Human embryonic stem cells (hESCs) provide an important means to effectively study soluble and cell-bound mediators that regulate development of early blood and endothelial cells in a human model system. Here, several complementary methods are used to demonstrate canonical Wnt signaling is important for development of hESC-derived cells with both hematopoietic and endothelial potential. Analyses using both standard flow cy-tometry, as well the more detailed high-throughput image scanning flow cytometry, characterizes sequential development of distinct early developing CD34brightCD31+Flk1+ cells and a later population of CD34dimCD45+ cells. While the CD34brightCD31+Flk1+ have a more complex morphology and can develop into both endothelial cells and hematopoietic cells, the CD34dimCD45+ cells have a simpler morphology and give rise to only hematopoietic cells. Treatment with dickkopf1 to inhibit Wnt signaling results in a dramatic decrease in development of cells with hematoendothelial potential. In addition, activation of the canonical Wnt signaling pathway in hESCs by coculture with stromal cells that express Wnt1, but not use of noncanonical Wnt5-expressing stromal cells, results in an accelerated differentiation and higher percentage of CD34brightCD31+Flk1+ cells at earlier stages of differentiation. These studies effectively demonstrate the importance of canonical Wnt signaling to mediate development of early hematoendothelial progenitors during human development.
Introduction
Hematopoietic and endothelial cells are mesoderm-derived lineages that demonstrate a close spatial, temporal, and genetic relationship during vertebrate embryogenesis.1 These properties have led to the hypothesis that these cell lineages originate from a common precursor, so-called hemangioblasts or hematogenic-endothelial cells.2,3 Mouse embryonic stem cells (mESCs) have been instrumental to define the phenotypic and developmental pathways that regulate endothelial and hematopoietic development.4,,,–8 For example, blast colony-forming cells (BL-CFCs) are thought to represent the functional equivalent of a common progenitor cell for both endothelial and hematopoietic cells after differentiation of mESCs.5 Importantly, similar cells with hematoendothelial potential have been identified in the posterior region of the primitive streak in mouse embryos, effectively translating in vitro differentiation from mESCs to in vivo embryonic development.9 However, recent in vivo lineage tracing studies of the developing yolk sac suggest that other mechanisms may also be involved.10 Continued studies are therefore needed, especially in a human model system where the relationship between hematopoietic and endothelial cells remains poorly characterized
Several reports of hematopoietic differentiation from human ESCs (hESCs) demonstrate that similar strategies used to study development of hematopoietic and endothelial lineages from mESCs can be transposed to the hESC system.11,,,,–16 This allows analysis of early cell fate specifications of endothelial and hematopoietic cells in a model system that is more directly relevant to human development. As with mESCs, there are 2 routine methods used to facilitate differentiation of hESCs: embryoid body (EB) formation and stromal cell coculture. Although the general kinetics of differentiation suggests a conserved pattern for development of endothelial and hematopoietic precursors between different methods of hESC differentiation, there are differences in the requirement for defined growth factors for development of hematopoietic precursors when hESCs are cocultured with stromal cells compared with EB differentiation.17 One study identified progenitor cells within hESC-derived EBs that express CD31, Flk1, and VE-cadherin but not CD45 (termed CD45negPFV cells), after approximately 7 to 10 days of differentiation, capable of generating both endothelial and hematopoietic cells.13 A similar study also identified hematogenic potential of endothelial cells from CD34+CD31+CD45− human EB-derived cells also after 10 days of differentiation.14 Another recent report demonstrates development of a cell population during EB-mediated differentiation of hESCs that express Flk1 (also termed KDR or VEGF-R2) and generate BL-CFCs similar to what has been observed for mESCs.15 Development of these human hemangioblast cells was observed earlier in the culture and was dependent on presence of bFGF, VEGF, and BMP4 during EB differentiation.15 Yet another group characterized a similar functional hemangioblast cell population, although these cells did not express CD34, CD31, or Flk1.16 The importance of VEGF and BMP4 for development of hematopoietic and endothelial cells has been shown previously.17,–19 However, the role of other exogenous factors regulating early cell fate specification of hematopoietic and endothelial precursors during human development still remains unclear.
Wnt proteins have many important roles during development including maintenance and/or proliferation of rare stem and progenitor cell populations, cell fate specification, segmentation, and dorsal-ventral patterning.20 In the mouse, canonical Wnt signaling is required for formation of the primitive streak, where distinct mesoderm subpopulations including the hemangioblast are generated.9 Mice lacking canonical Wnt ligands, the Wnt coreceptors Lrp5/6, or β-catenin do not develop primitive streak and fail to generate mesoderm from the epiblast.21,–23 A recent study has also demonstrated the requirement of Wnt signaling for the generation of in vitro primitive streak during mESC differentiation.24 Here, we characterize the phenotype of a hematogenic endothelium cell population derived from stromal cell–mediated hESC differentiation. These cells identified as CD34brightCD31+Flk1+ differentiate into both hematopoietic and endothelial cells. Development of these cells is largely dependent on functional canonical Wnt signaling, as inhibition of this signaling pathway results in a decreased generation of these cells. Complementary analyses of hESCs cocultured with stromal cells that overexpress Wnt1 demonstrates accelerated development of this human hematoendothelial cell population.
Methods
Cell culture
The hESC lines H1and H9 (obtained from Wicell, Madison, WI) were maintained as undifferentiated cells as previously described.11,25 Murine S17 stromal cells (kindly provided by Dr K. Dorshkind, University of California, Los Angeles, CA) were grown in RMPI 1640 (Invitrogen Life Technologies, Carlsbad, CA) containing 10% FBS (HyClone, Logan, UT), 2 mM l-glutamine (Cellgro/Mediatech, Herndon, VA), 1% penicillin-streptomycin (P/S; Invitrogen Life Technologies), 1% MEM–nonessential amino acids (Invitrogen Life Technologies), and 0.1 mM β-mercaptoethanol (Invitrogen Life Technologies). M210B4 stromal cells (American Type Culture Collection, Manassas, VA) were grown in RPMI 1640 containing 10% FBS and 1% P/S. Before coculture with hESCs, stromal cells were incubated with conditioned medium containing 10 μg/mL mitomycin C (American Pharmaceutical Partners, Los Angeles, CA) before attachment onto gelatin (Sigma-Aldrich, St Louis, MO)-coated 6-well plates (Nalge Nunc International, Rochester, NY). As indicated, S17 and M210B4 were transduced with a lentiviral construct with either Wnt1 or Wnt5 under control of an EF1α promoter, plus GFP expressed from a downstream IRES sequence. Control cells expressed GFP alone. Stable expressing cells were sorted for GFP, and immunohistochemistry was done to confirm expression of Wnt1 or Wnt5 (Santa Cruz Biotechnology, Santa Cruz, CA).
Differentiation of hESCs
Undifferentiated hESCs were transferred to confluent monolayer of S17 and M210 stromal cells as described previously.11,25 Cells were cultured in medium composed of RMPI 1640 supplemented with 15% defined FBS (HyClone), 2 mM l-glutamine, 0.1 mM 2-ME, 1% MEM–nonessential amino acids, and 1% P/S, with medium changes every 2 to 3 days. On the day of analysis, 1 to 2 wells were harvested by collagenase IV (Invitrogen Life Technologies) treatment, followed by incubation with trypsin/EDTA (0.05%; Cellgro/Mediatech) supplemented with 2% chick serum (Sigma-Aldrich).
Flow cytometry
Single-cell suspensions of differentiated hESCs from indicated number of days were stained with allophycocyanin (APC), PE- and FITC-coupled control Igs, or specific Abs against the following human surface antigens: CD34-APC or CD34-PE-Cy5, CD45-PE or CD45-APC, CD31-PE or CD31-FITC (all from BD Pharmingen, San Diego, CA); Flk1-PE (R&D Systems, Minneapolis, MN); VE-cadherin (Beckman Coulter, Fullerton, CA); CD31-APC, CXCR4-PE, and c-kit-PE (eBioscience, San Diego, CA). Analysis was done on FACSCalibur (BD Biosciences, San Jose, CA), and FACSAria (BD Biosciences) was used for cell sorting. Flow cytometric data were analyzed by FlowJo analysis software (Tree Star, Ashland, OR). Live cells were identified by 7-aminoactinomycin D (7AAD) exclusion.
ImageStream data acquisition and analysis
The ImageStream platform (Amnis, Seattle, WA) provides high throughput, high information content data for individual cells, and cell populations within a heterogeneous sample.26 This system allows effective discrimination of morphology and phenotype of individual cells and within defined cell populations. Differentiated hESCs (1-5 × 106 cells) were resuspended in 150 μL and filtered through 70-μm filter immediately before analysis. Cells probed with fluorescent antibody to specific cell surface antigens and 7-AAD were hydrodynamically focused, excited with a 488-nm laser light, and imaged on a time delay integration (TDI) CCD camera. Debris were excluded by the initial setup and 10 000 images were acquired. Images of cells collected on the ImageStream were analyzed using ImageStream Data Exploration and Analysis Software (IDEAS). Spectral compensation was digitally performed on a pixel-by-pixel basis prior to data analysis. In-focus cells were evaluated after gating on live, single cells.
Hematopoietic and endothelial differentiation
To induce endothelial differentiation, sorted cells were plated onto fibronectin-coated 24-well plates and cultured in EGM2 medium (Cambrex, Walkersville, MD). Medium was changed every 4 to 5 days and cells were analyzed by flow cytometry after 14 days in culture. Endothelial cells cultured in chamber slides were phenotyped for expression of CD31 and von Willebrad factor (VWF) by immunostaining and ability to take up acetylated LDL (acLDL). Expression of VWF protein (DAKO, Carpinteria, CA) was detected with a goat anti–rabbit IgG-FITC secondary antibody (DAKO). CD31 expression was detected by CD31-FITC (BD Pharmingen). Analysis of acLDL uptake was performed by diluting dil-acLDL (Invitrogen Life Technologies) in EGM2 medium. Tube formation in Matrigel (BD Biosciences) was done as previously described.27
Hematopoietic differentiation was induced by coculture of sorted hESC-derived cell populations with AFT024 stromal cells (kindly provided by Drs K. Moore and I. Lemischka, Princeton University, Princeton, NJ). Medium containing IMDM supplemented with 10% FBS (HyClone), P/S, 10 ng/mL Flt3-ligand (Peprotech, Rocky Hill, NJ), 10 ng/mL SCF (Peprotech), and 10 ng/mL Tpo (Peprotech) was changed every 4 to 5 days. Cells were analyzed by flow cytometry after 14 days in culture. Cells were also cultured for 14 days to quantify hematopoietic colony-forming cells (CFCs) in standard methylcellulose-based assay (StemCell Technologies, Vancover, BC).11,28
Evaluation of Wnt signaling
To generate medium containing dickkopf1 (DKK1), confluent 293T cells stably expressing DKK1 were cultured overnight in hESC differentiation medium described in “Differentiation of hESCs.” Conditioned DKK1 medium was collected and mixed 1:1 with regular hESC differentiation medium before use for differentiation cultures. Medium was changed every 2 to 3 days and differentiation was analyzed by flow cytometry. Conditioned medium from 293T cells expressing GFP only was harvested as described above and used as a control. The inhibition of the canonical Wnt signaling pathway was confirmed for DKK1 medium by TopFlash assay. Briefly, 293FT cells cultured overnight and hESCs were transiently transfected with Lipofectamine 2000 (Invitrogen Life Technologies). A total of 250 ng DNA was transfected in each well, including 0.1 ng pCMV-Renilla (to measure transfection efficiency) and 15 ng pSTF 19X (LEF/TCFβ-firefly luciferase reporter plasmid). pCS2-Wnt3a (10 ng; expressing constitutive levels of Wnt3a) was used as a control to measure assay sensitivity and pCS2+ acted as a control to equalize DNA amounts among the samples. After transfection, cells were cultured in serum-free DMEM (Invitrogen Life Technologies) for 3 hours followed by overnight incubation in 1:1 mixture hESC differentiation medium and conditioned medium from DKK1 or GFP-only 293T cells. Cells were assayed after 24 hours using the Dual Luciferase Reporter Assay (Promega, Madison, WI) according to the manufacturer's protocol. All luciferase readings were taken in a Lumat LB 9507 luminometer (Berthold Technologies, Bad Wildbad, Germany) and results were normalized to the renilla luciferase signal. Activity of DKK1-conditioned medium was compared with purified recombinant human DKK1 (R&D Systems).
The effect of Wnt activation was assessed by coculture of hESCs with S17 or M210 stromal cells stably expressing Wnt1 and Wnt5, or GFP alone as a control. To confirm activation of Wnt signaling in hESCs cultured on M210 or S17 stromal cells stably expressing Wnt proteins, stromal cells were first plated on a 24-well gelatin-coated plate one day prior to transfection. H9 and H1 hESCs maintained on matrigel in a 24-well plate were transfected with FugeneHD Transfection Reagent (Roche, Basel, Switzerland) according to the manufacturer's instructions. A total of 5 μg DNA was used in each transfection, including 0.05 μg pCMV-Renilla and 2.0 μg pSTF (19X). After a 3-hour incubation in DNA-lipid reagent complexes, cells were transferred onto transfected stromal cells and cocultured for 24 hours. Again, cells were assayed using the Dual Luciferase Reporter Assay 24 hours after transfection.
RT-PCR analysis
RNA was extracted from different samples using the Qiagen RNeasy Kit (Valencia, CA). Reverse-transcription (RT) reactions were done using Superscript RTIII (Invitrogen Life Technologies) according to the manufacturer's instructions. RT reactions were primed using oligo(dT) primers. Polymerase chain reactions (PCRs) were done with HotStarTaq (Qiagen) using 1 μL RT product per reaction according to the manufacturer's conditions. Conditions used for PCR were as follows: 10 minutes at 95°C, 20 to 40 cycles (actual number noted in Table 1) of 95°C for 30 seconds, annealing temperature (Ta, noted in Table 1) for 30 seconds, and 72°C for 30 seconds. A final 10 minutes at 72°C was included at the end. Human gene–specific primers used are shown in Table 1. Products were analyzed on 1.5% agarose gel and visualized with ethidium bromide staining. All human-specific primers were tested and found negative against mouse embryonic fibroblasts, and M210 and S17 stromal cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Primers and conditions used for RT-PCR
| Gene . | Primers . | Amplified product, bp . | Cycles . | Ta, °C . |
|---|---|---|---|---|
| ACTIN | S: 5′-atctggcaccacaccttctacaatgagctgcg-3′; AS: 5′-cgtcatactcctgcttgctgatccacatctgc-3′ | 838 | 40 | 63 |
| OCT4 | S: 5′-gggaaggtattcagccaaacg-3′; AS: 5′-ggttcgctttctctttcggg-3′ | 180 | 20 | 56 |
| BRACHYURY | S: 5′-cccgcgcactacacacccctcacc-3′; AS: 5′-ccttgggctgcggcgtcgtactg-3′ | 125 | 40 | 63 |
| SCL | S: 5′-atggtgcagctgagtcctcc-3′; AS: 5′-tctcattcttgctgagcttc-3′ | 331 | 40 | 63 |
| RUNX1B | S: 5′-cgtgcacatacattagtagcactacctttg-3′; AS: 5′-cttccacgaatcttgcttgcagaggttaag-3′ | 304 | 40 | 63 |
| RUNX1C | S: 5′-gaagtctgaacccagcatagtggtcagcag-3′; AS: 5′-gtggacgtctctagaaggattcattccaag-3′ | 231 | 40 | 63 |
| GATA-2 | S: 5′-agccggcacctgttgtgcaa-3′; AS: 5′-tgacttctcctgcatgcact-3′ | 244 | 30 | 58 |
| LMO2 | S: 5′-ggatcctgccggagagactatctc-3′; AS: 5′-gaattcagtgaacacctccgcaaa-3′ | 289 | 40 | 63 |
| C-MYB | S: 5′-ctcagacttggaaatgccttc-3′; AS: 5′-ccagtggtgtgagcagaaga-3′ | 406 | 40 | 63 |
| VE-CADHERIN | S: 5′-accggatgaccaagtacagc-3′; AS: 5′-acacactttgggctggtagg-3′ | 596 | 40 | 63 |
| FRIZZLED-3 | S: 5′-ccttggcctgaagatatgg-3′; AS: 5′-acaaggaggtgaacaactacg-3′ | 210 | 40 | 56 |
| FRIZZLED-4 | S: 5′-ccaacatggctgttgaaatg-3′; AS: 5′-tcacccaaccatttcctctc-3′ | 168 | 40 | 56 |
| FRIZZLED-5 | S: 5′-tgctaccagccgtccttcagt-3′; AS: 5′-ccatgccgaagaagtagaccag-3′ | 319 | 40 | 56 |
| WNT3A | S: 5′-ttcttactcctctgcagcctg-3′; AS: 5′-ctggatgccaatcttgatgc-3′ | 207 | 40 | 56 |
| WNT5 | S: 5′-cttcgcccaggttgtaattgaagc-3′; AS: 5′-ctgccaaaaacagaggtgttatcc-3′ | 273 | 40 | 56 |
| WNT10B | S: 5′-aatgcgaatccacaacaac-3′; AS: 5′-gggtctcgctcacagaagtc-3′ | 288 | 40 | 56 |
| GAPDH | S: 5′-gtcttctccaccatggagaaggct-3′; AS: 5′-catgccagtgagcttcccgttca-3′ | 277 | 40 | 50-63 |
| CYCLIN-D1 | S: 5′-gcggaggagaacaaacagat-3′; AS: 5′-tgtgaggcggtaggaca-3′ | 180 | 40 | 56 |
| Gene . | Primers . | Amplified product, bp . | Cycles . | Ta, °C . |
|---|---|---|---|---|
| ACTIN | S: 5′-atctggcaccacaccttctacaatgagctgcg-3′; AS: 5′-cgtcatactcctgcttgctgatccacatctgc-3′ | 838 | 40 | 63 |
| OCT4 | S: 5′-gggaaggtattcagccaaacg-3′; AS: 5′-ggttcgctttctctttcggg-3′ | 180 | 20 | 56 |
| BRACHYURY | S: 5′-cccgcgcactacacacccctcacc-3′; AS: 5′-ccttgggctgcggcgtcgtactg-3′ | 125 | 40 | 63 |
| SCL | S: 5′-atggtgcagctgagtcctcc-3′; AS: 5′-tctcattcttgctgagcttc-3′ | 331 | 40 | 63 |
| RUNX1B | S: 5′-cgtgcacatacattagtagcactacctttg-3′; AS: 5′-cttccacgaatcttgcttgcagaggttaag-3′ | 304 | 40 | 63 |
| RUNX1C | S: 5′-gaagtctgaacccagcatagtggtcagcag-3′; AS: 5′-gtggacgtctctagaaggattcattccaag-3′ | 231 | 40 | 63 |
| GATA-2 | S: 5′-agccggcacctgttgtgcaa-3′; AS: 5′-tgacttctcctgcatgcact-3′ | 244 | 30 | 58 |
| LMO2 | S: 5′-ggatcctgccggagagactatctc-3′; AS: 5′-gaattcagtgaacacctccgcaaa-3′ | 289 | 40 | 63 |
| C-MYB | S: 5′-ctcagacttggaaatgccttc-3′; AS: 5′-ccagtggtgtgagcagaaga-3′ | 406 | 40 | 63 |
| VE-CADHERIN | S: 5′-accggatgaccaagtacagc-3′; AS: 5′-acacactttgggctggtagg-3′ | 596 | 40 | 63 |
| FRIZZLED-3 | S: 5′-ccttggcctgaagatatgg-3′; AS: 5′-acaaggaggtgaacaactacg-3′ | 210 | 40 | 56 |
| FRIZZLED-4 | S: 5′-ccaacatggctgttgaaatg-3′; AS: 5′-tcacccaaccatttcctctc-3′ | 168 | 40 | 56 |
| FRIZZLED-5 | S: 5′-tgctaccagccgtccttcagt-3′; AS: 5′-ccatgccgaagaagtagaccag-3′ | 319 | 40 | 56 |
| WNT3A | S: 5′-ttcttactcctctgcagcctg-3′; AS: 5′-ctggatgccaatcttgatgc-3′ | 207 | 40 | 56 |
| WNT5 | S: 5′-cttcgcccaggttgtaattgaagc-3′; AS: 5′-ctgccaaaaacagaggtgttatcc-3′ | 273 | 40 | 56 |
| WNT10B | S: 5′-aatgcgaatccacaacaac-3′; AS: 5′-gggtctcgctcacagaagtc-3′ | 288 | 40 | 56 |
| GAPDH | S: 5′-gtcttctccaccatggagaaggct-3′; AS: 5′-catgccagtgagcttcccgttca-3′ | 277 | 40 | 50-63 |
| CYCLIN-D1 | S: 5′-gcggaggagaacaaacagat-3′; AS: 5′-tgtgaggcggtaggaca-3′ | 180 | 40 | 56 |
S indicates forward; and AS, reverse.
Statistical analysis
Differences between groups were compared using Student t test. Statistical analysis was performed using Prism 4 (GraphPad Software, San Diego, CA). Results were considered significant at P values of .05 or less.
Results
Kinetics of hESC differentiation on S17 stromal cells
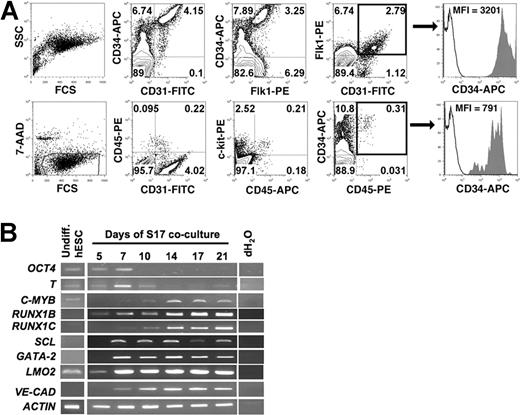
We have previously used detailed flow cytometric analysis for expression of endothelial- and hematopoietic-associated cell surface antigens to demonstrate that 2 waves of CD34+ hESC-derived cells develop after stromal cell coculture.11,28 The first wave consists of CD34+CD31+ cells that peak at approximately day 14 of differentiation. Multicolor analysis by flow cytometry demonstrates that the majority of these cells also express another endothelial cell surface marker, Flk1 (also termed KDR or VEGF-R2) (Figure 1A). Here we more closely evaluate the CD31+ cells that demonstrate a heterogeneous level of CD34 expression. A minority of the CD31+ cells are CD34−, while most CD31+ cells express high levels of CD34 and also express Flk1 (Figure 1A). In fact, all CD31+Flk1+ cells display a homogeneous bright expression of CD34, generating a population of CD34brightCD31+Flk1+ cells. A second wave of CD34+CD45+ cells develop starting at approximately 14 days of differentiation and peak at approximately day 21 of differentiation. The majority of the CD45+ cells also express CD31, as well as c-kit (Figure 1A). We and others have previously demonstrated development of CD34+CD45+ hESC-derived cells corresponds with development of CFCs, as in a standard methylcellulose assay, and sorting for these cells significantly enriches for myeloid and lymphoid progenitor cells.29,30 By examining individual flow cytometric plots, we found that the 2 waves of hESC-derived cells differed substantially in their level of CD34 expression. Compared with the homogenous bright expression found for CD34brightCD31+Flk1+ cells with a mean fluorescence intensity (MFI) of 3201, the CD34+CD45+ cells have uniformly lower CD34 expression with an MFI of 791 (Figure 1A).
Expression of hESC surface antigens and gene transcripts during stromal cell–mediated differentiation. (A) Flow cytometric analysis of hESCs differentiated on S17 stromal cells for 14 days demonstrates distinct populations of CD34bright cells that costain with both CD31 and Flk1, and CD34dim cells that coexpress CD45. Arrow shows histogram of CD34 expression (CD34-APC, shaded) of regions showing CD31+Flk1+ (top panel) and CD45+ (bottom panel) hESC-derived cells after 14 days of differentiation. Isotype control is shown in open histograms. Mean fluorescence intensity (MFI) of CD34 expression is indicated and demonstrates substantially higher CD34 expression in the CD34brightCD31+Flk1+ cell population compared with the CD34dimCD45+ cell population. (B) RT-PCR analysis of hematopoietic and endothelial gene expression during S17-mediated differentiation of hESCs over a time course, ranging from undifferentiated hESCs to day 21 of differentiation.
Expression of hESC surface antigens and gene transcripts during stromal cell–mediated differentiation. (A) Flow cytometric analysis of hESCs differentiated on S17 stromal cells for 14 days demonstrates distinct populations of CD34bright cells that costain with both CD31 and Flk1, and CD34dim cells that coexpress CD45. Arrow shows histogram of CD34 expression (CD34-APC, shaded) of regions showing CD31+Flk1+ (top panel) and CD45+ (bottom panel) hESC-derived cells after 14 days of differentiation. Isotype control is shown in open histograms. Mean fluorescence intensity (MFI) of CD34 expression is indicated and demonstrates substantially higher CD34 expression in the CD34brightCD31+Flk1+ cell population compared with the CD34dimCD45+ cell population. (B) RT-PCR analysis of hematopoietic and endothelial gene expression during S17-mediated differentiation of hESCs over a time course, ranging from undifferentiated hESCs to day 21 of differentiation.
Expression of hematopoietic and endothelial transcription factors in hESCs differentiated on S17 stromal cells parallels the development of the phenotypic cell populations described above (Figure 1B). Specifically, OCT4, a transcription factor essential for the maintenance of hESCs in the undifferentiated state, is down-regulated at early time points during coculture with S17 stromal cells. This is followed by a transient up-regulation of BRACHYURY (T), a mesoderm marker, between days 3 to 7. SCL, RUNX1, and GATA-2, transcription factors important for both hematopoietic and endothelial development, are expressed after 7 days of differentiation, as well as endothelial-associated VE-CADHERIN. c-MYB, a hematopoietic-specific transcript, is expressed at later stages of differentiation and appears at a similar time point as the first CD45+ cells.28 As previously reported, we find distinct patterns of expression of 2 RUNX1 isoforms.14
CD34 expression levels separate 2 morphologically distinct progenitor cell populations
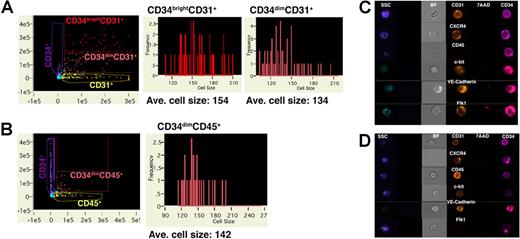
To better define the 2 waves of phenotypic CD34+ cells developing after coculture with S17 stromal cells, we analyzed differentiated hESCs by image scanning flow cytometry.26 This novel instrument allows concurrent analysis for expression of specific cell surface antigens and individual cellular morphology in a high throughput manner. Population analysis using image scanning flow cytometry confirmed analysis by standard flow cytometry, where CD34+CD31+ cells could be divided into both bright and dim CD34-expressing cells (Figure 2A), whereas CD34+CD45+ cells were exclusively CD34dim (Figure 2B). One nontrivial result of these studies is to clearly demonstrate that phenotypic double-positive cell populations are not due to cell doublets or other aggregates. Indeed, since the frequency of CD34+CD45+ cells can be relatively low at early time points during differentiation of hESCs, this finding that is typically assumed, but not always definitively demonstrated by standard flow cytometric analysis, becomes especially important. Image scanning flow cytometry also clearly demonstrates by histograms based on cell size distribution that cells within the CD34brightCD31+ population had a higher mean cell size compared with cells within the CD34dimCD31+ and CD34dimCD45+ cell populations, providing a novel means to depict morphologic differences between the CD34bright and CD34dim hESC-derived cells (Figure 2A,B).
Analysis of differentiated hESCs by image scanning flow cytometry. hESCs allowed to differentiate on S17 stromal cells were analyzed by image scanning flow cytometry to provide concurrent data on cell phenotype and morphology. Cells were stained for (A) CD34 and CD31 and (B) CD34 and CD45 with cell size distribution of cells gated for CD34brightCD31+ and CD34dimCD45+ cells as indicated. Mean signal intensity for each cell population is shown. (C,D) Images of cells gated for (C) CD34bright and (D) CD34dim expression. Brightfield (BF) channel indicates cell morphology, side scatter (SSC) shown in blue indicates granularity, and exclusion of 7-AAD is illustrated by absence of red staining. Pink staining is indicative of CD34 expression, and orange color demonstrates expression of indicated cell-surface antigens.
Analysis of differentiated hESCs by image scanning flow cytometry. hESCs allowed to differentiate on S17 stromal cells were analyzed by image scanning flow cytometry to provide concurrent data on cell phenotype and morphology. Cells were stained for (A) CD34 and CD31 and (B) CD34 and CD45 with cell size distribution of cells gated for CD34brightCD31+ and CD34dimCD45+ cells as indicated. Mean signal intensity for each cell population is shown. (C,D) Images of cells gated for (C) CD34bright and (D) CD34dim expression. Brightfield (BF) channel indicates cell morphology, side scatter (SSC) shown in blue indicates granularity, and exclusion of 7-AAD is illustrated by absence of red staining. Pink staining is indicative of CD34 expression, and orange color demonstrates expression of indicated cell-surface antigens.
Individual cell images generated from image scanning flow cytometry confirmed that the 2 cell populations also are morphologically distinct. Specifically, the CD34bright cells have a large cell size, complex cytoplasm, and irregular cell shape, which is morphologically consistent with endothelial cells (Figure 2C). In contrast, the CD34dim cells display a smaller cell size, less complex cytoplasm, and a homogeneous spherical/round cell shape, corresponding to a phenotype for hematopoietic stem and progenitor cells (Figure 2D).31 Comparison of the 2 cell populations finds Flk1+ and CD45+ cells are exclusively expressed on the CD34bright and CD34dim cells, respectively. However, expression of several other surface antigens, including CD31, c-kit, CXCR4, and VE-cadherin, is conserved between the 2 populations. These phenotypes demonstrate similarities with what is observed for endothelial and hematopoietic cells during mouse development, where VE-cadherin, typically considered an endothelial cell–specific surface antigen, can also be found expressed on early mouse hematoendothelial cells.31 Taken together, this suggests that the CD34brightCD31+Flk1+ cells found in the first wave of cells developing from hESCs after stromal coculture could represent early hematoendothelial cells.
Gene expression analysis of sorted cell populations
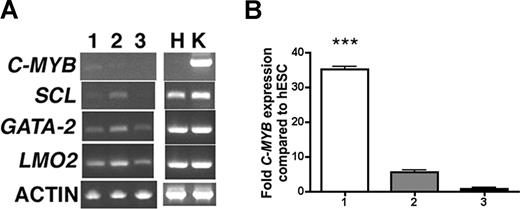
To understand the relationship between the hESC-derived cell populations described above, we analyzed expression of transcripts important for hematopoietic and endothelial differentiation in specific sorted cell populations identified at distinct time points during hESC differentiation. After 10 days of differentiation, no CD45+ cells have yet developed, but there are distinct populations that can be separated based on CD34, CD31, and Flk1 expression, as in Figure 1A. When these cell populations were analyzed, we found CD34brightCD31+Flk1+ cells to express transcripts involved in both hematopoietic and endothelial differentiation (Figure 3A). Most notably, the hematopoietic-specific transcript c-MYB is found within this CD34brightCD31+Flk1+ cell population. Quantitative RT-PCR (Q-RT-PCR) analysis further demonstrates that c-MYB expression is significantly higher in this cell population compared with the CD34+CD31−Flk1+ and CD34−CD31−Flk1− cell populations (Figure 3B).
RT-PCR analysis of sorted hESC-derived cell populations. (A) hESCs differentiated for 10 days were sorted for (lane 1) CD34brightCD31+Flk1+, (lane 2) CD34+CD31−Flk1+, and (lane 3) CD34−CD31−Flk1− cells and analyzed for expression of hematopoietic and endothelial transcripts. Human umbilical vein endothelial cells (H) and K562 cells (K) were used as positive controls. (B) Q-RT-PCR analysis for expression of C-MYB from sorted populations: column 1 = CD34brightCD31+Flk1+, column 2 = CD34+CD31−Flk1+, and column 3 = CD34−CD31−Flk1− cells. All samples were normalized to GAPDH expression and compared with C-MYB expression in undifferentiated hESCs. Error bars indicate plus or minus SEM; ***P < .001.
RT-PCR analysis of sorted hESC-derived cell populations. (A) hESCs differentiated for 10 days were sorted for (lane 1) CD34brightCD31+Flk1+, (lane 2) CD34+CD31−Flk1+, and (lane 3) CD34−CD31−Flk1− cells and analyzed for expression of hematopoietic and endothelial transcripts. Human umbilical vein endothelial cells (H) and K562 cells (K) were used as positive controls. (B) Q-RT-PCR analysis for expression of C-MYB from sorted populations: column 1 = CD34brightCD31+Flk1+, column 2 = CD34+CD31−Flk1+, and column 3 = CD34−CD31−Flk1− cells. All samples were normalized to GAPDH expression and compared with C-MYB expression in undifferentiated hESCs. Error bars indicate plus or minus SEM; ***P < .001.
CD34brightCD31+Flk1+ cells differentiate into both hematopoietic and endothelial cells
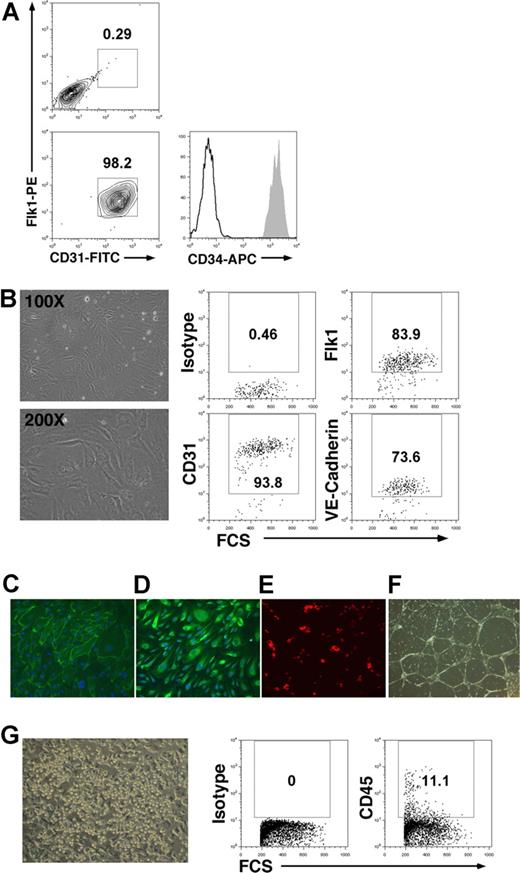
To more conclusively demonstrate the CD34brightCD31+Flk1+ cell population has dual hematoendothelial developmental potential, these cells were sorted and transferred to cultures supporting either endothelial or hematopoietic differentiation. Prior to fluorescence-activated cell sorting (FACS), the differentiated hESCs were first enriched for CD34+ cells by magnetic sorting. Postsort analysis of CD31+Flk1+ sorted cells demonstrates these to be homogenous CD34bright cells (Figure 4A). As expected, CD34brightCD31+Flk1+ cells gave rise to cells with endothelial cell phenotype when cultured in endothelial conditions (Figure 4B). Further analysis of the endothelial cells generated from CD34brightCD31+Flk1+ cells demonstrates that they express CD31 and von Willebrand factor, are able to take up acetylated LDL, and form vascular tube network in matrigel (Figure 4C-F). More importantly, they also generated CD45+ cells and clusters of hematopoietic cells when cultured in hematopoietic-inducing conditions (Figure 4G), as well as forming myeloid colonies in a standard methylcellulose assay (Figure S2). Therefore, this CD34brightCD31+Flk1+ cell population is thus likely very similar to the CD45negPVF and CD34+CD45−CD31+ hESC-derived cells from EB-mediated differentiation, a population with characteristics of hematogenic endothelium observed during mammalian development.13,14 To calculate the frequency of cells with hematopoietic and endothelial potential, sorted cells were plated in limiting dilution. CD34brightCD31+Flk1+ cells displayed effective endothelial differentiation (1:691), with a lower but reproducible frequency of hematpoietic potential (1:3208) (Figure S3). This frequency of hematopoietic cells is similar to what was observed for hESC-derived hemangioblast cells generated by EB differentiation.16 As expected, a higher frequency of hematopoietic potential was observed for CD34dimCD31+Flk1− cells, as these cells contain more committed hematpoietic progenitor cells with little to no endothelial potential (Figures S2,S3).
Bipotent differentiation potential of CD34brightCD31+Flk1+ cells. (A) Flow cytometric analysis of hESCs allowed to differentiate on S17 cells for 14 days and sorted for CD31+Flk1+ cells demonstrates high level of CD34 coexpression. (B) Endothelial differentiation of CD34brightCD31+Flk1+ cells in EGM2 medium supplemented with 10 ng/mL VEGF. Brightfield images (magnification: top panel, 100×; bottom panel, 200×) and flow cytometry analysis of cells cultured for 14 days in endothelial differentiation are shown. (C-F) Endothelial cells derived from CD34brightCD31+Flk1+ cells analyzed for expression of (C) CD31, (D) VWF, (E) dil-acLDL uptake, and (F) tube formation. (G) Hematopoietic differentiation of CD34brightCD31+Flk1+ cells by coculture with AFT024 stromal cells in medium supplemented with 10 ng/mL SCF, FL, and Tpo. Flow cytometry after 14 days of culture in hematopoietic differentiation identifies CD45+ hematopoietic cells. Representative image of clusters of hematopoietic-like cells is shown (magnification 100×). Similar results were observed for 3 independent experiments. Brightfield images were acquired on Olympus CKX41 microscope with 2×/0.05, 10×/0.25 PhP, and 20×/0.40 PhP objectives using Olympus DP2 camera. Fluorescent images were acquired with a Zeiss (Thornwood, NY) model Axiovert 200M microscope with a 20×/0.30 Ph1 objective at room temperature and photographed with Axiovision v4.3 (Zeiss). All images were processed using Adobe Photoshop 7.0 (San Jose, CA).
Bipotent differentiation potential of CD34brightCD31+Flk1+ cells. (A) Flow cytometric analysis of hESCs allowed to differentiate on S17 cells for 14 days and sorted for CD31+Flk1+ cells demonstrates high level of CD34 coexpression. (B) Endothelial differentiation of CD34brightCD31+Flk1+ cells in EGM2 medium supplemented with 10 ng/mL VEGF. Brightfield images (magnification: top panel, 100×; bottom panel, 200×) and flow cytometry analysis of cells cultured for 14 days in endothelial differentiation are shown. (C-F) Endothelial cells derived from CD34brightCD31+Flk1+ cells analyzed for expression of (C) CD31, (D) VWF, (E) dil-acLDL uptake, and (F) tube formation. (G) Hematopoietic differentiation of CD34brightCD31+Flk1+ cells by coculture with AFT024 stromal cells in medium supplemented with 10 ng/mL SCF, FL, and Tpo. Flow cytometry after 14 days of culture in hematopoietic differentiation identifies CD45+ hematopoietic cells. Representative image of clusters of hematopoietic-like cells is shown (magnification 100×). Similar results were observed for 3 independent experiments. Brightfield images were acquired on Olympus CKX41 microscope with 2×/0.05, 10×/0.25 PhP, and 20×/0.40 PhP objectives using Olympus DP2 camera. Fluorescent images were acquired with a Zeiss (Thornwood, NY) model Axiovert 200M microscope with a 20×/0.30 Ph1 objective at room temperature and photographed with Axiovision v4.3 (Zeiss). All images were processed using Adobe Photoshop 7.0 (San Jose, CA).
Effect of Wnt signaling on generation of hematoendothelial cell population from hESCs
Previous reports have shown an effect of many distinct exogenous factors on hESC differentiation by both EB and stromal cell coculture.15,17,18 However, little is know about the role of Wnt signaling on early cell specification into endothelial and hematopoietic lineages. Frizzled receptors and Wnt proteins are expressed in both undifferentiated and differentiated hESCs (Figure S4A).32 To study a possible role of Wnt signaling on hESC differentiation after coculture with M210B4 mouse bone marrow–derived stromal cells, we cultured cells with dickkopf 1 (DKK1), a specific inhibitor of the canonical Wnt signaling pathway. The ability of DKK1 to inhibit canonical Wnt signaling activity was confirmed by TopFlash assay monitoring expression of β-catenin–responsive luciferase reporter gene in 293 cells and undifferentiated hESCs (Figure 5A). After 7 or more days, hESCs differentiated by stromal cell coculture in medium containing DKK1 had a dramatic reduction in CD34+, CD34+CD31+, CD34+Flk1+, and CD31+Flk1+ cells (Figure 5B-E) compared with cells cultured in control medium. This is most evident between day 7 and 14 of differentiation, where 40% to 75% reduction of these cells is observed. We also investigated the effect on more committed hematopoietic progenitors and observed a reduction of CD34+CD45+ cells as well as colony-forming cells (CFCs) when cells were cultured in DKK1-containing medium (Figure 5F,G). To confirm that the effect observed was due to DKK1 and not a nonspecific effect of the conditioned medium, purified DKK1 was titrated to the concentration of the conditioned medium and analyzed for effect on hESC differentiation (Figure S4B,C). Use of the purified DKK1 also resulted in a dramatic reduction in CD34+, CD34+CD31+, CD34+Flk1+, and CD31+Flk1+ cells (Figure S4C). This analysis demonstrates the specificity of this ability to inhibit development of these phenotypic cell populations with hematopoietic and endothelial potential is due to the DKK1 and not a nonspecific effect of the conditioned medium. These results suggest that Wnt proteins (either produced by hESCs themselves or by mouse bone marrow–derived stromal cells) and active canonical Wnt signaling are critical to support differentiation of hESCs and to generate cells with these hematogenic endothelium phenotypes.
Inhibition of canonical Wnt signaling reduces hematogenic endothelium cell population. (A) Evaluation of control- and DKK1-conditioned medium in 293 cells (left) and hESCs (right) transfected with a β-catenin–responsive reporter system as a measure of Wnt activity. (B-E) hESCs were differentiated by stromal cell coculture in control medium (○) or medium containing dickkopf1 (DKK1) (■). Results from cocultures with S17 and M210B4 stromal cells were combined for the analysis (n = 3 experiments). Cells were analyzed by flow cytometry for presence of (B) CD34+, (C) CD34+CD31+, (D) CD34+Flk1+, and (E) CD31+Flk1+ cells over a time course of differentiation. Error bars indicate plus or minus SEM of 3 independent experiments. (F) Effect of DKK1 on formation of more committed hematopoietic progenitors was evaluated by flow cytometry for presence of CD34+CD45+ cells (n = 1 experiment). (G) Development of CFCs was evaluated for hESC-derived cells cultured with M210 stromal cells in control medium ( ) or DKK1-conditioned medium (
) or DKK1-conditioned medium ( ) for the indicated number of days (n = 1 experiment). ***P < .001; **P < .01; *P < .05.
) for the indicated number of days (n = 1 experiment). ***P < .001; **P < .01; *P < .05.
Inhibition of canonical Wnt signaling reduces hematogenic endothelium cell population. (A) Evaluation of control- and DKK1-conditioned medium in 293 cells (left) and hESCs (right) transfected with a β-catenin–responsive reporter system as a measure of Wnt activity. (B-E) hESCs were differentiated by stromal cell coculture in control medium (○) or medium containing dickkopf1 (DKK1) (■). Results from cocultures with S17 and M210B4 stromal cells were combined for the analysis (n = 3 experiments). Cells were analyzed by flow cytometry for presence of (B) CD34+, (C) CD34+CD31+, (D) CD34+Flk1+, and (E) CD31+Flk1+ cells over a time course of differentiation. Error bars indicate plus or minus SEM of 3 independent experiments. (F) Effect of DKK1 on formation of more committed hematopoietic progenitors was evaluated by flow cytometry for presence of CD34+CD45+ cells (n = 1 experiment). (G) Development of CFCs was evaluated for hESC-derived cells cultured with M210 stromal cells in control medium ( ) or DKK1-conditioned medium (
) or DKK1-conditioned medium ( ) for the indicated number of days (n = 1 experiment). ***P < .001; **P < .01; *P < .05.
) for the indicated number of days (n = 1 experiment). ***P < .001; **P < .01; *P < .05.
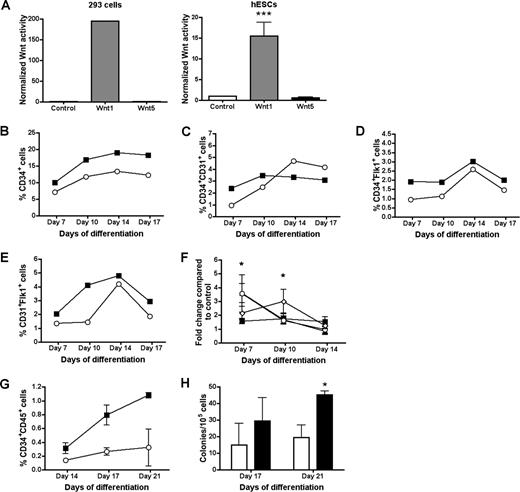
Next, we investigated whether overexpression of Wnt proteins would enhance hESC differentiation. Here, hESCs were cocultured with either S17 or M210 stromal cells that overexpress Wnt1 plus GFP, or GFP alone as a negative control. Expression of Wnt1 protein was confirmed by immunostaining for Wnt1 (Figure S5). The effect of the Wnt1 overexpression was validated by TopFlash reporter assay for β-catenin activation (Figure 6A). To demonstrate that undifferentiated hESCs are responsive to canonical Wnt signaling, hESCs transiently transfected with TopFlash reporter construct were cocultured with GFP only–, Wnt1-, or Wnt5-expressing stromal cells. When assayed for activation of β-catenin–responsive reporter gene, hESCs cocultured with Wnt1 stromal cells had a markedly increased activation of this canonical Wnt signaling reporter compared with coculture with control GFP– or Wnt5-expressing stromal cells (Figure 6A). The effect of Wnt1 was further evaluated over a time course of differentiation. Coculture with Wnt1-expressing stromal cells resulted in an increased percentage of CD34+, CD34+CD31+, CD34+Flk1+, and CD31+Flk1+ cells at early time points between day 7 and day 10 of differentiation (Figure 6B-F). Comparing these populations to hESCs cocultured with GFP control stromal cells, we found a significant increase in CD34+CD31+ cells at day 7 of differentiation and CD34+Flk1+ cells at day 10 of differentiation (P < .05), but not at later time points (days 14 and 17) (Figure 6F). Coculture with Wnt1-expressing stromal cells also resulted in an increased percentage of CD34+CD45+ cells as well as higher frequency of CFCs (Figure 6G,H). No change in hESC differentiation kinetics was observed when hESCs were cocultured with stromal cells overexpressing Wnt5 (data not shown). Similar results were seen using Wnt1-expressing S17 or M210 cells, demonstrating this effect is not limited to one specific stromal cell type. To confirm effect of modulated Wnt signaling on downstream target genes during differentiation of hESCs, expression of CYCLIN-D1 was monitored by Q-RT-PCR (Figure S6).33 This study demonstrates effective up-regulation (by Wnt1) and down-regulation (by DKK1) of CYCLIN-D1 expression during hESC differentiation. Taken together, these results indicate that activation of canonical Wnt signaling via Wnt1, but not noncanonical Wnt activation (Wnt5), results in accelerated differentiation of hESCs into hematoendothelial cells.
Activation of Wnt signaling results in accelerated differentiation of hESCs. (A) Evaluation of β-catenin activity in 293 cells (left) and undifferentiated hESCs (right) transfected with TopFlash plasmid and cultured on stromal cells expressing either GFP (control), Wnt1, or Wnt5. (B-E) hESCs were differentiated by coculture with stromal cells expressing GFP only as a control (○) or Wnt1 (■) over a defined time course. Cells were analyzed by flow cytometry for CD34+, CD34+CD31+, CD34+Flk1+, or CD31+Flk1+ cells, as indicated. Results from cocultures with S17 and M210B4 stromal cells were combined for the analysis (n = 6 experiments, except for CD31+Flk1+ cells where n = 3). P > .05 for all cell populations. (F) Change in CD34+ (■), CD34+CD31+ (▴), CD34+Flk1+ (○), and CD31+Flk1+ (◇) cell populations of hESCs cocultured with Wnt1-expressing cells compared with GFP control cells. Error bars indicate SEM. A significant increase in CD34+CD31+ cells at day 7 of differentiation and CD34+Flk1+ at day 10 of differentiation was found for Wnt1 compared with control stromal cells (P < .05). (G) Effect of Wnt1 on generation of CD34+CD45+ cells was analyzed by flow cytometry. Error bars indicate SEM of 2 independent experiments. (H) CFCs derived at the indicated days of differentiation with control stromal cells ( ) or Wnt1-expressing stromal cells (
) or Wnt1-expressing stromal cells ( ). *P < .05.
). *P < .05.
Activation of Wnt signaling results in accelerated differentiation of hESCs. (A) Evaluation of β-catenin activity in 293 cells (left) and undifferentiated hESCs (right) transfected with TopFlash plasmid and cultured on stromal cells expressing either GFP (control), Wnt1, or Wnt5. (B-E) hESCs were differentiated by coculture with stromal cells expressing GFP only as a control (○) or Wnt1 (■) over a defined time course. Cells were analyzed by flow cytometry for CD34+, CD34+CD31+, CD34+Flk1+, or CD31+Flk1+ cells, as indicated. Results from cocultures with S17 and M210B4 stromal cells were combined for the analysis (n = 6 experiments, except for CD31+Flk1+ cells where n = 3). P > .05 for all cell populations. (F) Change in CD34+ (■), CD34+CD31+ (▴), CD34+Flk1+ (○), and CD31+Flk1+ (◇) cell populations of hESCs cocultured with Wnt1-expressing cells compared with GFP control cells. Error bars indicate SEM. A significant increase in CD34+CD31+ cells at day 7 of differentiation and CD34+Flk1+ at day 10 of differentiation was found for Wnt1 compared with control stromal cells (P < .05). (G) Effect of Wnt1 on generation of CD34+CD45+ cells was analyzed by flow cytometry. Error bars indicate SEM of 2 independent experiments. (H) CFCs derived at the indicated days of differentiation with control stromal cells ( ) or Wnt1-expressing stromal cells (
) or Wnt1-expressing stromal cells ( ). *P < .05.
). *P < .05.
Discussion
There is a striking conserved pattern of differentiation to hematopoietic and endothelial cell lineages derived from hESCs using either the EB or stromal cell coculture systems.11,13,–15,25 Similar to studies using EB-mediated differentiation of hESCs, we find development of hematogenic endothelial cells between days 10 and 14 of coculture with murine bone marrow–derived stromal cells.13,14 Phenotypic characterization demonstrates CD34brightCD31+Flk1+ with endothelial morphology and promiscuous expression of both hematopoietic and endothelial transcription factors. These cells can generate distinct hematopoietic and endothelial cell populations after culture in conditions defined for each cell lineage. Two recent studies have identified CD45negPVF cells and CD34+CD31+CD45− cells derived from EB differentiation with similar bipotent differentiation potential. Therefore, the hematogenic endothelial cell population described by our studies is likely either identical or nearly identical to these phenotypic cell populations derived by EB-mediated differentiation.13,14 However, the relationship of these cells to BL-CFCs characterized from mouse ESCs and recently hESCs is not known.5,15,16 By image scanning flow cytometry, we can further distinguish these CD34brightCD31+Flk1+ hematogenic endothelial cells from CD34dimCD45+ hESC-derived cells restricted to the hematopoietic lineage, and show that they are morphologically distinct. Although not done routinely, we also show that VE-cadherin is expressed on both CD34bright and CD34dim cells (Figure 2C,D). As VE-cadherin is a trypsin/Ca2+-sensitive cell surface antigen and requires more laborious digestion protocols for identification by standard flow cytometry, the phenotypic hematogenic endothelial cells described here can be isolated more efficiently and reliably.
The role of Wnt signaling for development of the earliest human endothelial and hematopoietic progenitors is poorly characterized. Here we identify a crucial role of active canonical Wnt signaling for generation of hematogenic endothelial cell population from hESCs as demonstrated by a dramatic decrease in these cells after coculture in medium supplemented with DKK1. DKK1 acts by blocking the ability of canonical Wnt ligands to bind to the coreceptor Lrp5/6, which is necessary for activation of the canonical Wnt signaling pathway. Specifically, generation of CD34+, CD34+CD31+, CD34+Flk1+, and CD31+Flk1+ cells was reduced between 30% to 80% over a time course of differentiation (Figure 5). Conversely, the generation of this cell population was accelerated when hESCs were cocultured with stromal cells overexpressing Wnt1, a Wnt ligand for the canonical Wnt signaling pathway (Figure 6).
Several studies have recently described a role for canonical Wnt signaling during mouse ESC differentiation. Analysis of gene expression initially suggested a role of Wnt signaling for development of both endothelial and hematopoietic progenitors.34,35 Several components of Wnt signaling were found differentially expressed in Flk1+ cells, a phenotype typically used to identify mESC-derived hemangioblast cells.35 Activation of Wnt signaling resulted in an increase in these cells, whereas suppression of Wnt signaling using secreted frizzled-like protein 1 (SFRP1) resulted in diminished endothelial differentiation. Similar studies identified a regulated expression of Wnt3 during mESC differentiation that correlated with hematopoietic differentiation potential.34 Wnt3 overexpression resulted in increased hematopoietic differentiation and correlated with up-regulation of Brachyury, suggesting Wnt3 enhanced mesoderm commitment during mEB differentiation resulting in an increased hematopoietic differentiation. When mESCs are differentiated in presence of DKK1, they fail to generate Flk1+ cells, and gene expression analysis suggested a lack of germ layer induction that normally occurs in vivo.36 In addition, a recent study found Wnt signaling promotes cardiac differentiation (also a mesodermal lineage) from mouse ESCs at early stages of differentiation.37 These studies clearly demonstrate the importance of Wnt signaling during mouse development, and taken together with our findings demonstrate a conserved role for canonical Wnt signaling in promoting mesoderm formation and generation of hematoendothelial progenitors.
It is likely that the environmental regulation of Wnt signaling plays an important role in determining the effect on target cells. Wnt signaling has been found to enhance maintenance of mESCs and hESCs in the undifferentiated state.38,–40 However, maintenance of undifferentiated hESCs also requires presence of bFGF and feeder cell–derived factors, as Wnt activation in the absence of these factors resulted in enhanced differentiation and proliferation of hESCs.40,41 Undifferentiated and differentiated hESCs are responsive to activation and inhibition as indicated by attenuation of downstream targets by TopFlash assay for stabilization of cytoplasmic β-catenin (Figures 5A,6A) and Q-RT-PCR for CYCLIN-D1 (Figure S6).33 Our results demonstrate an accelerated differentiation and more efficient generation of hematoendothelial cells when hESCs were cocultured with Wnt1-overexpressing cells. Kinetic analysis has shown that these cells are transient cell populations not maintained in the coculture as they plateau between day 10 and day 14 of differentiation (Figures 5,6) for control cells. The accelerated differentiation facilitated by Wnt1 thus also induces an earlier subsequent decline of these cell populations at later time points. Wnt1 has also previously been shown to antagonize neural differentiation of mESCs.42 BMP4, a factor important for generation of hematopoietic and endothelial cells during EB differentiation of hESCs in serum-free conditions,18 acts cooperatively with Wnt signaling to up-regulate expression of Brachyury in mouse EBs.36 BMP4 is not required for generating hematopoietic progenitors when hESCs are cocultured with bone marrow–derived stromal cells in serum-free conditions, suggesting that coculture provides BMP4.17 This could explain why DKK1 does not completely abolish generation of hematoendothelial cells in our coculture model, as was observed with mESCs during EB formation in DKK1-containing medium.36 The requirement for intricate regulation of Wnt activity is clearly shown in the hematopoietic system. Engraftment and multilineage differentiation of transplanted wild-type hematopoietic stem cells (HSCs) can be improved after in vivo injection of Wnt5a and glycogen synthase kinase-3 (GSK-3) inhibitor, both resulting in activating of Wnt signaling pathway.43,44 However, constitutive activation of β-catenin in HSCs blocks their differentiation potential and abolishes HSC activity in vivo.45,46
Wnt proteins are secreted proteins that carry a lipid modification essential for their function.20 Our studies used a coculture system with stromal cells engineered to express Wnt proteins to provide stable and active Wnt activity without requiring addition of soluble Wnts to the media. As Wnt proteins likely act in an autocrine and paracrine manner, the close contact with stromal cells in this culture system is likely beneficial for more effective and consistent Wnt signaling. Several stromal cells have been reported to support development of hematopoietic and endothelial progenitors from mESCs and hESCs. S17 and M210-B4 are murine bone marrow–derived stromal cells that have been shown by our laboratory to support similar efficient and reproducible generation of hematopoietic and endothelial cells in FBS-only medium.11,25 Others have shown similar results using coculture with murine OP9 stromal cells, human fetal liver cells, and human bone marrow stroma.12,47,48 Understanding the role of secreted and cell-bound factors associated with these stromal cells and their role in promoting differentiation of hESCs will be important to further advance lineage-specific differentiation from hESCS in defined conditions. The studies described here demonstrate the importance of Wnt signaling to generate hematoendothelial progenitors from hESCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grant R01 HL77923 (D.S.K.) and a University of Minnesota Grant-in-Aid Award (D.S.K.). R.T.M. acknowledges support as an Investigator of the HHMI.
We gratefully acknowledge Greg Veltri and the University of Minnesota Flow Core for assistance with ImageStream analysis, Rizwan Romme for assistance with TopFlash assays, R&D Systems for providing reagents, and Paul Marker for FACSAria sorting.
National Institutes of Health
Authorship
Contribution: P.S.W. designed and performed experiments and wrote the paper; J.K.M., M.S.P., and R.K.M. performed experiments; A.D.K., T.L.B., and R.T.M. provided essential reagents and assisted with experimental design; D.S.K. designed experiments, and wrote and edited the paper. All authors read and approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dan S. Kaufman, Stem Cell Institute and Department of Medicine, University of Minnesota, Translational Research Facility, 2001 6th St SE, Minneapolis, MN 55455; e-mail: kaufm020@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal