Abstract
The tumor suppressor Smad4 mediates signaling by the transforming growth factor beta (TGF-β) superfamily of ligands. Previous studies showed that several TGF-β family members exert important functions in hematopoiesis. Here, we studied the role of Smad4 in adult murine hematopoiesis using the inducible Mx-Cre/loxP system. Mice with homozygous Smad4 deletion (Smad4Δ/Δ) developed severe anemia 6 to 8 weeks after induction (mean hemoglobin level 70 g/L). The anemia was not transplantable, as wild-type mice reconstituted with Smad4Δ/Δ bone marrow cells had normal peripheral blood counts. These mice did not develop an inflammatory disease typical for mice deficient in TGF-β receptors I and II, suggesting that the suppression of inflammation by TGF-β is Smad4 independent. The same results were obtained when Smad4 alleles were deleted selectively in hematopoietic cells using the VavCre transgenic mice. In contrast, lethally irradiated Smad4Δ/Δ mice that received wild-type bone marrow cells developed anemia similar to Smad4Δ/Δ mice that did not receive a transplant. Liver iron stores were decreased and blood was present in stool, indicating that the anemia was due to blood loss. Multiple polyps in stomach and colon represent a likely source of the bleeding. We conclude that Smad4 is not required for adult erythropoiesis and that anemia is solely the consequence of blood loss.
Introduction
The members of the transforming growth factor beta (TGF-β) superfamily of ligands modulate cell proliferation, differentiation, apoptosis, adhesion, and cell migration.1 These ligands, including TGF-β, activins, and bone morphogenetic proteins (BMPs), bind cell-surface receptors, classified as type I and II receptors, that contain an intracellular serine/threonine protein kinase domain. Upon ligand activation, the type II and type I receptors form an active ligand-receptor complex that phosphorylates members of the Small mutants (Caenorhabditis elegans) and mothers against the decapentaplegic homolog (Smad) family of proteins. The Smad family members that directly interact with the receptors are called receptor Smads (R-Smad). The type I receptors for TGF-β, activin, nodal, and myostatin phosphorylate R-Smad2 and 3, whereas the BMPs phosphorylate R-Smad1, 5, and 8. The R-Smads associate with Smad4, also called common partner Smad (co-Smad), and as a complex enter the nucleus to regulate transcription. Smad6 and Smad7 TGF-β inhibit signaling through multiple mechanisms and are called inhibitory Smads (I-Smad).2
Hematopoiesis is a tightly balanced process consisting of cell self-renewal, differentiation, and apoptosis of hematopoietic cells. The effects of TGF-β signaling on hematopoiesis are cell and context specific. TGF-β1 has an inhibitory function in early expansion of committed hematopoietic precursors,3 and BMP4 is implicated in mesoderm induction and hematopoietic commitment during embryogenesis.4 Mice deficient for TGF-β1 die 3 to 4 weeks after birth due to an inflammatory syndrome,5,6 whereas the knockouts of the TGF-β receptors I and II are embryonically lethal during midgestation.7,8 Cells taken from embryos deficient for TGF-β receptors I and II display an increase in erythroid colony-forming cells, consistent with an inhibitory effect of TGF-β in early expansion of committed hematopoietic precursors.8 Smad1−/− and Smad5−/− mice showed defects of hematopoietic and vascular development.9,10 Smad1 expression is sufficient to expand the number of cells that commit to the hemangioblast fate.11 Smad5 is dispensable for hematopoiesis in the adult mouse.12 Overexpression of Smad7 promotes self-renewal capacity of hematopoietic stem cells (HSCs) in vivo.13
Since Smad4 is necessary for signaling by both the TGF-β and the BMP families of ligands, Smad4−/− mice could be expected to show severe defects in hematopoiesis. However, Smad4−/− mice die during embryogenesis before the onset of hematopoiesis.14,15 To directly investigate the role of Smad4 in hematopoiesis, we crossed mice with a conditional Smad4 knockout allele (Smad4fl/fl)16 and a strain containing a Cre-recombinase gene controlled by the interferon-inducible Mx1 promoter (Mx-Cre).17 The Mx-Cre–inducible mouse was widely used in studies of hematopoiesis and showed high efficiency of recombination in bone marrow.17,18 Upon induction of Mx-Cre expression, the conditional Smad4fl/fl alleles (fl/fl) recombined to yield dysfunctional Smad4 alleles (Δ/Δ) and these mice developed severe anemia by 6 to 8 weeks after induction. To inactivate the Smad4fl/fl conditional allele in hematopoietic cells only, we crossed the Smad4 mice to the VavCre strain, which expresses Cre selectively in hematopoietic cells under the control of the Vav promoter.19 We show that erythropoiesis was not directly affected by the loss of Smad4. Rather, anemia is the consequence of blood loss from polyps that rapidly form in the stomach and colon of these mice.
Materials and methods
Mice
All mice used in this study were kept under specific pathogen-free conditions and in accordance with Swiss federal regulations. The Smad4fl/fl mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) were crossed with the Mx-Cre mice, which contain Cre recombinase under control of the interferon-inducible Mx1 promoter.16,17 Smad4 mice were genotyped using the primers S1 (ACTTTACAGGATGATGGTTA) and S2 (GGTCAAGCAGATTACAGCAA), which yield a 360-bp fragment for the floxed Smad4 allele (Smad4fl) and 310-bp fragment for the wild-type Smad4 allele (Smad4+), and also in parallel with the primers S3 (GGGCAGCGTAGCATATAAGA) and S4 (GACCCAAACGTCACCTTCAC), which produce a 450-bp fragment for Smad4fl and a 390-bp fragment for Smad4+. The alleles carrying the Cre-induced deletion of Smad4 (Smad4Δ) were detected with primer S5 (TCCCACATTCCTCTTAGTTTTGA) and primer S6 (CCAGCTTCTCTGTCCAGGTAGTA), yielding a 500-bp fragment for Smad4Δ. The efficiency of the Cre-mediated excision was assessed by Southern blot using a probe generated by the primers CTCGAGTAGGTTAACAAGG and CTTTATATACGCGCTTGGG located in intron 8 and exon 9 of the floxed Smad4 allele (Figure S1). Genotyping of Mx-Cre mice was performed with the primers AGGTGTAGAGAAGGCACTTAGC and CTAATCGCCATCTTCCAGCAGG, which amplify a 300-bp fragment. Mx-Cre expression was induced by the intraperitoneal injection of 300 μg polyinosine-polycytosine (pIpC) 3 times every 2 days. VavCre mice were kindly provided by Dr Dimitris Kioussis.19 These mice were crossed to obtain VavCre;Smad4fl/fl mice. Genotyping of VavCre mice was performed with the primers CTCTGACAGATGCCAGGACA and TGATTTCAGGGATGGACACA, yielding a 500-bp fragment.
Blood analysis
Blood was collected from the tail vein or by cardiac puncture and blood counts were determined by the Advia 120 Hematology Analyzer using the Multispecies Software, version 2.3.01-MS (Bayer, Leverkusen, Germany). The concentration of transferrin in mouse blood plasma was determined by a mouse serum transferrin enzyme-linked immunosorbent assay (ELISA) kit (ADI Alpha Diagnostic, San Antonio, TX). Direct antiglobulin test (DAT) was used for detecting IgG/IgM autoantibodies on the membrane of erythrocytes. Phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–labeled goat anti–mouse IgG/IgM was from Pharmingen BD (Erembodegen, Belgium). Blood from New Zealand Black (NZB) mice served as positive controls and Smad4fl/fl as negative controls for DAT. Serum erythropoietin levels were measured with the Quantikine Mouse/Rat Epo Immunoassay kit (R&D Systems, Abingdon, United Kingdom).
Red blood cell half-life measurement
Mouse blood cells were labeled by intravenous injection of 3 mg NHS-X-Biotin (Sigma, Deisenhofen, Germany). For analysis, capillary blood obtained by tail puncture was diluted in 3.8% sodium citrate, spun down by 180g, resuspended in 500 μL PBS-FACS buffer (0.5% BSA, 0.02% NaN3), and incubated 30 minutes at 4°C with PE-conjugated streptavidin (Becton Dickinson, Heidelberg, Germany). The cells were centrifuged and the pellet was resuspended in 1 mL PBS-FACS buffer for flow-cytometric analysis on a FACSCalibur (Becton Dickinson) to determine the fraction of labeled red blood cells (RBCs) remaining.
Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was isolated from tissue using Trizol (Peqlab, Erlangen, Germany) and reverse transcribed after random hexamer priming using the Omniscript RT kit (Qiagen, Hilden, Germany). Quantitative measurement of gene expression was carried out with SYBR Green PCR master mix on an ABI Prism 7000 (Applied Biosystems, Foster City, CA). The mouse RPL19 mRNA assessed with the primers ATCCGCAAGCCTGTGACTGT and TCGGGCCAGGGTGTTTTT was used for normalization.20 Relative expression values were calculated by the delta-delta cycle threshold (ΔΔCT) method using 1 bone marrow sample as a calibrator that was set to the value of 1.21,22 The primer pairs used for quantification of specific mRNAs are listed in Table 1 and were in part described previously.23-25
Sequences of primers used for quantitative RT-PCR
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| Smad4 | GTTCAGGTAGGAGAGACGTTTAAGGT | CCTTTACATTCCAACTGCACTCCT |
| Hepc | CCTATCTCCATCAACAGATG | AACAGATACCACACTGGGAA |
| Fpn | AAGGATTGACCAGCTAACCAACA | CAGCCAATGACTGGAGAACCA |
| Dcytb | GCAGCGGGCTCGAGTTTA | TTCCAGGTCCATGGCAGTCT |
| Dmt1 | AACCAACAAGCAGGTGGTTGA | CTTTGTAGATGTCCACAGCCA |
| Tf | TTGTGCCATCCCATCACAAC | CTAGTGTCCGATGCCTTCACC |
| Heph | TTGTCTCATGAAGAACATTACAGCAC | CATATGGCAATCAAAGCAGAAGA |
| Hfe | CTGAAAGGGTGGGACTACATGTTC | GGACACCACTCCCAACTTCGT |
| Tfr1 | CAGAAAGTTCCTCAGCTCAACCA | GTTCAATTCAACGTCATGGGTAAG |
| Tfr2 | AGCTGGGACGGAGGTGACTT | TCCAGGCTCACGTACACAACA |
| Sft | CTGTGCTCATTGAAGAGGACCTT | TCTGGTTGCTTTCTCAGTCACG |
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| Smad4 | GTTCAGGTAGGAGAGACGTTTAAGGT | CCTTTACATTCCAACTGCACTCCT |
| Hepc | CCTATCTCCATCAACAGATG | AACAGATACCACACTGGGAA |
| Fpn | AAGGATTGACCAGCTAACCAACA | CAGCCAATGACTGGAGAACCA |
| Dcytb | GCAGCGGGCTCGAGTTTA | TTCCAGGTCCATGGCAGTCT |
| Dmt1 | AACCAACAAGCAGGTGGTTGA | CTTTGTAGATGTCCACAGCCA |
| Tf | TTGTGCCATCCCATCACAAC | CTAGTGTCCGATGCCTTCACC |
| Heph | TTGTCTCATGAAGAACATTACAGCAC | CATATGGCAATCAAAGCAGAAGA |
| Hfe | CTGAAAGGGTGGGACTACATGTTC | GGACACCACTCCCAACTTCGT |
| Tfr1 | CAGAAAGTTCCTCAGCTCAACCA | GTTCAATTCAACGTCATGGGTAAG |
| Tfr2 | AGCTGGGACGGAGGTGACTT | TCCAGGCTCACGTACACAACA |
| Sft | CTGTGCTCATTGAAGAGGACCTT | TCTGGTTGCTTTCTCAGTCACG |
Smad4 indicates small mutants (C elegans) and mothers against decapentaplegic homolog 4 (Drosophila); Hepc, hepcidin; Fpn, ferroportin; Dcytb, cytochrome b reductase 1; Dmt1, divalent metal transporter 1; Tf, transferrin; Heph, hephaestin; Hfe, major histocompatibility complex class I–like protein; Tfr1, transferrin receptor 1; Tfr2, transferrin receptor 2; and Sft, stimulator of Fe transport.
Liver iron analysis
Total iron in the liver tissues was determined by flame atomic absorption spectroscopy under alkaline conditions on a Varian SpectrAA220 spectrometer (Varian, Zug, Switzerland) following solubilization by tetramethylammonium hydroxide.26
Feces analysis
The feces of mice were collected over several weeks with sampling intervals of 3.5 days. The presence of blood in feces was detected by Hemoccult-R (Beckman Coulter, Krefeld, Germany).
Flow cytometry
FITC- and PE-conjugated monoclonal antibodies against TER119, B220, Mac-1, GR-1, CD45.1, CD45.2, and mouse IgG and IgM (Pharmingen, BD, San Diego, CA) were diluted in PBS/1% calf serum and used for staining of single-cell suspensions derived from bone marrow and spleen. In order to get single-cell suspensions, organs were grinded and cells were filtered through 70-μm nylon mesh.
Bone marrow transplantation
Bone marrow cells from femora and tibiae were filtered through 40-μm mesh, and 3 × 106 cells in 200 μL HBSS (1 × Hanks balanced salt solution with 20 mM Hepes buffer) were injected into the tail vein of lethally irradiated (1100 cGy in 2 doses, separated by 3 hours) 7- to 10-week-old recipient mice. Mx-Cre;Smad4fl/fl and control mice received pIpC 10 days before being used as bone marrow donors. C57BL/6SJL-PtprcaPep3b/BoyJ (B6.CD45.1) mice were used as the recipients. In the converse experiment, B6.CD45.1 wild-type donor bone marrow cells were transplanted into irradiated Mx-Cre;Smad4fl/fl or control mice. The recipient mice received pIpC 5 weeks after transplantation. Chimerism of recipient mice was analyzed by flow-cytometric analysis of CD45.1+ and CD45.2+ cells.
Histology
Stomach, small intestine, and colon were isolated, fixed in 4% formalin, and paraffin embedded, and 4-μm sections were stained with hematoxylin and eosin (H&E). The images were viewed and captured with a Leica DMZ75 microscope (Leica, Wetzlar, Germany) and Leica DFC480 R2 camera with 1.0× plan objective, and Zeiss AX10 microscope (Carl Zeiss, Oberkochen, Germany) and Axio CamHR camera objective Plan-Neofluar 2.5×/0.075, Plan-Apochromat 5×/0.16, and Plan-Apochromat 1.0×/0.45. Images were acquired using Leica FireCam software version 1.5 and Axio CamHR version 4.6. Brightness/contrast and color balance were adjusted using Adobe Photoshop Elements 2.0 (Adobe Systems, San Jose, CA).
Results
Mice with induced deletion of Smad4 develop severe anemia
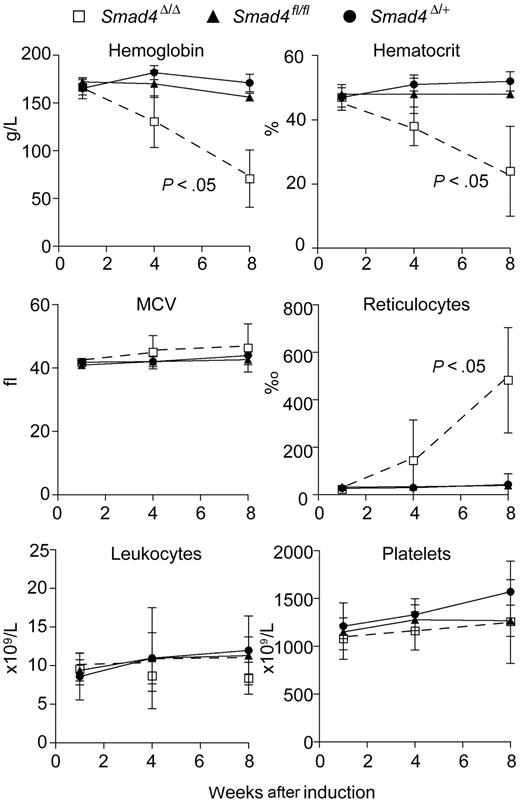
Four weeks after Mx-Cre–induced excision of Smad4fl/fl, the resulting Smad4Δ/Δ mice developed anemia and after 8 weeks the hemoglobin concentration and hematocrit were decreased to 30% of normal values (Figure 1). The mean corpuscular volume (MCV) was unchanged, but the reticulocyte count was strongly increased. The white blood count and platelet levels remained unchanged. The Smad4Δ/Δ alleles were detectable by PCR in 39 of 40 bone marrow–derived colonies (not shown). The apparent half-life of erythrocytes from Smad4Δ/Δ mice was reduced to 10 to 12 days, whereas the half-life in Smad4fl/fl control mice was 23 days (data not shown). A direct antiglobulin test showed no evidence for IgM or IgG surface antibodies on erythrocytes from Smad4Δ/Δ mice, arguing against autoimmune antibody–meditated hemolytic anemia (not shown). The apparent reduction of the erythrocyte half-life in circulation can be explained by compensatory increase in regeneration, marked by massive reticulocytosis (Figure 1) and increased erythropoietin serum levels (> 5000 pg/mL, n = 5; normal range 50-200 pg/mL, n = 4).
Smad4Δ/Δ mice developed anemia after induced Smad4 deletion. Hemoglobin level, hematocrit, reticulocyte count, leukocyte count, mean corpuscular volume (MCV), and platelet count in Smad4Δ/Δ (n = 3), Smad4fl/fl (n = 4), and Smad4Δ/+ (n = 8) mice were plotted against weeks after induction. The P values were calculated by Student t test. The error bars indicate standard deviation.
Smad4Δ/Δ mice developed anemia after induced Smad4 deletion. Hemoglobin level, hematocrit, reticulocyte count, leukocyte count, mean corpuscular volume (MCV), and platelet count in Smad4Δ/Δ (n = 3), Smad4fl/fl (n = 4), and Smad4Δ/+ (n = 8) mice were plotted against weeks after induction. The P values were calculated by Student t test. The error bars indicate standard deviation.
Smad4 is dispensable for adult murine erythropoiesis
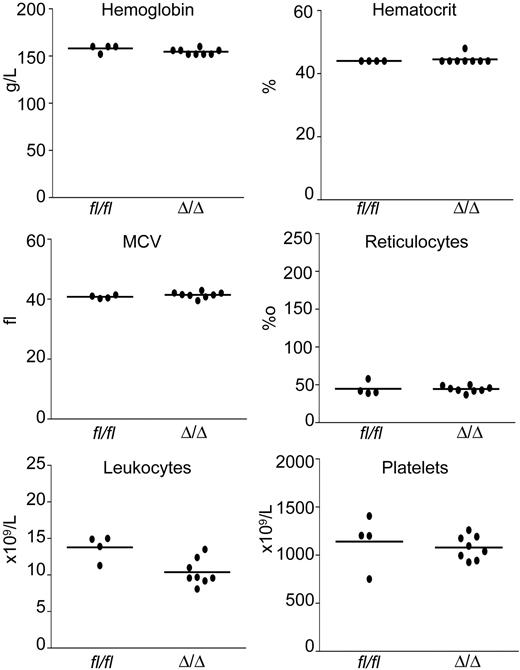
To determine whether the observed anemia was cell autonomous, bone marrow cells from pIpC-induced Mx-Cre;Smad4fl/fl mice and controls were transplanted into lethally irradiated recipient mice. Eight weeks after bone marrow transplantation, the mice were killed and peripheral blood counts were performed (Figure 2). The red blood cell parameters as well as white blood cell counts and platelet levels remained normal (Figure 2). The recipients of Smad4Δ/Δ bone marrow cells did not show any signs of inflammatory disease typical for knockouts of the TGF-β receptors (ie, absence of weight loss, leukocytosis, signs of inflammation of the eyes, and upon autopsy absence of organ damage). Chimerism of recipient mice was determined in peripheral blood by assessing the ratio of CD45.2 donor cells to CD45.1 recipient cells by flow cytometry. Both groups of mice displayed a ratio of donor to recipient cells of greater than 100:1 (Table 2). No differences in B cells (B220), T cells (CD3), or myeloid cells (Gr-1) were detected in bone marrow. Deletion of the floxed Smad4 alleles was found by PCR in DNA from peripheral blood cells of the recipient mice (not shown). Thus, anemia was not transplantable with Smad4-deficient bone marrow cells, indicating that Smad4 is dispensable for adult murine erythropoiesis.
Transplantation of Smad4Δ/Δ bone marrow cells into wild-type recipient mice did not lead to the development of anemia. Hemoglobin level, hematocrit, MCV, reticulocyte count, leukocyte count, and platelet count remained stable in the control and experimental groups. Eight recipients of Smad4Δ/Δ bone marrow cells and 4 recipients of Smad4fl/fl controls were analyzed 8 weeks after transplantation. Dots represent the values of individual mice; horizontal lines indicate the mean.
Transplantation of Smad4Δ/Δ bone marrow cells into wild-type recipient mice did not lead to the development of anemia. Hemoglobin level, hematocrit, MCV, reticulocyte count, leukocyte count, and platelet count remained stable in the control and experimental groups. Eight recipients of Smad4Δ/Δ bone marrow cells and 4 recipients of Smad4fl/fl controls were analyzed 8 weeks after transplantation. Dots represent the values of individual mice; horizontal lines indicate the mean.
Hematopoietic lineage distribution in bone marrow of wild-type recipient mice that received Smad4Δ/Δ or Smad4fl/fl bone marrow
| . | Smad4Δ/Δ . | Smad4fl/fl . |
|---|---|---|
| n | 4 | 4 |
| CD45.2+ | 87.4 ± 3.3 | 91.1 ± 3.2 |
| CD45.1+ | 0.24 ± 0.1 | 0.50 ± 0.4 |
| B220+ | 8.4 ± 2.1 | 5.8 ± 0.2 |
| CD3 | 2.5 ± 0.9 | 2.4 ± 0.6 |
| Gr1 | 14.8 ± 1.2 | 14.4 ± 1.3 |
| . | Smad4Δ/Δ . | Smad4fl/fl . |
|---|---|---|
| n | 4 | 4 |
| CD45.2+ | 87.4 ± 3.3 | 91.1 ± 3.2 |
| CD45.1+ | 0.24 ± 0.1 | 0.50 ± 0.4 |
| B220+ | 8.4 ± 2.1 | 5.8 ± 0.2 |
| CD3 | 2.5 ± 0.9 | 2.4 ± 0.6 |
| Gr1 | 14.8 ± 1.2 | 14.4 ± 1.3 |
Donor cells (CD45.2+), recipient cells (CD45.1+).
To confirm this observation in a system not depending on transplantation, we generated mice with a hematopoietic-specific deletion of Smad4. The VavCre strain has been shown to excise loxP target sequences in hematopoietic cells only.19 The resulting VavCre;Smad4fl/fl mice had normal blood counts and showed no symptoms of inflammation (Figure 3). We also confirmed complete excision of Smad4 in peripheral blood cells of these mice by PCR (Figure S1E). These results implied that host factors might be causing the anemia phenotype.
Absence of anemia in VavCre;Smad4fl/fl mice. Peripheral blood parameters were determined in 10-week-old mice. VavCre;Smad4fl/fl (Δ/Δ), VavCre;Smad4fl/+ (Δ/+), and Smad4fl/fl (fl/fl). Dots represent the values of individual mice; horizontal lines indicate the mean.
Absence of anemia in VavCre;Smad4fl/fl mice. Peripheral blood parameters were determined in 10-week-old mice. VavCre;Smad4fl/fl (Δ/Δ), VavCre;Smad4fl/+ (Δ/+), and Smad4fl/fl (fl/fl). Dots represent the values of individual mice; horizontal lines indicate the mean.
Anemia of Smad4Δ/Δ mice is non–cell-autonomous
To determine if anemia is caused by the host environment, bone marrow from wild-type C57BL/6J mice was transplanted into lethally irradiated Mx-Cre;Smad4fl/fl and Smad4fl/fl control mice. From weeks 2 to 4 after pIpC-induced deletion of Smad4, recipients began developing anemia (Figure 4). Interindividual differences in the severity of anemia were observed in Smad4Δ/Δ mice. The control Smad4fl/fl recipient mice remained healthy without any changes in blood parameters. These results demonstrate that anemia of Smad4Δ/Δ mice is caused by alterations outside of the hematopoietic system.
Transplantation of wild-type bone marrow into Smad4Δ/Δ recipients resulted in anemia. Hemoglobin level, hematocrit, MCV, reticulocyte count, leukocyte count, and platelet count are shown. Three individual Smad4Δ/Δ recipient mice (#1 [○], #2 [▵], and #3 [◇]) developed anemia with individual differences in severity and kinetics. *Significant differences at 4 weeks (P ≤ .03; Student t test). The values for the Smad4fl/fl control recipient mice (▴) are shown as the mean of 6 mice with standard deviation. The error bars indicate standard deviation.
Transplantation of wild-type bone marrow into Smad4Δ/Δ recipients resulted in anemia. Hemoglobin level, hematocrit, MCV, reticulocyte count, leukocyte count, and platelet count are shown. Three individual Smad4Δ/Δ recipient mice (#1 [○], #2 [▵], and #3 [◇]) developed anemia with individual differences in severity and kinetics. *Significant differences at 4 weeks (P ≤ .03; Student t test). The values for the Smad4fl/fl control recipient mice (▴) are shown as the mean of 6 mice with standard deviation. The error bars indicate standard deviation.
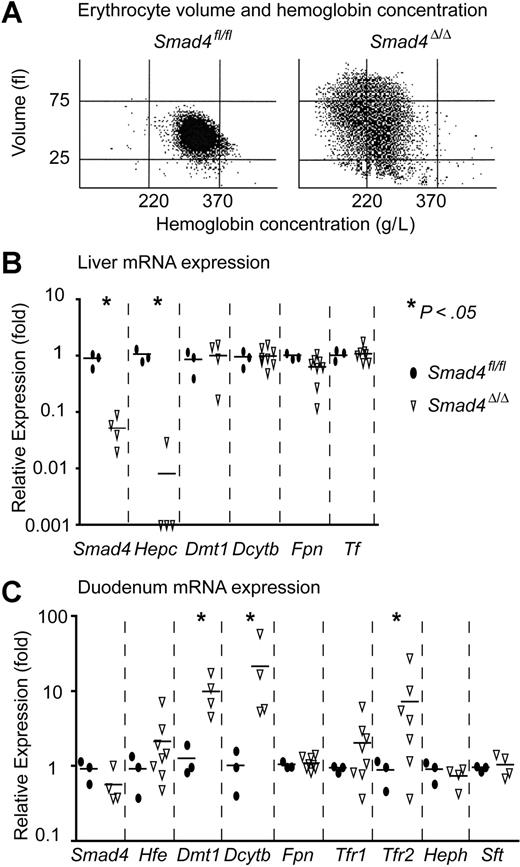
Smad4Δ/Δ mice show severe iron deficiency
The presence of hypochromic erythrocytes at later stages of anemia in Smad4Δ/Δ mice (Figure 5A) suggested that iron deficiency could be involved in the pathogenesis of anemia. Liver iron in Smad4Δ/Δ mice 4 weeks after pIpC induction was decreased to 23% (2.6 ± 0.3 μmol/g, n = 6) compared with liver iron of Smad4fl/fl mice (11.5 ± 4.0 μmol/g, n = 6) or wild-type C57BL/6J mice (16.6 ± 4.7 μmol/g, n = 3). No differences in plasma transferrin (Tf), determined by ELISA specific for mouse Tf, were found between Smad4Δ/Δ mice (1.9 ± 0.2 μg/L, n = 6) and Smad4fl/fl (1.9 ± 0.9 μg/L, n = 10) or wild-type BL6 mice (1.7 ± 0.08 μg/L, n = 3). We determined the expression of genes that are involved in iron metabolism by quantitative PCR (Figure 5B-C). The efficiency of Cre-mediated excision in the livers of Smad4Δ/Δ mice measured by Southern blot was ranging from 66% to 96% (not shown) and the expression of Smad4 mRNA was severely reduced (Figure 5B). To assure that only the full-length mRNA was measured, the forward primer (Table 1) used for the quantification of the full-length Smad4 mRNA was placed in exon 8, which is deleted by Cre-mediated excision. We also determined the expression of genes involved in the regulation of iron metabolism. Hepcidin (Hepc) mRNA was severely decreased, whereas the expression of divalent metal transporter 1 (Dmt1), cytochrome b reductase 1 (Dcytb), ferroportin 1 (Fpn), and transferrin (Tf) remained unchanged (Figure 5B). In the duodenum, the floxed Smad4 allele was excised by only 4% to 8% (not shown) and Smad4 mRNA was just merely decreased, whereas Dmt1 and Dcytb increased 5- to 50-fold and transferrin receptor 2 (Tfr2) increased 2- to 20-fold (Figure 5C). Other iron-related genes, such as major histocompatibility complex class I–like protein (Hfe), transferrin receptor 1 (Tfr1), hephaestin (Heph), and stimulator of Fe transport (Sft) showed no significant changes in expression (Figure 5C). Taken together, these changes fit well with a state of increased iron uptake and demand.
Smad4Δ/Δ mice display severe iron deficiency anemia. (A) Hypochromic erythrocytes in Smad4Δ/Δ mice. Cell volume was plotted against hemoglobin concentration. (Left) Normal control. (Right) Hypochromic red blood cells (< 220 g/L) and volume increase (> 75 fl) due to reticulocytosis in Smad4Δ/Δ. (B) In liver, Smad4 and hepcidin (Hepc) expression are almost abrogated, and ferroportin 1 (Fpn) is slightly decreased. Cytochrome b reductase 1 (Dcytb), divalent metal transporter 1 (Dmt1), and transferrin (Tf) were unchanged. (C) In duodenum, Smad4, Fpn, hephaestin (Heph), major histocompatibility complex class I–like protein (Hfe), transferrin receptor 1 (Tfr1), and stimulator of Fe transport (Sft) were unchanged, and Dmt1, Dcytb, and transferrin receptor 2 (Tfr2) were dramatically increased. Smad4fl/fl littermates were chosen as controls. The P values were calculated by Student t test.
Smad4Δ/Δ mice display severe iron deficiency anemia. (A) Hypochromic erythrocytes in Smad4Δ/Δ mice. Cell volume was plotted against hemoglobin concentration. (Left) Normal control. (Right) Hypochromic red blood cells (< 220 g/L) and volume increase (> 75 fl) due to reticulocytosis in Smad4Δ/Δ. (B) In liver, Smad4 and hepcidin (Hepc) expression are almost abrogated, and ferroportin 1 (Fpn) is slightly decreased. Cytochrome b reductase 1 (Dcytb), divalent metal transporter 1 (Dmt1), and transferrin (Tf) were unchanged. (C) In duodenum, Smad4, Fpn, hephaestin (Heph), major histocompatibility complex class I–like protein (Hfe), transferrin receptor 1 (Tfr1), and stimulator of Fe transport (Sft) were unchanged, and Dmt1, Dcytb, and transferrin receptor 2 (Tfr2) were dramatically increased. Smad4fl/fl littermates were chosen as controls. The P values were calculated by Student t test.
Polyps in stomach and colon cause blood loss in Smad4Δ/Δ mice
Histopathology of the gastrointestinal (GI) tract of Smad4Δ/Δ mice revealed polyps in stomach and colon (Figure 6) but less frequently in the small intestine. Most of them were histologically characterized by a branching architecture reminiscent of hyperplastic lesions, mostly with foci of low- and/or high-grade dysplasia. We classified these lesions as mixed hyperplastic/adenomatous polyps. Moreover, “serrated” aspects of the polyps were also detected focally. Additionally, colon polyps frequently displayed cystic changes. None of the gastric or colon polyps fulfilled the criteria of “juvenile polyps,” as they lacked the typical histologic features of such lesions (eg, prominent stroma overgrowth).
Stomach and colon polyp formation in Smad4Δ/Δ mice. The left panel shows a Smad4fl/fl control mouse, right panel shows a Smad4Δ/Δ mouse. Gross macroscopy of stomach (A,B), histologic hematoxylin-eosin staining of stomach (C-F), and colon (G-J) with magnified view of the boxed areas. Magnifications for A,B: 2.5×; C,D: 50×; E-H: 100×; I,J: 200×.
Stomach and colon polyp formation in Smad4Δ/Δ mice. The left panel shows a Smad4fl/fl control mouse, right panel shows a Smad4Δ/Δ mouse. Gross macroscopy of stomach (A,B), histologic hematoxylin-eosin staining of stomach (C-F), and colon (G-J) with magnified view of the boxed areas. Magnifications for A,B: 2.5×; C,D: 50×; E-H: 100×; I,J: 200×.
To show that iron deficiency in Smad4Δ/Δ mice is due to GI bleeding, we collected stool over several weeks and determined the presence of heme by the hemoccult assay (Figure 7). Bleeding was detectable in all mice but with variable onset and duration. In some mice, bleeding started 17 days after pIpC induction of Smad4 deletion, whereas in others the onset was delayed until 31 days. At the time of the first detectable bleeding, the mice did not yet display severe anemia and the severity of anemia did not correlate with the time of onset of bleeding.
Fecal occult blood test in Smad4Δ/Δ mice. A total of 13 mice were analyzed (numbered in y-axis). Time in weeks after first pIpC injection is shown on the x-axis. Horizontal bars represent the duration of the stool collection; □, negative hemoccult tests; and ■, positive hemoccult tests.
Fecal occult blood test in Smad4Δ/Δ mice. A total of 13 mice were analyzed (numbered in y-axis). Time in weeks after first pIpC injection is shown on the x-axis. Horizontal bars represent the duration of the stool collection; □, negative hemoccult tests; and ■, positive hemoccult tests.
Discussion
Our results from transplantation of Smad4Δ/Δ bone marrow into wild-type recipients and deletion of Smad4 selectively in hematopoietic cells in VavCre;Smad4fl/fl mice demonstrate that Smad4 signaling is dispensable for adult erythropoiesis in vivo (Figures 2,3). The red blood cell parameters in peripheral blood were normal. Our study is consistent with the results of an in vitro study that examined cultured human CD34+ hematopoietic stem/progenitor cells under shRNA-mediated Smad4 knock-down.27 We also found normal megakaryopoiesis in our study, arguing that the decrease in circulating platelet counts and increase in megakaryocyte numbers in mice injected with TGF-β protein in vivo are mediated by Smad4-independent signaling.28 The white blood cell counts of neutrophil, basophil, and eosinophil granulocytes as well as monocytes and lymphocytes were normal. The severe anemia observed in Smad4Δ/Δ mice is caused by blood loss.
TGF-β signaling plays an important role in immune surveillance. By producing the immunosuppressive cytokine TGF-β, tumors may escape from immune surveillance via inhibiting the expression of cytolytic genes.29 TGF-β receptor II (TBRII) dominant-negative approaches led to autoimmune inflammatory disease and spontaneous T-cell activation.30 Mice with TBRII deletion in bone marrow not only showed increased CD8+ proliferation in vivo but also developed a lethal inflammatory disease,18,31 and the TBRII-deficient cells of hematopoietic origin, most likely T cells, could induce multifocal inflammatory disease in a dominant way. On the contrary, our Smad4Δ/Δ bone marrow recipients and VavCre;Smad4fl/fl mice were healthy and did not show any signs of inflammatory disease. This result implies that T-cell–mediated suppression of inflammation via TGF-β signaling is Smad4 independent. It remains to be determined whether this effect is mediated by the traditional TGF-β pathway with an alternative downstream mediator like TIF1 or by crosstalk with other signaling through MAPK, JNK, PI3K, or other mediators.
Smad4 is a tumor suppressor gene, and Smad4 mutations are frequently detected in pancreatic cancer, colon cancer, gastric polyps, and adenocarcinomas.32-37 Mice heterozygous for the Smad4 knockout appear normal but develop gastrointestinal polyps after a long latency with loss of heterozygosity in the epithelial cells.38,39 Our Smad4Δ/Δ mice, which have complete excision in bone marrow as well as partially in stomach (10%-50%), started bleeding around day 20 after pIpC induction (Figure 7) and quickly developed a severe anemia and significant polyps in the GI tract without formation of real tumors before they needed to be killed. Ablation of TGF-β by T-cell–specific overexpression of dominant-negative TBRII could accelerate azoxymethane-induced colon carcinogenesis through increased T-cell production of IL-6.40 Recently, it was reported that T-cell specific deletion of Smad4 leads to spontaneous epithelial tumors throughout the GI tract. Mice in this study displayed weight loss starting 3 months after birth and eventually developed clinical features of systemic illness. In contrast, mice with epithelial-specific deletion of Smad4 remained free of polyps, suggesting that polyp formation and tumorigenesis are induced by Smad4-deficient T cells.41 Our Mx-Cre;Smad4Δ/Δ mice developed polyps with a much faster kinetics and since they suffered from severe bleeding anemia, we could not examine tumor progression. At age 10 weeks, our VavCre;Smad4fl/fl mice did not yet show any signs of weight loss or systemic disease. Furthermore, when Smad4Δ/Δ mice were used as recipients for the transplantation of normal bone marrow cells, we observed rapid evolution of anemia (Figure 4). However, we cannot exclude that some host T cells deficient for Smad4 remained functional in these mice.
Recently, shRNA-mediated knockdown of bone morphogenic protein type II receptor (Bmpr2), another member of the TGF-β receptor family, resulted in severe mucosal hemorrhage, gastrointestinal hyperplasia, and blood loss.42 This phenotype was related to vascular dysmorphogenesis. Other studies suggested that malformed vessels are present in polyps of patients with juvenile polyposis, found exclusively in patients carrying a mutation up to codon 415 of SMAD4.43 In contrast, no malformations of blood vessels were observed in polyps from our Smad4Δ/Δ mice.
Our Smad4Δ/Δ mice showed an almost complete loss of hepcidin mRNA expression in liver. This could be due to 2 mechanisms: (1) liver-specific Smad4 deletion results in markedly decreased hepcidin transcription44 ; and (2) increased erythropoiesis due to anemia suppresses the production of hepcidin.45 In our mice, both mechanisms are likely to be contributing to the strongly suppressed hepcidin expression.
Our results demonstrate that Smad4 is dispensable for adult erythropoiesis and suggest that polyp formation in mice with homozygous deletion of Smad4 is not entirely T-cell dependent. The same results were recently reported by Karlsson et al.46 The Smad4Δ/Δ mice can be used to further study polyp formation and the interplay between the inflammatory response and alterations in the epithelial cells and the stroma of the GI tract.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Martin Hersberger (University Hospital Zürich, Switzerland) for determining iron in liver samples, Alois Gratwohl (Basel) and Andre Tichelli (Basel) for helpful discussions, and Ralph Tiedt (Basel) and Alexandre Theocharides (Basel) for comments on the manuscript. This work was supported by a grant from the Roche Foundation of Anemia Research (RoFAR) (R.C.S. and D.P.).
Authorship
Contribution: D.P. performed research, analyzed data, and wrote the paper; T.S. performed research and analyzed data; C.P.K. performed flow cytometric analysis; L.M.T. performed pathology analysis; K.H. and W.K. performed bone marrow transplantations; H.H.-S. performed genotyping; C.D. analyzed data; and R.C.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Radek C. Skoda, Department of Research, Experimental Hematology, University Hospital Basel, Hebelstrasse 20, 4031 Basel, Switzerland; e-mail: radek.skoda@unibas.ch.


![Figure 4. Transplantation of wild-type bone marrow into Smad4Δ/Δ recipients resulted in anemia. Hemoglobin level, hematocrit, MCV, reticulocyte count, leukocyte count, and platelet count are shown. Three individual Smad4Δ/Δ recipient mice (#1 [○], #2 [▵], and #3 [◇]) developed anemia with individual differences in severity and kinetics. *Significant differences at 4 weeks (P ≤ .03; Student t test). The values for the Smad4fl/fl control recipient mice (▴) are shown as the mean of 6 mice with standard deviation. The error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/8/10.1182_blood-2007-02-074393/4/m_zh80210708740004.jpeg?Expires=1764958254&Signature=TUkXJYnZxwDagCcGT-gUXNGUjRy4NH7cHxNjl~6-H5AhotAzbfx0bCNL73LdWWIwqoEQI5sjyW0P1KEAkvmWXz4AlQwuOzXJOEtcPjQ0ylxgTl5f9JRGcbVfwcAXv-ChM~P~RrE2BLjRLmSOUTjBKuI~eRCd-8TZ-Xc0L~n2eH21Dhe7H5CEtvvvi8DUWakJazrZwwcV8GAwvy8bFA7QukvQMb5xC7OgmmiLh3bWgFUXTms5-mkzTtT0bEADVVUXbfyfYum1SDwRErhXHxrFNwAv~ohB7WIolHUJ~iEK9II8RiKE2crf46P~TZ~3ig3XYEK5ASe1arhjI8MqrP6kNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)