Hematopoiesis is a highly regulated process resulting in the formation of all blood lineages. Aberrant regulation of phosphatidylinositol-3-kinase (PI3K) signaling has been observed in hematopoietic malignancies, suggesting that regulated PI3K signaling is critical for regulation of blood cell production. An ex vivo differentiation system was used to investigate the role of PI3K and its downstream effector, protein kinase B (PKB/c-akt) in myelopoiesis. PI3K activity was essential for hematopoietic progenitor survival. High PKB activity was found to promote neutrophil and monocyte development, while, conversely, reduction of PKB activity was required to induce optimal eosinophil differentiation. In addition, transplantation of β2-microglobulin (−/−) NOD/SCID mice with CD34+ cells ectopically expressing constitutively active PKB resulted in enhanced neutrophil and monocyte development, whereas ectopic expression of dominant-negative PKB induced eosinophil development in vivo. Inhibitory phosphorylation of C/EBPα on Thr222/226 was abrogated upon PKB activation in hematopoietic progenitors. Ectopic expression of a nonphosphorylatable C/EBPα mutant inhibited eosinophil differentiation ex vivo, whereas neutrophil development was induced, demonstrating the importance of PKB-mediated C/EBPα phosphorylation in regulation of granulopoiesis. These results identify an important novel role for PKB in regulation of cell fate choices during hematopoietic lineage commitment.
Introduction
The development of blood cells is a complex series of events regulated by cytokines at multiple levels, including proliferation, survival, and differentiation. Hematopoietic stem cells (HSCs) are defined as cells that can both self-renew and differentiate toward all blood lineages. The HSC pool can be divided into 2 different subpopulations based on their functionality: long-term reconstituting HSCs, and short-term reconstituting HSCs (ST-HSC), which can subsequently differentiate to multipotent progenitors that are capable of differentiating toward a subset of hematopoietic lineages.1 These multipotent progenitors include both the common lymphoid progenitor2 and the common myeloid progenitor.3 The common lymphoid progenitor gives rise to B and T lymphocytes as well as NK cells, whereas the common myeloid progenitor can differentiate toward the erythroid, megakaryocytic, monocytic, and granulocytic lineages. Although the role of transcription factors in regulation of hematopoiesis has been extensively investigated, the cytokine-mediated intracellular signal transduction pathways regulating transcription factor activity remain to be determined
Phosphatidylinositol 3-kinase (PI3K) has been demonstrated to play a critical role in the survival and proliferation of a plethora of cell types, and recent evidence suggests that PI3K may also play a role in regulating hematopoiesis.4 Furthermore, PI3K is aberrantly regulated in hematopoietic malignancies, including both acute and chronic myeloid leukemia, and myelodysplastic syndromes.5,6 Deletion of discrete P13K isoforms has revealed that p110δ, p110γ, and p85α play an important role in regulation of B and T lymphocyte development.7,–9 Furthermore, regulation of PI3K activity has been demonstrated to be required for erythropoietin (EPO)–induced erythropoiesis from CD34+ hematopoietic progenitors as well as for EPO-mediated survival of erythroid precursors.10 These studies have not, however, addressed the critical question as to whether the reduction in mature cells is caused by inducing apoptosis in the progenitor cells rather than blocking the intrinsic differentiation program. This has implications for potential clinical therapies using pharmacological modulators of this signaling pathway.
Protein kinase B (PKB/c-akt), a serine/threonine kinase, is an important effector of PI3K signaling.11,12 To date, 3 highly homologous PKB isoforms have been described to be expressed in mammalian cells: PKBα, PKBβ, and PKBγ, the PKBα isoform being ubiquitously expressed. Similar to PI3K, PKB has been demonstrated to play an important role in regulation of cell survival and proliferation in a variety of systems.4 Analysis of PKB-deficient mice has revealed that PKB plays an important role in multiple developmental processes, including organismal growth13,,–16 and thymus, skin, cardiovascular, and nervous system development.17 However, studies have not investigated whether regulation of hematopoiesis also is perturbed.
We have investigated the role of PI3K/PKB in regulation of myelopoiesis using an ex vivo differentiation system as well as an in vivo mouse transplantation model. PI3K activity was found to be essential for hematopoietic progenitor survival, while modulation of PKB activity regulated lineage development. This was found to involve regulation of C/EBPα phosphorylation. These results indicate that PKB plays an important role in regulation of lineage choice decisions during myelopoiesis, at least in part by regulation of C/EBPα phosphorylation.
Methods
Isolation and culture of human CD34+ cells
Mononuclear cells were isolated from umbilical cord blood by density centrifugation over a Ficoll-Paque solution (density 1.077 g/mL). MACS immunomagnetic cell separation (Miltenyi Biotech, Auburn, CA) using a hapten conjugated antibody against CD34, which was coupled to beads, was used to isolate CD34+ cells. CD34+ cells were cultured in Iscove modified Dulbecco medium (Gibco, Paisley, United Kingdom) supplemented with 9% fetal calf serum (FCS), 50 μM β-mercaptoethanol, 10 U/mL penicillin, 10 μg/mL streptomycin, and 2 mM glutamine at a density of 0.3 × 106 cells/mL. Cells were differentiated toward eosinophils upon addition of stem cell factor (SCF) (50 ng/mL), FLT-3 ligand (50 ng/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF) (0.1 nmol/L), interleukin 3 (IL-3) (0.1 nmol/L), and IL-5 (0.1 nmol/L). Every 3 days, cells were counted and fresh medium was added to a density of 0.5 × 106 cells/mL. After 3 days of differentiation, only IL-3 and IL-5 were added to the cells. Neutrophil differentiation was induced upon addition of SCF (50 ng/mL), FLT-3 (50 ng/mL) ligand, GM-CSF (0.1 nmol/L), IL-3 (0.1 nmol/L), and G-CSF (30 ng/mL). After 6 days of culture, only G-CSF was added to the cells. CD34+ cells used in transplantation studies were cultured for 3 days in IMDM containing the cytokines SCF (50 ng/mL), FLT-3 ligand (50 ng/mL), and thrombopoietin (TPO) (10 ng/mL). Cells were cultured either in absence or presence of pharmacolocigal inhibitors including 10 μM LY294002 (Biomol, Plymouth Meeting, PA) to inhibit PI3K, and 20 μM 1L-6-Hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (HIMO) (Calbiochem, San Diego, CA) to inhibit PKB. 10 μM SB216763 (Calbiochem) was used to inhibit GSK-3 activity.
Viral transduction of CD34+ cells
A bicistronic retroviral DNA construct was used, expressing either myrPKB or PKBcaax (kindly provided by Dr H. Spits, Amsterdam, the Netherlands), and an internal ribosomal entry site (IRES) followed by the gene encoding for eGFP (LZRS-eGFP). LZRS-eGFP retrovirus was produced by transient transfection of the retroviral packaging cell line, Phoenix-ampho, by calcium phosphate co-precipitation. Cells were plated in 6-cm dishes, 24 hours before transfection. A total of 10 μg of DNA was used per transfection. Medium was refreshed 16 hours after transfection. After an additional 24 hours, viral supernatants were collected and filtered through a 0.2-μm filter. CD34+ cells were transduced in 24-well dishes precoated with 10 μg/cm2 recombinant human fibronectin fragment CH-296 (RetroNectin; Takara, Otsu, Japan) for 2 hours, and 2% bovine serum albumin (BSA) for 30 minutes. Transduction was performed by addition of 0.5 mL viral supernatant to 0.5 mL medium containing 0.5 × 106 cells. Twenty-four hours after transduction, 0.7 mL medium was removed from the cells, and 0.5 fresh virus supernatant was added together with 0.5 mL fresh medium.
Histochemical staining of hematopoietic cells
May-Grunwald Giemsa staining was used to analyze myeloid differentiation. Cytospins were prepared from 5 × 104 differentiating granulocytes and were fixed in methanol for 3 minutes. After fixation cytospins were stained in a 50% eosin methylene blue solution according to May-Grunwald (Sigma-Aldrich, Seelze, Germany) for 20 minutes, rinsed in water for 5 seconds, and the nuclei were counterstained with 10% Giemsa solution (Merck kGaA, Darmstadt, Germany) for 15 minutes. During eosinophil differentiation, cells could be characterized as differentiating from blast cells toward promyelocyte type I, promyelocyte type II, myelocyte, metamyelocyte, and finally mature eosinophils with segmented nuclei. These stages can be distinguished by the size of the cells, ratio of cytoplasm versus nucleus, presence of azurophilic granules, appearance of eosinophilic granules, and the shape of the nuclei. “Differentiated eosinophils” were characterized as cells belonging to the stages of myelocyte, metamyelocyte, and mature eosinophils. Neutrophil differentiation also can be characterized by distinct stages from myeloblast, promyelocyte I, promyelocyte II, myelocyte, and metamyelocytes toward neutrophils with banded or segmented nuclei. Differentiated neutrophils were characterized as cells containing either banded or segmented nuclei. Micrographs were acquired by staining with May-Grunwald Giemsa solution, with an Axiostar plus microscope (Carl Zeiss, Sliedrecht, the Netherlands) fitted with a 100×/1.3 NA EC Plan Neofluor oil objective using Immersol 518F oil (Carl Zeiss), a Canon Powershot G5 camera (Canon Nederland, Hoofddorp, the Netherlands), and Canon Zoombrowser EX image acquisition software. Photoshop CS2 was used for image processing (Adobe Systems Benelux, Amsterdam, the Netherlands).
The percentage of eosinophil differentiation was also determined by Luxol Fast Blue (Avocado Res Chem, Heysham, United Kingdom), a dye specifically staining eosinophil granules. Cytospins were prepared from cells. Slides were fixed in dry-acetone for 10 minutes. Slides were stained with 0.15% w/v Luxol fast blue in urea-saturated ethanol for 2 hours.
Immunohistochemical staining of hematopoietic cells
Neutrophil differentiation also was analyzed by intracellular staining of lactoferrin. Cells were first washed in phosphate-buffered saline (PBS) and resuspended in 100 μL 0.5% formaldehyde. After 15 minutes' incubation at 37°C, 900 μL of ice-cold methanol was added to the cells. Cells were washed with PBS after 30 minutes of incubation on ice and resuspended in 0.5% BSA. After 10 minutes of incubation at room temperature, cells were washed, and neutrophil progenitors were resuspended in phycoerythrin (PE)–conjugated antilactoferrin (Immunotech, Marseille, France) and incubated for another 25 minutes. Cells were again washed, and lactoferrin-positive cells were detected by FACS analysis (FACS Vantage; Becton Dickinson, Alphen a/d Rijn, the Netherlands).
B-cell development was determined using a CD19-PE antibody. A CD14-PE antibody was used to determine monocyte development, and a CD49d-PE antibody was used to determine both eosinophil and monocyte development (all from Becton Dickinson).
Western blot analysis
Western blot analysis was performed using standard techniques. In brief, differentiating granulocytes were lysed in Laemmli buffer (0.12 M Tris HCl pH 6.8, 4% SDS, 20% glycerol, 0.05 μg/μL bromophenol blue, and 35 mM β-mercaptoethanol) and boiled for 5 minutes. Equal amounts of total lysate were analyzed by 10% SDS-polyacrylamide gel electrophoresis. Proteins were transferred to Immobilon-P and incubated with blocking buffer (Tris buffered saline/Tween20) containing 5% low-fat milk for 16 hours at 4°C before incubating with antibodies against phosphorylated PKB (Cell Signaling Technology, Danvers, MA), C/EBPα (Santa Cruz Biotechnology, Santa Cruz, CA), and an antibody against β-actin (Santa Cruz) overnight in the same buffer. Before incubation with antibodies against phosphorylated C/EBPα (Cell Signaling Technology) for 16 hours, blots were incubated for 1 hour at 4°C in blocking buffer containing 5% BSA. Subsequently, blots were incubated with peroxidase conjugated secondary antibodies for 1 hour. Enhanced chemical luminescence (ECL) was used as a detection method according to the manufacturers' protocol (Amersham Pharmacia, Amersham, United Kingdom).
Transplantation of human CD34+ cells into β2-microglobulin (−/−) NOD/SCID mice
The β2-microglulin (−/−) nonobese diabetic/severe combined immune deficient (NOD/SCID) mice were bred and maintained under sterile conditions in microisolator cages and provided with autoclaved food and acidified water containing 111 mg/L ciprofloxacin (Ciproxin). Eight- to 10-week-old mice, sublethally irradiated with 350 cGy administered from a 137Cs source, received transplants via tail vein injections with approximately 500 000 unsorted retrovirally transduced cord blood derived hematopoietic progenitors along with 106 irradiated (1500 cGy) CD34-depleted cord blood–derived accessory cells. After 6 weeks, the mice were killed, and both tibiae and femora were flushed. EGFP-positive bone marrow cells were sorted on a FACS Vantage and cytospins prepared. May-Grunwald Giemsa staining was used to analyze mature eosinophils, neutrophils, monocytes, and erythrocytes.
Statistics
A Levene test for equality of variances was performed in all experiments. Subsequently, an independent sample T test was performed to compare the differences in proliferation, differentiation, and annexin-positive cells between the controls and cells transduced with eGFP, myrPKB, or PKBcaax. The same assay was performed to compare cells cultured either in absence or presence of pharmacological inhibitors. A P value of .05 or less was considered significant.
Results
Regulation of myelopoiesis by protein kinase B
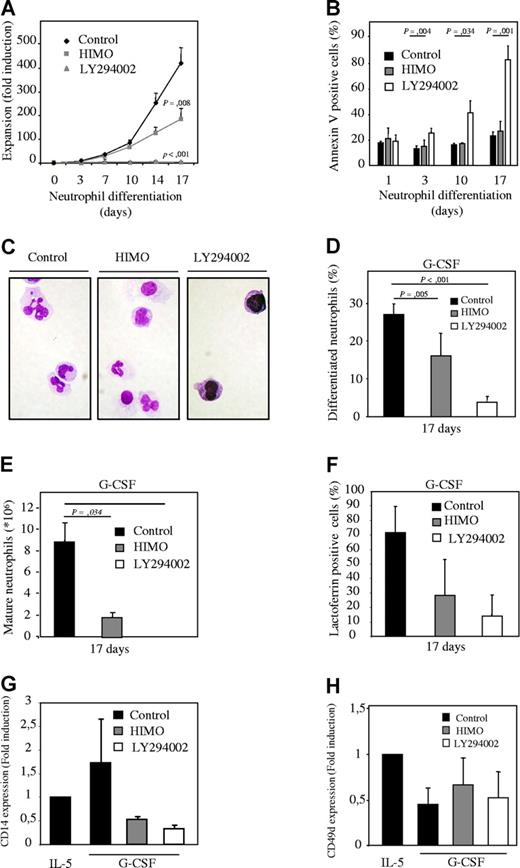
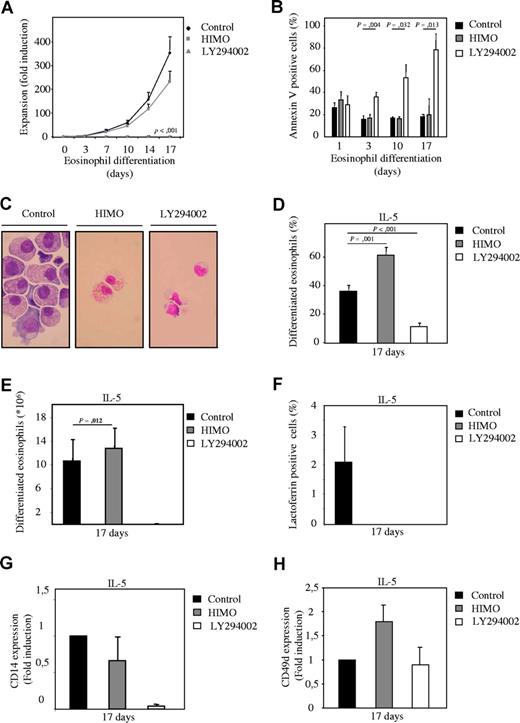
To determine whether PI3K signaling is involved in regulation of myelopoiesis, an ex vivo differentiation system was used. Human CD34+ hematopoietic progenitor cells, isolated from umbilical cord blood, were cultured in the presence of either G-CSF or IL-3 and IL-5 to induce neutrophil and eosinophil differentiation, respectively.21,22 Cells were cultured either in absence or presence of pharmacological inhibitors of the PI3K signaling module, and differences in expansion, survival, and differentiation were analyzed. The PI3K inhibitor LY294002 (Figure S3A, available on the Blood website; see the Supplemental Materials link at the top of the online article) completely abrogated expansion during both neutrophil and eosinophil differentiation (Figures 1A, 2A) and dramatically increased the percentage of apoptotic cells after extended culture (Figures 1B, 2B). To determine whether PI3K plays a critical role in granulocyte differentiation per se, cytospins were prepared from the low numbers of surviving cells after 14 and 17 days of differentiation, and the morphology of the differentiating granulocytes was analyzed after May-Grunwald Giemsa staining (Figures 1C,D, 2C,D). Inhibition of PI3K dramatically inhibited maturation of both lineages. Thus, PI3K activity is critical not only for survival but also for normal development of hematopoietic progenitors during granulopoiesis.
Inhibition of PKB with a pharmacological inhibitor inhibits neutrophil differentiation. (A) CD34+ cells were cultured in presence of G-CSF to induce neutrophil differentiation during 17 days. Cells were cultured either in absence or presence of 10 μM LY294002 or 20 μM 1L-6-Hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (HIMO), a PKB inhibitor. Expansion was determined by counting the trypane blue–negative cell population. (B) During the 17-day culture period the percentage of apoptotic cells was determined by annexin V staining. (C) After 17 days of neutrophil differentiation, cytospins were made and stained with May-Grunwald Giemsa solution. (D) Data were expressed as both the percentage of differentiated neutrophils (E) and as absolute numbers. (F) Lactoferrin, (G) CD14, and (H) CD49d expression was analyzed by FACS to determine neutrophil, monocyte, and eosinophil/monocyte development, respectively. Error bars represent SEM.
Inhibition of PKB with a pharmacological inhibitor inhibits neutrophil differentiation. (A) CD34+ cells were cultured in presence of G-CSF to induce neutrophil differentiation during 17 days. Cells were cultured either in absence or presence of 10 μM LY294002 or 20 μM 1L-6-Hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (HIMO), a PKB inhibitor. Expansion was determined by counting the trypane blue–negative cell population. (B) During the 17-day culture period the percentage of apoptotic cells was determined by annexin V staining. (C) After 17 days of neutrophil differentiation, cytospins were made and stained with May-Grunwald Giemsa solution. (D) Data were expressed as both the percentage of differentiated neutrophils (E) and as absolute numbers. (F) Lactoferrin, (G) CD14, and (H) CD49d expression was analyzed by FACS to determine neutrophil, monocyte, and eosinophil/monocyte development, respectively. Error bars represent SEM.
To investigate whether PKB, a major downstream PI3K effector, could mediate the effect of PI3K on myelopoiesis, a pharmacological inhibitor (HIMO) was used. Similar to PI3K, inhibition of PKB with HIMO reduced expansion during both neutrophil and eosinophil differentiation (Figures 1A, 2A). However, inhibition of PKB did not have a detrimental effect on progenitor survival (Figures 1B, 2B). Terminal differentiation of neutrophils was inhibited in cells cultured in presence of the PKB inhibitor (Figure 1C-E). In contrast, however, inhibition of PKB dramatically enhanced eosinophil differentiation, and terminally differentiated eosinophils could be observed in these cultures (Figure 2C-E).
Inhibition of PKB with a pharmacological inhibitor induces eosinophil differentiation. (A) CD34+ cells were cultured in presence of IL-3 and IL-5 to induce eosinophil differentiation during 17 days. Cells were cultured either in absence or presence of 10 μM LY294002 or 20 μM 1L-6-Hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (HIMO), a PKB inhibitor. Expansion was determined by counting the trypane blue–negative cell population. (B) During the 17-day culture period the percentage of apoptotic cells was determined by annexin V staining. (C) After 17 days of eosinophil differentiation, cytospins were made and stained with May-Grunwald Giemsa solution. Data were expressed as both the percentage of differentiated eosinophils (D) and as absolute numbers (E). (F) Lactoferrin, (G) CD14, and (H) CD49d expression was analyzed by FACS to determine neutrophil, monocyte, and eosinophil/monocyte development, respectively. Error bars represent SEM.
Inhibition of PKB with a pharmacological inhibitor induces eosinophil differentiation. (A) CD34+ cells were cultured in presence of IL-3 and IL-5 to induce eosinophil differentiation during 17 days. Cells were cultured either in absence or presence of 10 μM LY294002 or 20 μM 1L-6-Hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (HIMO), a PKB inhibitor. Expansion was determined by counting the trypane blue–negative cell population. (B) During the 17-day culture period the percentage of apoptotic cells was determined by annexin V staining. (C) After 17 days of eosinophil differentiation, cytospins were made and stained with May-Grunwald Giemsa solution. Data were expressed as both the percentage of differentiated eosinophils (D) and as absolute numbers (E). (F) Lactoferrin, (G) CD14, and (H) CD49d expression was analyzed by FACS to determine neutrophil, monocyte, and eosinophil/monocyte development, respectively. Error bars represent SEM.
Flow cytometric analysis also was performed to support the morphological observations. Inhibition of PKB activity reduced expression of both lactoferrin, a neutrophil marker (Figures 1F, 2F), and CD14, a monocyte marker (Figures 1G, 2G) during neutrophil and eosinophil development. Expression of CD49d, a monocyte/eosinophil marker, was induced upon inhibition of PKB activity (Figures 1H, 2H).
These results suggest that PKB plays an important, but differential, role in lineage choice decisions during myelopoiesis.
Activation of PKB enhances neutrophil development but inhibits eosinophil differentiation
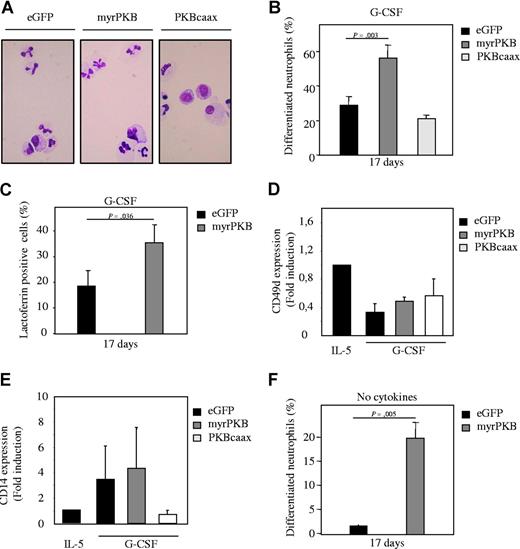
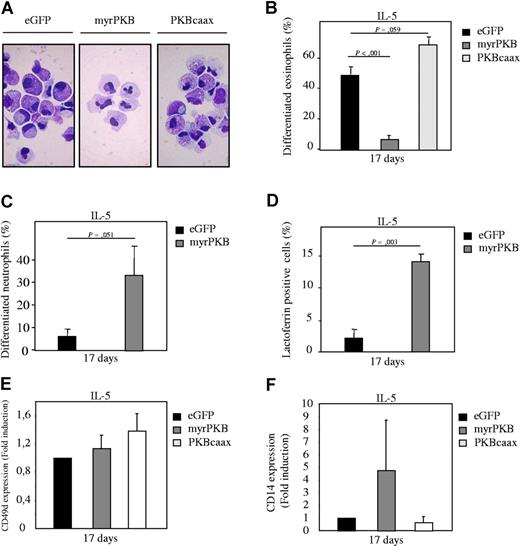
To confirm the results obtained using a pharmacological inhibitor of PKB, a bicistronic retroviral DNA construct was used co-expressing eGFP and either a constitutively active form of PKBα (myrPKB) or a dominant-negative form of PKBα (PKBcaax). Retrovirus was generated and used to infect CD34+ cells, which were subsequently differentiated toward either eosinophils or neutrophils. Ectopic expression of myrPKB and PKBcaax in progenitors was determined by Western blot analysis (Figure S2A, S3B). After 17 days of differentiation, eGFP-positive cells were sorted by FACS from the nontransduced cells and cytospins prepared. The morphology of the differentiating cells was subsequently analyzed after May-Grunwald Giemsa staining. Ectopic expression of myrPKB resulted in increased percentages of neutrophils with banded or segmented nuclei, whereas PKBcaax modestly inhibited neutrophil differentiation (Figure 3A,B). Similar results were obtained using the neutrophil-specific granule protein lactoferrin as a marker for neutrophil differentiation (Figure 3C). Conversely, ectopic expression of myrPKB dramatically inhibited eosinophil differentiation (Figure 4A,B), while PKBcaax induced eosinophil development (Figure 4A,B,E). Surprisingly, while normally requiring G-CSF, approximately 70% of cells had differentiated toward the neutrophil lineage in presence of IL-5, of which approximately 30% of these cells had banded or segmented nuclei (Figure 4A,C). Furthermore, the percentage of lactoferrin-positive cells also increased in cells cultured in presence of IL-5 and ectopically expressing myrPKB (Figure 4D). Surprisingly, a discrepancy between the percentage of lactoferrin-positive cells and the percentage of differentiated neutrophils was observed in these cultures, suggesting that IL-5, PKB, or retroviral transduction can modulate lactoferrin expression. Similar data were obtained culturing cells ectopically expressing myrPKB in absence of cytokines (Figure 3F). Activation of PKB in the absence of G-CSF is thus sufficient to induce neutrophil production. In addition, ectopic expression of myrPKB induced CD14 expression both in presence of G-CSF and IL-5 (Figures 3E, 4F). These data clearly demonstrate that PKB activity is not only critical for myelopoiesis, but also for defining lineage commitment decisions ex vivo.
Ectopic expression of PKB regulates neutrophil differentiation ex vivo. CD34+ cells were retrovirally transduced with myrPKB, PKBcaax, or eGFP alone. (A) Retrovirally transduced CD34+ cells were cultured in presence of G-CSF to induce neutrophil differentiation. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (B) Data were expressed as the percentage of differentiated neutrophils. (C) Lactoferrin, (D) CD49d, and (E) CD14 expression was analyzed by FACS to determine neutrophil, eosinophil/monocyte, and monocyte development, respectively, in presence of G-CSF. (F) CD34+ cells were retrovirally transduced with myrPKB or eGFP alone. Retrovirally transduced CD34+ cells were cultured in absence of cytokines. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. Data were expressed as the percentage of differentiated neutrophils. Error bars represent SEM.
Ectopic expression of PKB regulates neutrophil differentiation ex vivo. CD34+ cells were retrovirally transduced with myrPKB, PKBcaax, or eGFP alone. (A) Retrovirally transduced CD34+ cells were cultured in presence of G-CSF to induce neutrophil differentiation. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (B) Data were expressed as the percentage of differentiated neutrophils. (C) Lactoferrin, (D) CD49d, and (E) CD14 expression was analyzed by FACS to determine neutrophil, eosinophil/monocyte, and monocyte development, respectively, in presence of G-CSF. (F) CD34+ cells were retrovirally transduced with myrPKB or eGFP alone. Retrovirally transduced CD34+ cells were cultured in absence of cytokines. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. Data were expressed as the percentage of differentiated neutrophils. Error bars represent SEM.
Ectopic expression of PKB regulates eosinophil differentiation ex vivo. CD34+ cells were retrovirally transduced with myrPKB, PKBcaax, or eGFP alone. (A) Retrovirally transduced CD34+ cells were cultured in presence of IL-5 to induce eosinophil differentiation. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (B) Data were expressed as the percentage of differentiated eosinophils. (C) Data were expressed as the percentage of differentiated neutrophils that developed during the 17-day culture period in presence of IL-3 and IL-5. (D) Lactoferrin, (E) CD49d, and (F) CD14 expression was analyzed by FACS to determine neutrophil, eosinophil/monocyte, and monocyte, respectively in presence of IL-3 and IL-5. Error bars represent SEM.
Ectopic expression of PKB regulates eosinophil differentiation ex vivo. CD34+ cells were retrovirally transduced with myrPKB, PKBcaax, or eGFP alone. (A) Retrovirally transduced CD34+ cells were cultured in presence of IL-5 to induce eosinophil differentiation. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (B) Data were expressed as the percentage of differentiated eosinophils. (C) Data were expressed as the percentage of differentiated neutrophils that developed during the 17-day culture period in presence of IL-3 and IL-5. (D) Lactoferrin, (E) CD49d, and (F) CD14 expression was analyzed by FACS to determine neutrophil, eosinophil/monocyte, and monocyte, respectively in presence of IL-3 and IL-5. Error bars represent SEM.
PKB activity regulates myelopoiesis in vivo
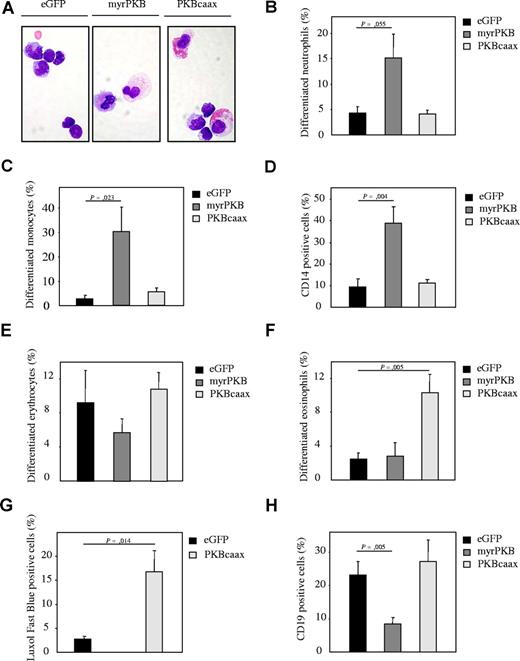
The data so far demonstrate that PKB plays a critical role in regulation of myelopoiesis ex vivo. To address the question whether regulation of PKB activity could also affect myelopoiesis in vivo, sublethally irradiated β2 microglobulin (−/−) NOD/SCID mice received transplants of retrovirally transduced human CD34+ progenitors. Progenitors were transduced with myrPKB, PKBcaax, or eGFP alone. Six weeks after injection, eGFP-positive cells were sorted and cytospins prepared (“Isolation and culture of human CD34+ cells”). The morphology of the cells was analyzed by May-Grunwald Giemsa staining (Figure 5A). Transplantation of mice with human CD34+ cells ectopically expressing myrPKB resulted in enhanced neutrophil (Figure 5B) and monocyte (Figure 5C,D) development and inhibition of erythrocyte differentiation (Figure 5E), whereas ectopic expression of PKBcaax induced eosinophil development in vivo (Figure 5F,G). Interestingly, ectopic expression of myrPKB also dramatically reduced the percentage of CD19-positive B cells (Figure 5H), suggesting that regulation of PKB activity plays a role in regulation of not only myelopoiesis but also lymphopoiesis.
Regulation of PKB activity in human CD34+ hematopoietic progenitors affects lineage development in β2-microglobulin (−/−) NOD/SCID mice. (A) CD34+ cells, cultured in presence of the cytokines SCF, FLT-3, ligand and TPO, were transduced with empty vector alone, myrPKB, or PKBcaax. After 3 days of culture, cells were injected into β2-microglobulin (−/−) NOD/SCID mice. Six weeks after injection, mice were killed, eGFP-positive human cells were sorted, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. Lineage development was depicted as the percentage of (B) neutrophils, (C) monocytes, (D) CD14-positive cells, (E) erythrocytes, and (F) eosinophils within the human, eGFP-positive bone marrow–derived cells. (G) To determine eosinophil development, the eGFP-positive population was sorted from the nontransduced cells and cytospins were made. The percentage of cells differentiated toward eosinophils was determined by Luxol Fast Blue staining, a dye specifically staining for eosinophilic granules. (H) The percentage of CD19-positive cells was determined by FACS analysis to determine B-cell development. Error bars represent SEM.
Regulation of PKB activity in human CD34+ hematopoietic progenitors affects lineage development in β2-microglobulin (−/−) NOD/SCID mice. (A) CD34+ cells, cultured in presence of the cytokines SCF, FLT-3, ligand and TPO, were transduced with empty vector alone, myrPKB, or PKBcaax. After 3 days of culture, cells were injected into β2-microglobulin (−/−) NOD/SCID mice. Six weeks after injection, mice were killed, eGFP-positive human cells were sorted, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. Lineage development was depicted as the percentage of (B) neutrophils, (C) monocytes, (D) CD14-positive cells, (E) erythrocytes, and (F) eosinophils within the human, eGFP-positive bone marrow–derived cells. (G) To determine eosinophil development, the eGFP-positive population was sorted from the nontransduced cells and cytospins were made. The percentage of cells differentiated toward eosinophils was determined by Luxol Fast Blue staining, a dye specifically staining for eosinophilic granules. (H) The percentage of CD19-positive cells was determined by FACS analysis to determine B-cell development. Error bars represent SEM.
PKB phosphorylates of C/EBPα
Although our data demonstrate that PKB activity plays a critical role in lineage choice decisions, the molecular mechanisms underlying these observations remain to be defined. One of the key transcription factors that regulates lineage choices during myelopoiesis is the CCAATT/enhancer binding protein α (C/EBPα).23,24 It has recently been demonstrated that phosphorylation of C/EBPα on Ser21 can inhibit granulocyte differentiation.25 Furthermore, the serine/threonine kinase GSK-3 was found to phosphorylate C/EBPα on Thr222 and Thr 226 in adipocytes, thereby affecting adipocyte development.26 Since GSK-3 is directly inhibited by PKB activation, it was of interest to investigate whether modulation of PKB activity could also affect C/EBPα phosphorylation in primary human hematopoietic progenitors.
Inhibition of both PI3K and PKB with pharmacological inhibitors induced phosphorylation of C/EBPα in neutrophil progenitors (Figure 6A, lanes 2,3), whereas inhibition of GSK-3, as previously described,26 inhibited C/EBPα phosphorylation (Figure 6A, lane 4). In addition, cells were retrovirally transduced to ectopically express dominant-negative PKB (PKBcaax) and cultured in presence of IL-5 to induce eosinophil differentiation. After 7 days of culture, protein lysates were made from the eGFP-positive population, and C/EBPα phosphorylation was analyzed. Ectopic expression of PKBcaax induced the level of phosphorylated C/EBPα in hematopoietic progenitors (Figure 6B). These data demonstrate that PKB can regulate C/EBPα phosphorylation.
C/EBPα phosphorylation is regulated by PI3K/PKB. (A) Cells were starved overnight in absence of cytokines and in presence of 0.5% FCS. Cells were left untreated (lane 1) or treated with 10 μM LY294002 (lane 2), 20 μM HIMO (lane 3), and 10 μM SB216763 (lane 4) for 1 hour before protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated C/EBPα, total C/EBPα, and as a control for equal loading an antibody against β-actin. (B) CD34+ cells were retrovirally transduced with eGFP as a control (lane 1) or PKBcaax (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated C/EBPα (Τ221/226) and an antibody against β-actin. Vertical line(s) have been inserted to indicate a repositioned gel lane. (C) CD34+ cells were retrovirally transduced with eGFP as a control (lane 1) or myrPKB (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated JunB and an antibody against β-actin (D).CD34+ cells were retrovirally transduced with eGFP as a control (lane 1) and constitutively active GSK-3 (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated C/EBPα (Τ221/226), JunB, and an antibody against β-actin. (E) CD34+ cells were retrovirally transduced with eGFP (lane 1) or C/EBPαT222/226A (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against C/EBPα (Τ221/226), C/EBPα JunB, and an antibody against β-actin. Vertical line(s) have been inserted to indicate a repositioned gel lane.
C/EBPα phosphorylation is regulated by PI3K/PKB. (A) Cells were starved overnight in absence of cytokines and in presence of 0.5% FCS. Cells were left untreated (lane 1) or treated with 10 μM LY294002 (lane 2), 20 μM HIMO (lane 3), and 10 μM SB216763 (lane 4) for 1 hour before protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated C/EBPα, total C/EBPα, and as a control for equal loading an antibody against β-actin. (B) CD34+ cells were retrovirally transduced with eGFP as a control (lane 1) or PKBcaax (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated C/EBPα (Τ221/226) and an antibody against β-actin. Vertical line(s) have been inserted to indicate a repositioned gel lane. (C) CD34+ cells were retrovirally transduced with eGFP as a control (lane 1) or myrPKB (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated JunB and an antibody against β-actin (D).CD34+ cells were retrovirally transduced with eGFP as a control (lane 1) and constitutively active GSK-3 (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against phosphorylated C/EBPα (Τ221/226), JunB, and an antibody against β-actin. (E) CD34+ cells were retrovirally transduced with eGFP (lane 1) or C/EBPαT222/226A (lane 2). After 7 days of differentiation in presence of IL-3 and IL-5 to induce eosinophil differentiation, transduced cells were separated from the nontransduced cells by FACS, and protein lysates were made. Western blot analysis was performed with an antibody against C/EBPα (Τ221/226), C/EBPα JunB, and an antibody against β-actin. Vertical line(s) have been inserted to indicate a repositioned gel lane.
Ectopic expression of myrPKB also dramatically reduced the expression level of JunB (Figure 6C), a transcriptional target of C/EBPα. In contrast, ectopic expression of constitutively active GSK3 resulted in a modest but reproductive increase in JunB expression (Figure 6D). Moreover, ectopic expression of C/EBPαT222/226A, a C/EBPα mutant that cannot be phosphorylated by GSK-3, resulted in a reduction of JunB expression (Figure 6E). Since C/EBPα expression is up-regulated during early granulocyte differentiation (M.B., unpublished data, September 2002), C/EBPα expression was still relatively low and below detection level after 7 days of culture in eGFP-transduced cells (Figure 6E).
These data suggest that PKB-mediated regulation of C/EBPα phosphorylation may modulate hematopoietic lineage choice decisions.
PKB mediates lineage choice decisions via regulation of C/EBPα
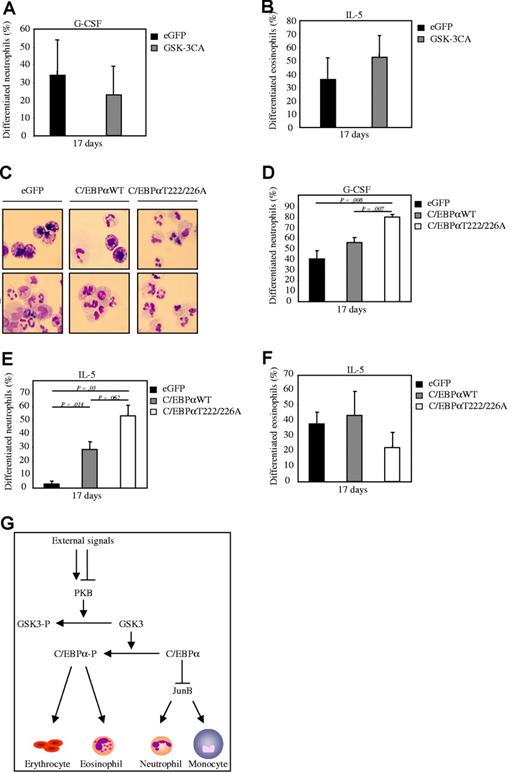
To investigate whether PKB can indeed regulate myelopoiesis through a GSK-3– and C/EBPα–dependent mechanism, bicistronic retroviral DNA constructs were used co-expressing eGFP and a constitutively active form of GSK-3 (GSK-3CA), wildtype C/EBPα (C/EBPαWT), or a C/EBPα mutant (C/EBPα T222/226A). Retrovirus was generated and used to infect CD34+ cells, which were subsequently differentiated toward either eosinophils or neutrophils. After 17 days of differentiation, eGFP-positive cells were sorted by FACS from the nontransduced cells and cytospins prepared. The morphology of the differentiating cells was subsequently analyzed after May-Grunwald Giemsa staining. Ectopic expression of GSK-3CA resulted in an inhibition of neutrophil development (Figure 7A), whereas eosinophil differentiation was induced (Figure 7B). In addition, ectopic expression of wildtype C/EBPα induced neutrophil development both in presence of G-CSF (Figure 7C-D) and IL-5 (Figure 7E), while eosinophil differentiation was inhibited (Figure 7F). Ectopic expression of the active C/EBPα T222/226A mutant further enhanced the observed induction of neutrophil development (Figure 7D) and further inhibited eosinophil differentiation compared with wildtype C/EBPα (Figure 7F). These data clearly demonstrate that PKB mediates lineage choice decisions at least in part through regulation of GSK-3 and C/EBPα activity.
PKB mediates lineage choice decisions via regulation of C/EBPα. (A) CD34+ cells were retrovirally transduced with GSK-3CA or eGFP alone. Retrovirally transduced CD34+ cells were cultured in presence of either (A) G-CSF to induce neutrophil development or (B) IL-5 to induce eosinophil differentiation After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (A-B) Data were expressed as the percentage of differentiated neutrophils or eosinophils. (C) CD34+ cells were retrovirally transduced with C/EBPαWT, C/EBPαT222/226A, or eGFP alone. Retrovirally transduced CD34+ cells were cultured in presence of either IL-5 to induce eosinophil differentiation or G-CSF to induce neutrophil development. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (D) Data were expressed as the percentage of differentiated neutrophils. (E) Data were expressed as the percentage of differentiated neutrophils that developed during the 17-day culture period in presence of IL-3 and IL-5. (F) Data were expressed as the percentage of differentiated eosinophils. (G)A schematic model showing the effect of regulation of PKB activity on regulation of myelopoiesis. Activation of PKB results in inhibitory phosphorylation of GSK-3. Active GSK-3 phosphorylates and thereby inhibits activation of C/EBPα. Unphosphorylated, active C/EBPα inhibits expression of JunB. Both activation of PKB and C/EBPα induces neutrophil differentiation, whereas eosinophil development is inhibited. Inhibition of PKB results in activation of GSK-3, phosphorylation of C/EBPα, and induction of eosinophil differentiation at the expense of neutrophil development. Error bars represent SEM.
PKB mediates lineage choice decisions via regulation of C/EBPα. (A) CD34+ cells were retrovirally transduced with GSK-3CA or eGFP alone. Retrovirally transduced CD34+ cells were cultured in presence of either (A) G-CSF to induce neutrophil development or (B) IL-5 to induce eosinophil differentiation After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (A-B) Data were expressed as the percentage of differentiated neutrophils or eosinophils. (C) CD34+ cells were retrovirally transduced with C/EBPαWT, C/EBPαT222/226A, or eGFP alone. Retrovirally transduced CD34+ cells were cultured in presence of either IL-5 to induce eosinophil differentiation or G-CSF to induce neutrophil development. After 17 days of culture, transduced cells were separated from the nontransduced cells by FACS, and cytospins were made. Cytospins were stained with May-Grunwald Giemsa solution. (D) Data were expressed as the percentage of differentiated neutrophils. (E) Data were expressed as the percentage of differentiated neutrophils that developed during the 17-day culture period in presence of IL-3 and IL-5. (F) Data were expressed as the percentage of differentiated eosinophils. (G)A schematic model showing the effect of regulation of PKB activity on regulation of myelopoiesis. Activation of PKB results in inhibitory phosphorylation of GSK-3. Active GSK-3 phosphorylates and thereby inhibits activation of C/EBPα. Unphosphorylated, active C/EBPα inhibits expression of JunB. Both activation of PKB and C/EBPα induces neutrophil differentiation, whereas eosinophil development is inhibited. Inhibition of PKB results in activation of GSK-3, phosphorylation of C/EBPα, and induction of eosinophil differentiation at the expense of neutrophil development. Error bars represent SEM.
Discussion
Although the PI3K/PKB signaling module has been demonstrated to play an important role in regulation of expansion and survival of hematopoietic cells, its role in regulation of differentiation of hematopoietic cells is unclear. In this study we have investigated the role of the PI3K signaling module in regulation of myelopoiesis. Our data demonstrate that PI3K and its downstream effector PKB play a critical though differential role in expansion, survival, and differentiation during granulopoiesis. Inhibition of PKB activity was required for eosinophil differentiation, whereas activation of PKB was sufficient to induce both neutrophil and monocyte development.
PKBα is the most ubiquitously expressed isoform, and null mutant mice display growth retardation and increased levels of apoptosis. Although the mice are viable, their life span upon exposure to genotoxic stress is shorter compared with wildtype mice.13,16 PKBβ is expressed at low levels in most organs but is highly expressed in insulin-responsive tissues. PKBβ-deficient mice primarily exhibited an insulin-resistant diabetic phenotype.27 PKBα/PKBβ double-knockout (DKO) mice exhibit severe growth deficiency and die shortly after birth. Analysis of these mice revealed that PKB plays an important role in multiple developmental processes, including organismal growth and thymus, skin, cardiovascular, and nervous system development.28 Since these mice die shortly after birth, it is not possible to examine the role of PKB in regulation of adult hematopoiesis in these mice. However, the observed growth retardation and impaired organ development suggests that PKB also may play a critical role in expansion and survival of hematopoietic progenitors. Indeed, expansion of granulocytic progenitors was abrogated upon inhibition of PKB (Figures 1,2). Survival of granulocyte progenitors, however, was not inhibited upon inhibition of PKB, suggesting that additional pathways are involved in this regulation of programmed cell death.
An important regulator of PI3K signaling is PTEN (phosphate and tensin homolog), a tumor-suppressor protein that dephosphorylates PtdIns(3,4,5)P,3 thereby reversing PI3K activity. Deletion of PTEN, resulting in activation of the PI3K pathway, resulted in increased levels of myeloid cells and T lymphocytes and subsequent formation of a myeloproliferative disorder.29 Although this study clearly indicates that PI3K is an important regulator of hematopoiesis, the downstream effectors of PI3K activity have not been defined.
Although our data demonstrate that differences in the level of PKB activity play a critical role in regulation of lineage choice decisions, both IL-5 and G-CSF can induce activation of PKB. However, it is unknown whether the level and kinetics of PKB activation in human CD34+ cells upon stimulation with both cytokines are identical. It could be hypothesized that additional signals, present in the bone marrow microenvironment, are required to inhibit PKB activity in vivo and to induce eosinophil maturation. It recently has been demonstrated that human bone marrow–derived mesenchymal stromal cells (MSCs) can inhibit activation of PKB in multiple tumor and primary cell lines upon cell-cell contact.37 We have observed that coculture of bone marrow–derived stromal cells with CD34+ progenitors cells can enhance final maturation of eosinophil differentiation (data not shown). And it is tempting to speculate that this is due to inactivation of PKB. Although the molecular mechanism underlying PKB inactivation by bone marrow–derived mesenchymal stromal cells remains to be investigated, several potential inhibitors of PKB activity have been described.
Rho GTPase, a member of the Ras superfamily of small GTP binding proteins, is capable of down-regulating PKB activity in human endothelial cells via Rho kinase (Rock).38 However, the molecular mechanism by which Rock can inhibit PKB activation is thus far unknown. Since RhoA can be activated by cell adhesion molecules such as integrins, it could be speculated that cell contact between stromal cells and granulocyte precursors induces activation of RhoA and subsequent inhibition of PKB activity, which is required to induce eosinophil differentiation. Another protein implicated to play an important role in cell fate determination is protein kinase C (PKC). In hematopoietic progenitors, transformed by the avian E26 leukemia virus, it has been demonstrated that high levels of PKC result in eosinophil differentiation, whereas low levels of PKC activity induce myeolomonocytic differentiation.39 It also has been demonstrated that various PKC isoforms can negatively regulate PKB activity. For example, inhibition of both PKCβ and PKCζ with biochemical inhibitors results in up-regulation of PKB activity.40,41 In addition, it has been demonstrated that PKCϵ inhibits G-CSF–mediated induction of PKB activity in 32D cells.42
We have demonstrated that PKB can regulate C/EBPα phosphorylation in CD34+ progenitor cells (Figure 6B). In addition, expression of JunB, a downstream target of C/EBPα, also was regulated by PKB (Figure 6C). The role of C/EBPα in regulation of hematopoiesis has been extensively investigated, and it is evident that expression of C/EBPα plays a critical role in the development of myeloid cells.23,24 Recently, it has been demonstrated that phosphorylation of C/EBPα on Ser21 inhibits granulopoiesis.43 Ross et al have previously demonstrated that GSK-3, itself a direct substrate of PKB, regulates phosphorylation of C/EBPα at Thr 222 and Thr 226 in 3T3-L1 adipocytes, resulting in conformational changes thought to affect association with critical co-factors.26 Although the precise effect of phosphorylation of Thr222 and Thr226 is at the moment incompletely understood, it is thought that it affects association with co-factors. Our results demonstrate that inhibition of C/EBPα phosphorylation of Thr 222 and Thr 226 can indeed modulate granulopoiesis (Figure 7C-F), thus providing a novel molecular mechanism for PKB-mediated regulation of hematopoiesis (Figure 7G).
Taken together, this is the first study that clearly implicates the PI3K/PKB signaling module in playing a critical role in regulation of hematopoietic lineage choices. Interestingly, in support of this hypothesis, recent findings demonstrated that treatment of NOD/SCID mice with a pharmacological inhibitor against GSK-3 accelerates neutrophil recovery upon transplantation with mouse Lin−Sca-1+C-kit+ hematopoietic stem cells,44 suggesting that pharmacological modulation of this signaling module may provide a clinical means of modulating bone marrow activity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr G. Nolan (Stanford University School of Medicine, Stanford, CA) for kindly providing us with the LZRS construct and the phoenix-ampho packaging cell line. We also thank Prof Dr H. Spits (AMC, Amsterdam, the Netherlands) for providing us with the LZRS constructs expressing myrPKB and PKBcaax. We thank Dr O. A. MacDougald (University of Michigan Medical School, Ann Arbor, MI) for providing us with the C/EBPα constructs.
This work was supported by a research grant from the Dutch Cancer Society. M.M., L.V., and H.V.D. were supported by a grant from the Dutch Cancer Society (UU2001-2491 and UU2005-3659). The Lund Stem Cell Center was supported by a Center of Excellence grant from the Swedish Foundation for Strategic Research.
Authorship
Contribution: M.B. designed and performed experiments, analyzed data, and wrote the paper. L.V., H.D., A.C., and S.V. performed experiments. L.K. and S.E.J. designed experiments and analyzed data. P.J.C. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul J. Coffer, Department of Immunology, KC.02.085.2 University Medical Center, Lundlaan 6, 3584 EA Utrecht, the Netherlands; e-mail: p.j.coffer@umcutrecht.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal