormal and irregular vessel growth, suggesting that EC migration and vessel guidance are dependent on galectin-1.7 In line with this, Nangia-Makker and colleagues showed that both in vitro and in vivo, galectin-3 acts as a chemoattractant for ECs.85,86 Galectin-9 has also been reported as a chemoattractant, although the direct effects of galectin-9 on EC migration have not been studied. Nevertheless, current observations suggest that galectins might be involved in migration and correct pathfinding of ECs. In the last few years, there have been many reports on proteins that share a regulatory activity in vascular and neuronal network formation (eg, robos/slits, ephs/ephrins, neuropilins/semaphorins, and unc5B/netrins).87,88 There are also several reports that galectin-1 plays an important role in axonal targeting. Galectin-1 was shown to stimulate the in vitro outgrowth of dorsal root ganglion (DRG) neurons and olfactory axons, to promote Schwann cell migration, and to enhance the regeneration of sensory and motor neurons in vivo.89-91 The galectin-1-null mice display defects that are consistent with a role for this lectin in neuronal pathfinding.89,90,92 For example, a subset of primary sensory olfactory axons fail to reach their appropriate target site in the absence of galectin-1.92 Based on these observations, together with the suggested role of galectin-1 in vascular guidance, it is tempting to speculate that galectin-1 is also a member of the group of proteins that regulate patterning of both vascular and neuronal networks. However, it remains to be elucidated how the apparent normal vascular development is regulated in mice that lack galectin-1 or even both galectin-1 and galectin-3. It might be that, similar to the neuronal growth and regeneration, small vascular abnormalities do occur in these mice that only become apparent after a pathophysiologic challenge such as cancer.
Altogether, the current literature indicates that galectins, and most notably galectin-1, are involved in different endothelial processes during angiogenesis, and that they can regulate tumor angiogenesis. Although the exact function and involvement of other galectins in angiogenesis still remains to be studied, it is likely that interfering with galectin function might be used to obstruct tumor angiogenesis.
Exploiting galectins in the tumor endothelium for combined cancer therapy
As described here, members of the galectin family are expressed by ECs and possibly contribute to tumor progression (Figure 3). This makes galectins in the tumor endothelium promising targets for cancer therapy. Lotan and coworkers showed that preincubation of liver ECs with an anti-galectin-1 antibody partially inhibited the attachment of liver-metastatic large-cell lymphoma cells in vitro.25 A similar observation was made by Clausse et al, who were able to inhibit the attachment of prostate carcinoma cells to cultured ECs by preincubating the latter with an anti-galectin-1 antibody.31 Thus, blocking galectin-1 might prevent heterotypic adhesion between tumor cells and ECs, thereby hampering metastasis. Unfortunately, the only in vivo study with a tumor model in galectin-1-null mice (ie, F9 teratocarcinoma cells) did not address tumor metastasis. The colonization of either lung or liver by the teratocarcinoma cells used in that study has been shown to depend on the cell differentiation status,93 and future experiments could disclose the role of endothelial galectin-1 in this process. Furthermore, experiments using different in vivo metastasis models could disclose the potential therapeutic applications of galectin-1-blocking agents on tumor metastasis.
Possible roles of endothelial galectins in 3 mainstays of tumorigenesis. Schematic overview of the mechanisms by which galectin expression by activated ECs might contribute to 3 key processes in tumor progression. (A) Galectin-1, galectin-3, and galectin-9 can modulate the antitumor immune response both positively and negatively. They mainly regulate T-cell survival, activation, and transendothelial migration. In addition, they could facilitate adhesion of neutrophils and eosinophils or act as chemoattractants for macrophages and eosinophils. The overall effect possibly allows tumor cells to escape from immune surveillance. (B) Endothelial galectins have also been suggested to facilitate metastasis formation by promoting heterotypic cell adhesion between circulating tumor cells and ECs. Furthermore, the adhesion of circulating cells to ECs could stimulate the endothelial expression of galectin-1 and galectin-3. (C) Galectin-1 and galectin-3 have been shown to be involved in tumor angiogenesis. They can influence EC migration and proliferation, thereby promoting tube formation. In addition, galectin-3 acts as a chemoattractant for ECs.
Possible roles of endothelial galectins in 3 mainstays of tumorigenesis. Schematic overview of the mechanisms by which galectin expression by activated ECs might contribute to 3 key processes in tumor progression. (A) Galectin-1, galectin-3, and galectin-9 can modulate the antitumor immune response both positively and negatively. They mainly regulate T-cell survival, activation, and transendothelial migration. In addition, they could facilitate adhesion of neutrophils and eosinophils or act as chemoattractants for macrophages and eosinophils. The overall effect possibly allows tumor cells to escape from immune surveillance. (B) Endothelial galectins have also been suggested to facilitate metastasis formation by promoting heterotypic cell adhesion between circulating tumor cells and ECs. Furthermore, the adhesion of circulating cells to ECs could stimulate the endothelial expression of galectin-1 and galectin-3. (C) Galectin-1 and galectin-3 have been shown to be involved in tumor angiogenesis. They can influence EC migration and proliferation, thereby promoting tube formation. In addition, galectin-3 acts as a chemoattractant for ECs.
For galectin-3, more evidence exists that it might be a target for antimetastatic cancer treatment. Peptide antagonists that specifically bind the CRD of galectin-3 were recently shown to inhibit the adhesion of MDA-MB-435 breast carcinoma cells to cultured EC monolayers.10 Blocking galectin-3 with lactose significantly inhibits the LAMP1-mediated adhesion of B16 melanoma cells to galectin-3 expressing lung ECs.37 These in vitro observations suggest that blocking specific galectins might hamper metastasis formation in vivo. Indeed, Glinskii et al demonstrated that blocking galectin-3 with antibodies inhibited the accumulation of metastatic cells in the lungs and bones of mice.81 Antimetastatic activity was also reported for another molecule that binds galectin-3, a modified form of citrus pectin. Modified citrus pectin (MCP), a galactosyl-rich polysaccharide, inhibits the in vitro adhesion of rat prostate cancer MAT-LyLu cells to rat ECs.94 While MCP had no effect on the growth of the primary tumor, rats with at least 0.1% MCP (wt/vol) in their drinking water displayed a significant decrease in the number of lung metastatic colonies.94 Comparable observations were reported using metastatic human breast carcinoma cells. Following injection of human metastatic tumor cells in mice on a MCP-rich diet, a decrease in lung metastasis was observed.86 Similar to galectin-1, studies in galectin-3-null mice might be used to further address the role of endothelial galectin-3 in tumor metastasis. In the latter study, there was also a significant decrease in tumor microvessel density, indicating that blocking galectin-3 had a direct effect on tumor angiogenesis.86 Rabinovich et al have shown that blocking galectins can indeed be an effective strategy to inhibit tumor angiogenesis.29 They developed synthetic lactulose amines that inhibited the binding of galectin-1 and galactin-3 to glycosylated 90K protein. These compounds selectively modulated tumor cell aggregation and apoptosis, and were able to inhibit in vitro tube formation of ECs. Others have tried antigalectin antibodies to interfere with galectin function in angiogenesis. However, the effects of these antibodies in in vitro angiogenesis assays are diverse, ranging from inhibition to no response, or even reversal of the inhibitory effects of synthetic blockers.29,36,85 These differences are most likely related to variations in the antibodies used, the experimental conditions, and the reading output.
We identified galectin-1 as an important target to inhibit tumor angiogenesis when screening for anginex receptors.7 Anginex, which is a synthetic 33-mer peptide that specifically homes to the tumor vasculature, inhibits tumor angiogenesis by inhibition of EC proliferation and migration. Secondary to this, EC death occurs through anoikis.95-97 It was suggested that anginex uses soluble fibronectin to home to the tumor vasculature.97 Using the artificial anginex-encoding gene98 in a yeast 2-hybrid analysis, we identified galectin-1 as the main binding partner in activated ECs. Indeed, interfering with endothelial expression of galectin-1 or treatment with anti-galectin-1 antibodies in vitro induced similar angiostatic effects compared with treatment with anginex.7 Furthermore, tumors in galectin-1-null mice were no longer responsive to anginex therapy, confirming that the antiangiogenesis activity of the peptide was mediated through galectin-1.7 This is in contradiction with the finding that anginex directly disrupts the EC membrane.99 However, the latter study was performed on cultured ECs in vitro, outside the context of a tumor vasculature.
Whether and how targeting of galectins in the endothelium affects the antitumor immune response is less well studied. Given the observation that increased expression of galectins could have immunosuppressive effects, interfering with galectin function might counteract the galectin-mediated tumor immune escape. In line with this, Sato et al showed that lactose treatment inhibited the galectin-3-mediated adhesion of neutrophils to ECs in vitro.62 In addition, we observed that incubation of cultured ECs with a galectin-1–blocking antibody restored the transendothelial migration of T cells.30 Thus, apart from preventing leukocyte apoptosis, blocking galectins in the endothelium might lead to an enhanced leukocyte infiltration of the tumor. However, in a tumor model in galectin-1-null mice, the infiltration of leukocytes was not different from tumors in wild-type animals.7 As described, studies using in vivo tumor models that can distinguish between the effects of endothelial galectins and galectins expressed by the tumor cells represent one of the challenges for future research.
Concluding remarks and future perspectives
In this review, we have summarized the current knowledge regarding the expression of galectins in ECs and their possible roles in cancer. It is well established that ECs are involved in several steps of tumor progression and that they provide a target for tumor-type independent treatment strategies. ECs express at least 3 members of the galectin family, and their expression is regulated during cell activation. Whether other galectins are also expressed by ECs, and how their expression relates to EC activation, remains to be investigated. The observation that endothelial galectin expression differs upon cell activation might be used to target activated ECs for diagnostic purposes in a similar fashion as αvβ3 or E-selectin targeting.100 The elevated expression of galectins on the surface of activated ECs could also open a window of opportunity to develop strategies for targeted delivery of liposomes carrying drugs or viral vectors for gene therapy.4 In line with this, we recently provided evidence that liposomes conjugated with the galectin-1-binding peptide anginex can indeed be used to target fluorescently labeled paramagnetic liposomes to activated ECs in vitro.101 Future experiments using different in vivo tumor models will have to determine the feasibility to apply such an approach for diagnostic imaging of tumor angiogenesis in human patients with cancer.
Beyond the possible applications in tumor vascular targeting, galectins in the endothelium are also suggested to play a role in key steps of cancer progression, including tumor angiogenesis, tumor immune escape, and tumor metastasis. As described, the galectin type, expression level, and even cellular localization can be altered upon EC activation. It can be hypothesized that alterations in the “galectin code” of ECs provides an excellent target for EC-based therapy, especially since such therapies might be tumor-type independent and less susceptible to drug-induced resistance (Figure 4). In fact, targeting endothelial galectins has already been shown to induce a therapeutic effect on tumor immunity, tumor metastasis, and tumor angiogenesis. Several compounds have been described that interfere with or completely block galectin function, including antibodies, peptides, and chemical-binding substrates. Recently, an approach was presented that allows the identification of glycopeptides that bind to and possibly inhibit galectins.102 Different libraries of fully randomized series of peptide hexamers were generated using both unsubstituted and glycosylated amino acids. Following screening against fluorescently labeled galectin-1 and galectin-3, the interacting hexa(glyco)peptides were further characterized, and their ability of inhibit galectin-binding to tumor cells was determined. Some of the interacting hexa(glyco)peptides were more effective inhibitors than lactose, suggesting that the inhibitory activity also involved binding of the nonsugar part of the glycopeptide.102 A better understanding of the inhibitory mechanisms of galectin-binding compounds might lead to the development of more specific and potent inhibitors. It is tempting to speculate that blocking a single galectin with 1 of those compounds could provoke a response in different steps of tumor progression, thereby preventing tumor metastasis, inhibiting tumor angiogenesis, and reversing tumor immune escape. However, for the rational development of galectin-based therapeutic and diagnostic applications, more insights in their exact function in endothelial biology is of utmost importance.
Targeting galectins in the tumor endothelium. (A) In quiescent ECs, the specific set of galectins, their expression level, and their cellular localization is represented by a “galectin code.” Having this galectin code, the ECs are angiogenically inactive, the immune surveillance is not impaired, and heterotypic cell adhesion of circulating cells is hampered. The net result is an environment that does not promote tumor progression. (B) In the presence of stimulatory growth factors, the ECs become activated, causing alterations in the repertoire, expression level, and even localization of galectins. This change in the galectin code enhances the angiogenic potential of the cells, hampers a proper immune response, and facilitates metastasis formation. Consequently, the tumor progression potential is high. From this, it can be speculated that changing the galectin code (eg, by altering galectin expression levels or by directly targeting the galectins with blocking antibodies or peptides), a combination of effects could be generated. This might involve inhibition of angiogenesis, reversal of EC anergy, and/or prevention of metastasis formation. Thus, the progressive phenotype of any tumor might be reversed by targeting the galectins that are present on the ECs.
Targeting galectins in the tumor endothelium. (A) In quiescent ECs, the specific set of galectins, their expression level, and their cellular localization is represented by a “galectin code.” Having this galectin code, the ECs are angiogenically inactive, the immune surveillance is not impaired, and heterotypic cell adhesion of circulating cells is hampered. The net result is an environment that does not promote tumor progression. (B) In the presence of stimulatory growth factors, the ECs become activated, causing alterations in the repertoire, expression level, and even localization of galectins. This change in the galectin code enhances the angiogenic potential of the cells, hampers a proper immune response, and facilitates metastasis formation. Consequently, the tumor progression potential is high. From this, it can be speculated that changing the galectin code (eg, by altering galectin expression levels or by directly targeting the galectins with blocking antibodies or peptides), a combination of effects could be generated. This might involve inhibition of angiogenesis, reversal of EC anergy, and/or prevention of metastasis formation. Thus, the progressive phenotype of any tumor might be reversed by targeting the galectins that are present on the ECs.
Authorship
Contribution: V.L.J.L.T., F.P., L.G.B., and A.W.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arjan W. Griffioen, Department of Pathology, Angiogenesis Laboratory, Maastricht University & University Hospital Maastricht, PO Box 5800, 6202 AZ Maastricht, the Netherlands; e-mail: aw.griffioen@path.unimaas.nl.