Efforts to reproducibly isolate tumor antigen–specific T cells from patients would be facilitated by removing immunoregulatory barriers. Using a human model for eliciting T-cell responses to tumor-associated antigens, we develop a novel strategy that eliminates nearly all Foxp3-expressing cells through the combination of CD25 depletion and IL-21 treatment resulting in a more than 150-fold decrease in Foxp3+ cells to virtually undetectable levels and a more than 200-fold increase in antigen-specific cytotoxic T lymphocytes (CTLs). The extent of Foxp3 elimination and degree of expansion of antigen-specific CTLs shown in this study have not previously been achievable and are unique to IL-21. We demonstrate for the first time a possible mechanism for IL-21–mediated expansion of antigen-specific CTLs that involves suppression of Foxp3-expressing cells and reversal of inhibition to tumor-associated antigen–specific CTL generation in vitro. Taken together, the combination of CD25 depletion and IL-21 exposure, by releasing regulatory constraints, leads to markedly enhanced CTL induction and represents a robust strategy for the ex vivo generation of antigen-specific T cells for adoptive cellular therapy.
Introduction
Adoptive cellular therapy of cancer involves ex vivo generation of autologous antigen-specific T cells, followed by in vitro expansion and infusion into patients in the hope that transferred T cells will traffic to and eradicate tumor.1,2 Recent studies have demonstrated tumor regression and measurable clinical benefit in patients who are otherwise refractory to conventional therapy.3,4 One major obstacle to adoptive therapy has been the feasibility of isolating tumor-reactive T cells. T cells recognizing tumor-associated antigens, which are often normal self-proteins, exist at very low frequency and exhibit predominantly low target avidity5,6 due to central and peripheral tolerance mechanisms. Moreover, such potentially autoreactive T cells may be suppressed by circulating regulatory cells (Tregs), that can lead to impaired proliferative response to antigenic stimulation.
Regulatory T cells (Tregs) have been shown to play a critical role in controlling immunologic tolerance to self-antigens as well as presenting a major barrier to the development of effective antitumor immunity in animal models.7,–9 In animal models, tumor antigen–specific CD8 cells failed to undergo normal functional maturation in the presence of Tregs and were rendered incapable of destroying specific tumor targets.10 Conversely, depletion of regulatory T cells controlled the growth of melanoma in most mice and promoted long-lasting CD8+ T-cell–dependent protective immunity,11 possibly through the recruitment of high-avidity antigen-specific cytotoxic T lymphocytes (CTLs).12 Recent evidence of elevated Tregs in the peripheral blood of patients with cancer13,,–16 and the finding that increased prevalence of tumor-associated Tregs in situ as a predictor for reduced survival17 suggest the importance of regulatory control of the endogenous antitumor immune response. The suppressive mechanisms at play in vivo18 may also limit the capacity to generate antigen-specific T cells in vitro.
Expression of the forkhead transcription factor, Foxp3, has been linked to the regulatory phenotype. Although Foxp3 is a reliable marker of naturally occurring Tregs, as an intracellular protein, it cannot be used as a practical method of sorting for viable Tregs. Instead, it has been shown that CD4+ cells constitutively expressing CD25hi are also Foxp3+. We and others have demonstrated that depletion of CD25+ cells in vitro can lead to enhanced generation of CD4+ T cells recognizing tumor-associated self-antigens,19 presumably by eliminating the inhibitory influence of CD25+ Tregs in the peripheral blood mononuclear cell (PBMC) responder population.
IL-21 belongs to the family of gamma-chain receptor cytokines that includes IL-2, IL-7, and IL-15—cytokines that all deliver their intracellular signal through the shared gamma-chain receptor and influence T-cell activation and differentiation.20,–22 Recently, we demonstrated that in vitro exposure to IL-21 (in contrast to other gamma-chain receptor cytokines) can lead to the generation of self-antigen–specific CTL in increased numbers and with enhanced avidity and function.23 In light of work demonstrating that barriers to optimal T-cell development may involve a regulatory component, we postulate that IL-21 may influence the regulatory control of cellular responses to tumor self-proteins in vitro.
Methods
This study was approved by the Institutional Review Board of Fred Hutchinson Cancer Research Center, Seattle, WA. Informed consent was obtained in accordance with the Declaration of Helsinki.
Depletion of CD25+ Tregs
Depletion of CD25+ T cells was performed using the CliniMACS CD25 MicroBeads (Miltenyi Biotech, Auburn, CA) according to manufacturer's instructions. In brief, the leukapheresis product was washed and resuspended in PBS/EDTA (2 mM; Invitrogen, Frederick, MD) supplemented with 0.5% human serum albumin (Life Technologies, Gaithersburg, MD). Appropriate amount of CD25 MicroBeads was added and incubated for 15 minutes at room temperature with gentle mixing every 5 minutes. Cells were washed, resuspended, and applied to the AutoMACS instrument with the CD25 depletion program selected. Upon completion of the depletion, an aliquot of cell fraction was stained for CD25 following FACScan analysis to check the efficiency of CD25 MicroBeads depletion.
Antibody plus peptide-MHC tetramer staining of T cells
APC-labeled peptide-MHC tetramer was produced in the immune monitoring laboratory at Fred Hutchinson Cancer Center based on previously described protocols.24 For sample analysis, 0.5 × 106 cells in 25 μL of 2% FCS/PBS were first stained with peptide tetramer APC (final concentration of 20 μg MHC/mL) for one hour at room temperature, followed by anti–CD8-FITC or PerCP (BD PharMingen, San Diego, CA) staining for 20 minutes at 4°C to analyze antigen-specific T-cell population. In some experiments, measure for phenotypes was carried out using anti–CD28-APC (BD PharMingen) or anti–CD28-FITC (Caltag Lab, Burlingame, CA), anti–CCR7-PE, and anti-CD45RO or anti–CD45RA-FITC (BD PharMingen) staining. After washing with PBS, cells were resuspended in PBS containing 2% FBS and DAPI was added. Data were acquired using a FACScalibur flow cytometer and CellQuest (BD PharMingen) and analyzed using FlowJo software (Tree Star, San Carlos, CA).
In vitro generation of human antigen-specific CD8+ T cells
Donor blood was typed by the HLA Typing Lab at the Puget Sound Blood Center (Seattle, WA). HLA-A2+ donors were used in the study. MART-1 M26, NY-ESO-1 NY157, and WT-1 WT126 peptide–specific T cells were generated in a manner similar to that previously described.3,23 Dendritic cells (DCs) were generated by exposing adherent PBMCs to IL-4 (500 U/mL; R&D Systems, Minneapolis, MN) and granulocyte-macrophage colony-stimulating factor (GM-CSF, 800 U/mL; Amgen, Thousand Oaks, CA) in AIM-V medium (Life Technologies) followed by maturation using IL-1β at 2 ng/mL, IL-6 at 1000 U/mL, TNF-α at 10 ng/mL (R&D Systems), and PGE-2 at 1 μg/mL (Sigma, St Louis, MO) for an additional 2 days. The mature DC population contained more than 90% CD83+ DCs on day 8 as determined by fluorescence-activated cell sorting (FACS) analysis.
Mature DCs were harvested and pulsed with 40 μg/mL synthesized peptides at 2 × 106 cell/mL in the presence of 3 μg/mL β2 microglobulin (Scripps Lab, San Diego, CA) in PBS with 1% human serum albumin (Life Technologies) for 4 hours at room temperature. After washing 3 times with sterile PBS (Life Technologies), DCs were cocultivated with responder T cells (unmanipulated or CD25 depleted) at 5 × 105 cells/well in 24-well plate in CTL medium, consisting of RPMI 1640 (Gibco, Carlsbad, CA), 25 mM HEPES, 4 mM l-glutamine, 50 μM 2-mercaptoethanol (Gibco), penicillin (50 U/mL), streptomycin (50 mg/mL) (Life Technologies), and 10% pooled human serum from healthy donors., IL-21 (30 ng/mL), was added to the experimental wells at culture initation. In some experiments, IL-2 (10 U/mL), IL-7 (10 ng/mL), or IL-15 (10 ng/mL) was added instead to compare the effect of IL-21. These concentrations of IL-2, IL-7, IL-15, and IL-21 were previously demonstrated to represent optimal dosing; higher concentrations led to no benefit or a detrimental effect in the generation of antigen-specific CTLs23 (and data not shown). For the second stimulation, irradiated autologous PBMCs pulsed with antigenic peptide were used. A total of 5 donors were evaluated, 3 of them for MART-1–specific responses and 2 other patients (ovarian cancer and NY-ESO–seropositive melanoma) for WT-1 and NY-ESO-1 responses, respectively. Unless otherwise specified, results were obtained at 7 days after the second in vitro stimulation.
Intracellular detection of Foxp3 protein by FACS analysis
Intracellular staining for Foxp3 protein used PE-conjugated antihuman Foxp3 staining set (clone PCH101; eBioscience, San Diego, CA) according to the manufacturer's instructions. Briefly, 106 cells were stained with FITC-conjugated anti-CD4 antibody (BD Pharmingen) first for 20 minutes on ice, washed, then resuspended in fixation/permeabilization buffer and incubated for 60 minutes at 4°C.
Immune suppression assay in vitro
Suppression assay was carried out based on the published protocol25 with minor modifications. CD4+CD25− and CD8+CD25− T cells (5 × 104 cells/well) were cocultured with Tregs (2.5 or 5 × 104 cells/well) with 100 ng/mL anti-CD3 antibody (OKT3) in the presence of irradiated (35 gray) autologous dendritic cells (0.5-1 × 104 cells/well) in a 96-well flat-bottom plate. Proliferation was assessed by [3H]thymidine (1 μCi [0.037 MBq] per well) incorporation.
Results
The combination of CD25 depletion and IL-21 markedly augments the expansion of antigen-specific CD8+ T cells following in vitro stimulation
MART-1–specific CTLs were generated according to methods described in “In vitro generation of human antigen-specific CD8+ T cells” under the following conditions: no manipulation (control cultures), IL-21 treatment alone, CD25 depletion alone, and IL-21 treatment with CD25-depleted responder PBMCs. IL-21 was used at a dose of 30 ng/mL, previously shown to be optimal for generating antigen-specific T cells.26 Concentrations of 10 ng/mL or less had no effect, and inhibition was observed when IL-21 concentration was used at 100 ng/mL or greater.
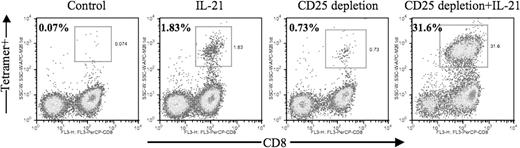
Exposure to IL-21 added once during the first in vitro stimulation led to a marked enhancement in the frequency of MART-1–specific CTLs that could be generated (Figure 1: 1.83% vs 0.07%), confirming our previous findings. CD25 depletion alone led to a 10-fold increase in the frequency of antigen-specific CD8+ T cells compared with control cultures (0.73% vs 0.07%). A striking more than 450-fold increase in the frequency of antigen-specific CD8+ T cells was observed through the combined use of CD25 depletion and IL-21 treatment (31.6% vs 0.07%). Data from 3 additional patients demonstrated a 160-fold to more than 300-fold increase in the absolute numbers of antigen-specific CTLs that could be generated with combined CD25 depletion and IL-21 treatment (Table 1: 1.45 vs 244.94, 1.68 vs 462.65, 0.45 vs 141.0). Antigen-specific T cells generated by CD25 depletion plus IL-21 demonstrated robust killing activity against antigen-expressing tumor target (> 50% lysis at an E/T ratio of 10:1) and IFN-gamma secretion upon recognition of specific peptide antigen in vitro assay (data not shown).
Combined use of IL-21 and CD25 depletion leads to significantly enhanced generation of antigen-specific cytotoxic T lymphocytes. Unmanipulated or CD25-depleted PBMCs from HLA-A2+ patients with melanoma were stimulated in vitro with autologous mature dendritic cells pulsed with the MART-1 M26 peptide as described in “In vitro generation of human antigen-specific CD8+ T cells.” Where indicated, IL-21 (30 ng/mL) was added at culture initiation. After 2 cycles of stimulation, 5 × 105 cells from each experiment group were harvested and stained with 20 μg/mL peptide/MHC tetramer (PE, vertical axis) and FITC-conjugated CD4; a vital dye (DAPI) was also added to exclude dead cells. Data are expressed as percentage of tetramer-positive cells among gated lymphocytes on day 18 after second stimulation.
Combined use of IL-21 and CD25 depletion leads to significantly enhanced generation of antigen-specific cytotoxic T lymphocytes. Unmanipulated or CD25-depleted PBMCs from HLA-A2+ patients with melanoma were stimulated in vitro with autologous mature dendritic cells pulsed with the MART-1 M26 peptide as described in “In vitro generation of human antigen-specific CD8+ T cells.” Where indicated, IL-21 (30 ng/mL) was added at culture initiation. After 2 cycles of stimulation, 5 × 105 cells from each experiment group were harvested and stained with 20 μg/mL peptide/MHC tetramer (PE, vertical axis) and FITC-conjugated CD4; a vital dye (DAPI) was also added to exclude dead cells. Data are expressed as percentage of tetramer-positive cells among gated lymphocytes on day 18 after second stimulation.
Number of tetramer+ cells from representative melanoma patient
| . | Experiment 1 . | Experiment 2 . | Experiment 3 . |
|---|---|---|---|
| Control | 1.45/1 | 1.68/1 | 0.45/1 |
| IL-21 | 17.14/11.82 | 23.17/13.79 | 8.37/18.60 |
| CD 25 depletion | 8.20/5.66 | 12.64/7.52 | 4.35/9.67 |
| CD25 depletion + IL-21 | 244.94/168.92 | 462.65/275.38 | 141.00/313.33 |
| . | Experiment 1 . | Experiment 2 . | Experiment 3 . |
|---|---|---|---|
| Control | 1.45/1 | 1.68/1 | 0.45/1 |
| IL-21 | 17.14/11.82 | 23.17/13.79 | 8.37/18.60 |
| CD 25 depletion | 8.20/5.66 | 12.64/7.52 | 4.35/9.67 |
| CD25 depletion + IL-21 | 244.94/168.92 | 462.65/275.38 | 141.00/313.33 |
The absolute number in millions of tetramer+ cells corresponding to each experimental culture from a representative melanoma patient, depicted in Figure 1, and the fold increase in absolute numbers of CD25 depletion and/or IL-21 treated to untreated cultures (control). Representative results from 3 separate experiments are presented.
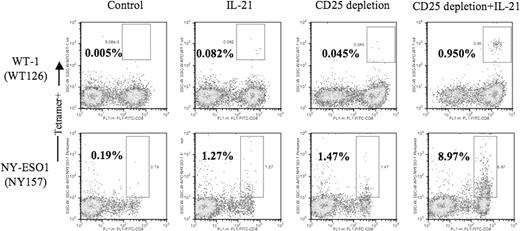
For these initial studies, we used a canonical tumor antigenic epitope: M26 peptide of the melanoma-associated self-antigen, MART-1. To evaluate the generality of this finding, we examined CTL responses to 2 other tumor-associated antigens, WT-1 and NY-ESO-1 in HLA-A*0201+ patient donors. Representative results are shown for responses generated from the PBMCs of a patient donor with WT-1+ ovarian tumor and the PBMCs of an NY-ESO-1–seropositive patient donor with melanoma (Figure 2 and Table 2) Although the endogenous precursor frequency of CTLs specific for WT-1 (a self-antigen that is prevalent at low levels among normal tissues) is very low and the ability to enrich for this rare population of WT-1–specific CTLs somewhat elusive (0.005% after 2 in vitro stimulations), the positive influence of CD25 depletion and IL-21 exposure is similar to that seen for MART-1–specific CTL responses. An 8- to 20-fold increase in the numbers and frequency of WT-1–specific CTLs is observed with either IL-21 treatment or CD25 depletion alone compared with control, but more than 250-fold greater when cultures receive both CD25 depletion and IL-21 treatment. Similarly, for NY-ESO-1–specific responses, a relative 10-fold increase with either treatment alone increases to 100-fold with combined CD25 depletion and IL-21 exposure.
Combined use of IL-21 and CD25 depletion enhances generation of WT-1– and NY-ESO-1–specific CTL responses. Unmanipulated or CD25-depleted PBMCs from an HLA-A2+ patient with a WT-1+ ovarian cancer (top panels) and a patient with melanoma (NY-ESO seropositive; bottom panels) were stimulated as described in Figure 1.
Combined use of IL-21 and CD25 depletion enhances generation of WT-1– and NY-ESO-1–specific CTL responses. Unmanipulated or CD25-depleted PBMCs from an HLA-A2+ patient with a WT-1+ ovarian cancer (top panels) and a patient with melanoma (NY-ESO seropositive; bottom panels) were stimulated as described in Figure 1.
Number of WT-1– or NY-ESO-1–specific CTLs
| . | WT-1(WT126) . | NY-ESO1 (NY157) . |
|---|---|---|
| Control | 0.023/1 | 0.558/1 |
| IL-21 | 0.413/17.96 | 5.144/9.21 |
| CD25 depletion | 0.203/8.83 | 5.144/9.21 |
| CD25 depletion + IL-21 | 5.928/257.74 | 56.78/101.76 |
| . | WT-1(WT126) . | NY-ESO1 (NY157) . |
|---|---|---|
| Control | 0.023/1 | 0.558/1 |
| IL-21 | 0.413/17.96 | 5.144/9.21 |
| CD25 depletion | 0.203/8.83 | 5.144/9.21 |
| CD25 depletion + IL-21 | 5.928/257.74 | 56.78/101.76 |
The increase in absolute number of WT-1– or NY-ESO-1–specific CTLs as well as the fold increase in numbers compared with control cultures are shown.
IL-21 exposure leads to optimal expansion of antigen-specific CTLs following CD25 depletion
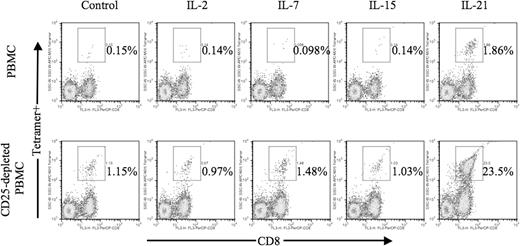
Among the γ-chain receptor cytokines, including IL-2, IL-7, and IL-15, IL-21 provided the greatest effect on antigen-specific CTL expansion following CD25 depletion (Figure 3). When using untreated PBMCs, IL-21 elicited a 10-fold or greater increase in antigen-specific CTLs compared with no cytokine or IL-2–, IL-7–, or IL-15–treated cultures in congruence with our previous results.23 When using CD25-depleted PBMCs, IL-21–exposed cultures yielded a 20-fold greater increase in antigen-specific CTL frequency compared with other CD25-depleted cultures.
IL-21 is unique among γ-chain receptor cytokines in its ability to induce expansion of antigen-specific CTLs. Unmanipulated or CD25-depleted PBMCs from HLA-A2+ patients with melanoma were stimulated in vitro with autologous mature dendritic cells pulsed with the MART-1 M26 peptide as described in “In vitro generation of human antigen-specific CD8+ T cells.” Optimal concentrations of IL-2 (12.5 U/mL), IL-7 (10 ng/mL), IL-15 (30 U/mL), and IL-21 (30 ng/mL) were added to individual cultures at time of stimulation on day 1. Cells (5 × 105) from each experiment group were harvested on day 7 and stained with 20 μg/mL peptide/MHC tetramer PE- and PerCP-conjugated CD8. Data are expressed as percentage of tetramer-positive cells among gated lymphocytes.
IL-21 is unique among γ-chain receptor cytokines in its ability to induce expansion of antigen-specific CTLs. Unmanipulated or CD25-depleted PBMCs from HLA-A2+ patients with melanoma were stimulated in vitro with autologous mature dendritic cells pulsed with the MART-1 M26 peptide as described in “In vitro generation of human antigen-specific CD8+ T cells.” Optimal concentrations of IL-2 (12.5 U/mL), IL-7 (10 ng/mL), IL-15 (30 U/mL), and IL-21 (30 ng/mL) were added to individual cultures at time of stimulation on day 1. Cells (5 × 105) from each experiment group were harvested on day 7 and stained with 20 μg/mL peptide/MHC tetramer PE- and PerCP-conjugated CD8. Data are expressed as percentage of tetramer-positive cells among gated lymphocytes.
IL-21 exposure enriches for a population of CD28+ CCR7− CD8+ memory CTLs
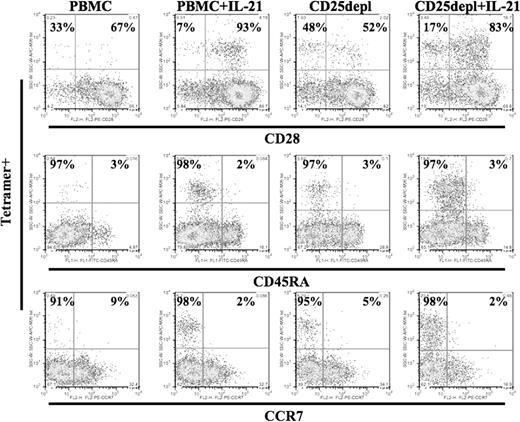
The surface phenotype of antigen-specific CTLs generated in cultures under the 4 conditions (control, IL-21 treatment, CD25 depletion, and combined IL-21 treatment and CD25 depletion) was evaluated by staining for CD28, CCR7, CD45RA expression (Figure 4). A unique memory phenotype, CD28+, CCR7−, was observed only among the IL-21–exposed cultures (PBMC+ IL-21 and CD25depl+ IL-2). Results were obtained 7 days after the second in vitro stimulation. This phenotype was stable for more than 4 weeks when maintained with IL-2 and IL-7 and without further exposure to IL-21. These findings are consistent with previous studies we have published demonstrating up-regulation of CD28 expression and helper independence among antigen-specific CTLs generated in IL-21–treated cultures.23
IL-21–treated cultures enrich for a population of CD28hi Ag-specific memory CTLs. Cells were collected 7 days after second stimulation with MART-1 peptide–pulsed autologous DCs and stained for MART-1 tetramer and simultaneously with CD28, CCR7, or CD45RA under the conditions described: PBMCs (untreated control culture), PBMCs + IL-21 (addition of IL-21 at 30 ng/mL during the first stimulation), CD25depl (CD25-depleted PBMCs used as a source of T cells), and CD25depl + IL-21 (combined CD25 depletion and IL-21 treatment). These results are representative of cultures from 3 donors.
IL-21–treated cultures enrich for a population of CD28hi Ag-specific memory CTLs. Cells were collected 7 days after second stimulation with MART-1 peptide–pulsed autologous DCs and stained for MART-1 tetramer and simultaneously with CD28, CCR7, or CD45RA under the conditions described: PBMCs (untreated control culture), PBMCs + IL-21 (addition of IL-21 at 30 ng/mL during the first stimulation), CD25depl (CD25-depleted PBMCs used as a source of T cells), and CD25depl + IL-21 (combined CD25 depletion and IL-21 treatment). These results are representative of cultures from 3 donors.
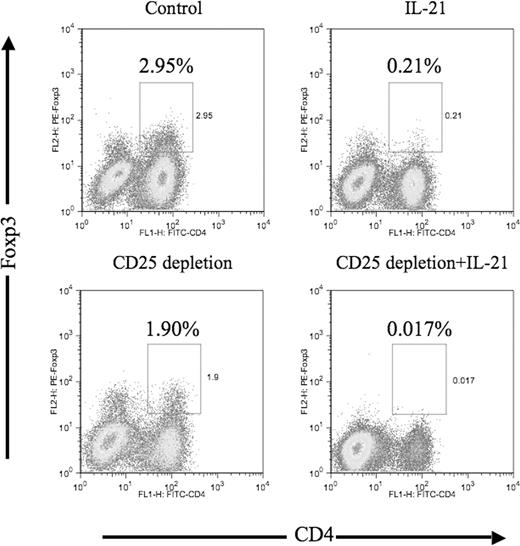
Exposure of CTL culture to IL-21 leads to a decrease in the frequency of Foxp3+ T cells
Foxp3+ cells were identified by intracellular staining following CD25 depletion and/or IL-21 treatment (Figure 5). CD25 depletion alone led to a measurable decrease in the fraction of Foxp3+ cells in culture. IL-21 treatment alone led to a 10-fold decrease in the fraction of Foxp3+ cells (2.95% to 0.21%). The combination of CD25 depletion and IL-21 treatment resulted in a drop in the CD4+ Foxp3+ population to an almost undetectable level (2.95% to 0.017%); further, a substantial decrease in the fraction of CD4− Foxp3+ cells was observed that was not seen with either IL-21 treatment or CD25 depletion alone.
The frequency of CD4+ Foxp3+ T cells is significantly decreased by IL-21 and CD25 treatment in stimulation culture. MART-1–specific T-cell stimulation cultures were established using unmanipulated PBMCs (control), IL-21–treated, CD25-depleted, and CD25-depleted/IL-21–treated PBMCs. Three weeks following initial in vitro stimulation, 5 × 105 cells from each experimental culture were stained with FITC-conjugated CD4, followed by intracellular staining for Foxp3 protein. Data are expressed as percentage of CD4+ Foxp3-positive cells among gated lymphocytes.
The frequency of CD4+ Foxp3+ T cells is significantly decreased by IL-21 and CD25 treatment in stimulation culture. MART-1–specific T-cell stimulation cultures were established using unmanipulated PBMCs (control), IL-21–treated, CD25-depleted, and CD25-depleted/IL-21–treated PBMCs. Three weeks following initial in vitro stimulation, 5 × 105 cells from each experimental culture were stained with FITC-conjugated CD4, followed by intracellular staining for Foxp3 protein. Data are expressed as percentage of CD4+ Foxp3-positive cells among gated lymphocytes.
IL-21 fails to induce Treg proliferation
To evaluate the possibility that the differential effects of IL-21, compared with the other gamma-chain receptor cytokines, IL-2, IL-7, and IL-15, were associated with cytokine-mediated Treg proliferation, sorted CD4+ 25+ Tregs were treated with IL-2, IL-7, IL-15, or IL-21 in the presence or absence of CD3 stimulation (Figure 6A). Anti-CD3 stimulation led to enhanced Treg proliferation (thymidine uptake) among IL-2, IL-7, and IL-15. IL-15 enhanced Treg expansion in an antigen-independent manner. IL-21, however, failed to significantly induce Treg proliferation, even after anti-CD3 stimulation and was no different from the no-cytokine culture.
IL-21 partially reverses Treg-mediated inhibition of CD8 T-cell proliferation. (A) Sorted Tregs (5 × 104/well) were cultured with IL-2 (12.5 U/mL), IL-7 (10 ng/mL), IL-15 (30 ng/mL), or IL-21 (30 ng/mL) in the presence or absence of anti-CD3 and autologous dendritic cells. (B) Sorted CD8+CD25− effector T cells (5 × 104/well) were cultured with Tregs at ratio of 1:1 (CD8+/Tregs) in the presence of anti-CD3 and autologous dendritic cells. IL-21 (30 ng/mL) was added as indicated. Proliferation was measured by [3H]thymidine incorporation pulsed on day 3 and harvested 16 to 20 hours later. The results of CPM were calculated from triplicate wells with standard error of the mean. The P value was obtained by applying paired sample t test to evaluate the influence of Tregs on the CD8 proliferation with and without IL-21. Error bars represent plus or minus standard error calculated from triplicate results for each data point.
IL-21 partially reverses Treg-mediated inhibition of CD8 T-cell proliferation. (A) Sorted Tregs (5 × 104/well) were cultured with IL-2 (12.5 U/mL), IL-7 (10 ng/mL), IL-15 (30 ng/mL), or IL-21 (30 ng/mL) in the presence or absence of anti-CD3 and autologous dendritic cells. (B) Sorted CD8+CD25− effector T cells (5 × 104/well) were cultured with Tregs at ratio of 1:1 (CD8+/Tregs) in the presence of anti-CD3 and autologous dendritic cells. IL-21 (30 ng/mL) was added as indicated. Proliferation was measured by [3H]thymidine incorporation pulsed on day 3 and harvested 16 to 20 hours later. The results of CPM were calculated from triplicate wells with standard error of the mean. The P value was obtained by applying paired sample t test to evaluate the influence of Tregs on the CD8 proliferation with and without IL-21. Error bars represent plus or minus standard error calculated from triplicate results for each data point.
IL-21 partially reverses Treg-mediated suppression of CD8 proliferation
When added to CD8+ T-cell cultures, Tregs inhibit proliferation by up to 66% when evaluated by thymidine incorporation assays (Figure 6B, *P < .01 vs CD8+ only). When IL-21 was added at optimal concentration, the suppressive effect of Tregs on CD8 T-cell proliferation was partially reversed (Figure 6B, #P < .01 vs CD8+ plus IL-21). Thus, IL-21 not only fails to induce Treg proliferation, but also reverses Treg-mediated suppression.
Discussion
Using a prototypic human self-antigen, we demonstrate for the first time that regulatory T-cell depletion can lead to substantially enhanced generation of tumor-associated antigen-specific CTLs. The combination of CD25 depletion and IL-21 resulted in a more than 100-fold depletion of Foxp3+ Tregs and augmented the frequency and absolute number of tumor antigen–specific CD8+ CTLs between 150- and 300-fold. This effect is much greater than that expected given the individual contribution of each factor and is unique for IL-21 since the addition of other γ-chain receptor cytokines such as IL-2, IL-7, and IL-15 did not have a similar effect.
Although CD25 T-cell depletion was nearly complete after anti-CD25 immunomagnetic depletion, more than 50% of the Foxp3+ population remained, likely due to the presence of Foxp3+ Tregs that do not constitutively express CD25. IL-21 treatment had a greater Foxp3-depleting effect (> 90% depletion) and was able to only partially reverse Treg-mediated suppression of CD8 T-cell proliferation. Perhaps it was the unexpectedly potent combination of both CD25 depletion and IL-21 treatment leading to more than 99.5% depletion of Foxp3+ cells that enabled a uniquely robust antigen-specific T-cell response. We also observed a decrease of Foxp3+ cells not only in the conventional CD4 compartment, but also among CD8+ (CD4−) T cells that may represent the putative CD8+ regulatory T-cell population recently described.27,28
The mechanism of action of IL-21 in this model is unclear. Upon activation, both CD4+ CD25+ Tregs and CD8+ T cells up-regulate IL-21 receptor expression but resting Tregs express very low levels of IL-21 receptor29 (and data not shown). Since Tregs are not directly activated in this model, the decrease in Foxp3+ cells following IL-21 treatment in vitro may be due to IL-21 engagement of very low-density of surface receptors, the indirect action of IL-21 through intermediate cell types, or expansion of effector cells contributing as a cytokine sink for Treg survival.
In recent studies, IL-21 appears to be a key element in the induction of Th17 cells responsible for proinflammatory and autoimmune responses.30,–32 IL-21–deficient T cells failed to differentiate into Th17 cells and were associated with the reciprocal development of Foxp3 Tregs, suggesting a possible role for IL-21 in mediating decreased levels of Foxp3 in our findings by enhancing the development of Th17 cells at the expense of Tregs. A genetic basis for these results involving the IDD3 gene, which regulates the function of Tregs, was suggested.33 These possible mechanisms are currently under investigation in our model.
The observation that IL-21 treatment leads to depletion of Foxp3+ cells contrasts with results recently published describing the absence of any Treg-depleting effect of IL-21 and no beneficial effect on CD8 T-cell proliferation in the presence of Tregs.29 Our studies have shown that the concentration of IL-21 used may produce confounding results. At concentrations greater or equal to 100 ng/mL, there is no significant beneficial in vitro effect and, in fact, an inhibitory effect. Peluso et al were also unable to address the influence of IL-21 on the generation of antigen-specific CTLs since antibodies were used to nonspecifically trigger the T-cell receptor to reproduce an “inflammatory” state.29 The use of anti-CD3 to stimulate through the TCR fails to address issues associated with the generation of tumor-associated, self-antigen–specific T-cell responses that arise from a naturally occurring low frequency and low avidity T-cell subset.
Our recent experience in human samples demonstrated a beneficial effect of IL-21 on the generation of helper-independent antigen-specific CTLs.23 The impact of IL-21 on Tregs has only recently been proposed and, until now, not previously shown to directly or indirectly deplete human Treg population. Neither the means to eliminate Foxp3+ cells to such a profound degree nor the capacity to enhance the generation of tumor-associated antigen–specific CTLs has previously been demonstrated. By removing regulatory constraints limiting antigen-specific expansion, this strategy may not only improve the prospects for generating tumor-reactive T cells for adoptive cellular therapy, but also uncover novel, tumor-associated targets that were previously below the threshold for generating in vitro T-cell responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grants CA 83636, CA104711, and MO1-RR-000037 (University of Washington General Clinical Research Center). C.Y. currently holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund. Y.L. was supported in part by the Rippel Foundation.
National Institutes of Health
Authorship
Contribution: C.Y. designed the research, analyzed the data, interpreted results, and wrote the paper; Y.L. codesigned and performed the studies, analyzed the data, interpreted results, and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cassian Yee, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D3-100, Seattle, WA 98109; e-mail: cyee@fhcrc.org.

![Figure 6. IL-21 partially reverses Treg-mediated inhibition of CD8 T-cell proliferation. (A) Sorted Tregs (5 × 104/well) were cultured with IL-2 (12.5 U/mL), IL-7 (10 ng/mL), IL-15 (30 ng/mL), or IL-21 (30 ng/mL) in the presence or absence of anti-CD3 and autologous dendritic cells. (B) Sorted CD8+CD25− effector T cells (5 × 104/well) were cultured with Tregs at ratio of 1:1 (CD8+/Tregs) in the presence of anti-CD3 and autologous dendritic cells. IL-21 (30 ng/mL) was added as indicated. Proliferation was measured by [3H]thymidine incorporation pulsed on day 3 and harvested 16 to 20 hours later. The results of CPM were calculated from triplicate wells with standard error of the mean. The P value was obtained by applying paired sample t test to evaluate the influence of Tregs on the CD8 proliferation with and without IL-21. Error bars represent plus or minus standard error calculated from triplicate results for each data point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/1/10.1182_blood-2007-05-089375/3/m_zh80030812130006.jpeg?Expires=1769105349&Signature=VtaA17VPu7S~iZ45C5gsP9R31Q~2z6Utj~-EDnMk42OH~ut774SHFFZfjPNvbvzVgwofaQppoBMw21sz4y8Zxil24hRljcOxD0tMQOwckjsGHbMKGw2qC3unLFNXcn9QmiQ2d0-a7DIaSR2c42n-wrkLRjzaBwISVprAmEkyz8X7x9Cgyl5YKx8E3McU4TvkPghWqjzdXfxIgPgBr4qvfP20~qt3lq6fAW8o0N7ZuZ~YHUBYLhEYbqBkPdL6cAnfSmERchESVrotFMTLkl2FUeIRsBosSpSrS2XuxIACAahtNnL8he7Yhv0SlpYIW2T-7lBNl~iy8fVt3gdEtwHNJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)