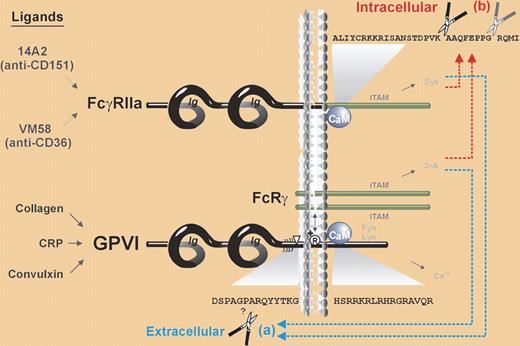
The interaction between the platelet and subendothelial matrix, a process mediated by platelet receptors GPVI for collagen and GPIb-IX-V for von Willebrand factor, is an initial event in thrombus formation on vessel injury. Although we know a lot about the events following receptor activation, our understanding is limited with respect to the mechanisms that down-regulate these responses once activated. Glycoprotein VI (GPVI) is a member of the immunoglobulin (Ig) receptor family with 2 extracellular Ig domains; it is noncovalently linked with an Fc receptor γ-chain (FcRγ) dimer, an association essential for GPVI surface expression. The cytoplasmic portion of FcRγ has an ITAM that is phosphorylated by Src family kinases on GPVI activation, culminating in the cleavage of the extracellular domain of GPVI and down-regulation of receptor function. The ITAM-dependent mechanisms are essential to this process. Also present on platelet surface is the IgG receptor FcγRIIa, which has 2 extracellular Ig domains and, importantly, an ITAM in the cytoplasmic domain. Ligation of this receptor by immune complexes induces platelet activation.
Gardiner and colleagues have explored the interplay of the 2 ITAM-bearing receptors to address whether signaling through FcγRIIa would also induce the cleavage of the GPVI ectodomain that was previously noted with GPVI ligation.1 They demonstrate that antibody ligation of platelet FcγRIIa results in the shedding of GPVI ectodomain mediated by a metalloproteinase, as well as cleavage of FcγRII intracellular domain by calpain. This parallel but differential cleavage of the 2 receptors was also observed on activation of GPVI, providing evidence of simultaneous down-regulation by distinct proteolytic cleavage of both receptors on ligation of either ITAM-bearing receptor. These observations extend our insights of the mechanisms that terminate signaling through these receptors in the context of hemostasis and thrombosis.
From the clinical perspective, there are important implications. Platelet FcγRIIa mediates the intense platelet activation that accompanies heparin-induced thrombocytopenia (HIT) induced by platelet binding of the immune complexes. Gardiner et al go on to demonstrate in vitro down-regulation of GPVI and FcγRIIa in platelets exposed to IgG from HIT patients. This predicts blunted collagen-induced platelet activation in HIT patients, a finding of considerable interest and in need of further supporting evidence. Also relevant in this context are the previous observations that GPVI antibody depletes platelet GPVI in vivo and decreases collagen responses in immune thrombocytopenic purpura (ITP) patients2,3 and in mouse models,4,5 and that GPVI antibody has an antithrombotic effect.5 Proteolytic cleavage of GPVI is the mechanism in at least some of these studies. Moreover, for both receptors studied by Gardiner et al, calmodulin is a major intermediary player and calmodulin inhibitors induce their cleavage, suggesting a potential role for these agents as antithrombotic strategies to down-regulate platelet receptor function, provided that their other effects on hematopoietic cells are not a limitation.
Scheme showing ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors.
Scheme showing ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal