Zebrafish, the tropical freshwater Danio rerio, has been an exceptional vertebrate model for investigating organogenesis and in particular the molecular regulation, differentiation, and development of vertebrate hematopoiesis.1,2 There are several advantages to using zebrafish as a model of developmental vertebrate hematopoiesis: (1) zebrafish fertilize a large number of eggs externally that can be readily collected and investigated; (2) zebrafish embryos are transparent, facilitating direct observation of organogenesis; (3) zebrafish embryonic development is rapid; and (4) a variety of technologies, including morpholino-induced gene knock-down, RNA in situ hybridization, unique methods of mutagenesis, and the study of transgenic zebrafish have provided critical insights into molecular regulation of vertebrate hematopoiesis.1 The zebrafish model has allowed the use of “forward genetics” to identify new and sometimes unanticipated genes that regulate developmental vertebrate hematopoiesis, identify the genetic basis of a few human hematopoietic disorders, provide insights into the genetic basis of myelodysplastic syndromes and leukemic transformation, and find potential novel targets for therapeutic interventions.
Hematopoiesis was originally thought to arise only from the intermediate cell mass (ICM) or caudal hematopoietic tissue (CHT) located in the posterior region between the notochord endoderm in primitive zebrafish. However, researchers have recently identified a second site of embryonic “primitive” hematopoiesis that originates in the anterior-lateral mesoderm (rostral blood island [RBI]) and eventually migrates to the kidney, which serves as the location of “definitive” hematopoiesis during the remainder of the zebrafish's development and lifespan.1,2 In contrast, in mammals, primitive hematopoiesis originates from mesoderm outside the embryo that migrates onto the embryonic yolk sac to develop blood islands; during the course of further development, it migrates to the aorta-gonad-mesonephros (AGM) region, fetal liver, and finally the bone marrow for “definitive” hematopoiesis.1,–3
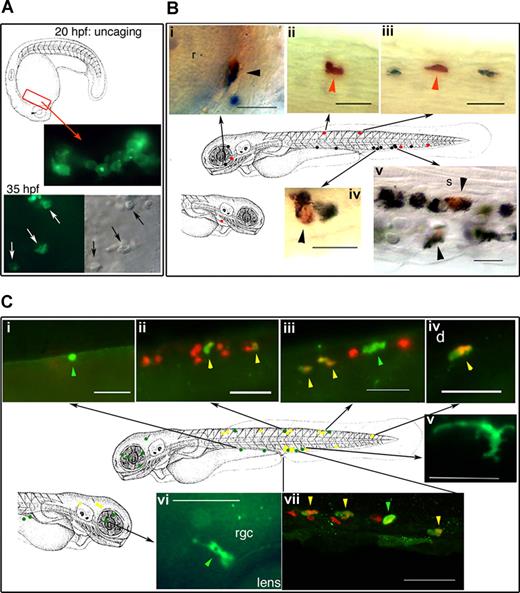
Macrophages have been identified as the first leukocytes to be derived during developmental myelopoiesis in vertebrate embryos, originate from the RBI, before dispersing throughout the embryonic mesenchyme prior to blood circulation in the developing zebrafish. Neutrophilic granulocytes appear next in primitive hematopoiesis and are identified in blood circulation and connective tissue (mesenchyme) by 48 hours after fertilization (hpf). Neutrophil development during mammalian hematopoiesis was thought to originate from primitive hematopoietic cells similar to macrophages but not from macrophages themselves. In this issue, Le Guyader and coworkers have identified a unique subset of neutrophilic granulocytes that originate from primitive macrophages from the RBI giving rise to precursor primitive myeloid progenitor cells. Furthermore, these neutrophilic granulocytes arising from primitive macrophages have unique properties, including expression of PU.1 and L-plastin, migrating toward invading microbes without phagocytosis, and are dispersed into mesenchyme and epidermis but not the blood circulation. Using a fluorescein dye to detect peroxidase activity of activated granulocytes by an ultraviolet laser, Le Guyader et al show, through cell tracing of sudan black (SB)–stained granulocytes, a population of neutrophilic granulocytes that were derived from myeloid progenitors originating from primitive macrophages of the RBI (see figure).
In vivo cell labeling with a photoactivatable cell tracer demonstrates the double potential of the primitive (rostral) myeloid progenitors. See the complete figure in the article beginning on page 132.
In vivo cell labeling with a photoactivatable cell tracer demonstrates the double potential of the primitive (rostral) myeloid progenitors. See the complete figure in the article beginning on page 132.
These novel findings of a unique population of neutrophils derived from primitive macrophages are contrary to the dogma of mammalian myelopoiesis and provide direction for future critical investigations. Does this unique population exist in the human neonate, providing a partial source for their immaturity in phagocytic immunity?4 What are the molecular mechanisms regulating this developmental process, and can they be exploited for ex vivo human myelopoiesis? What, if any, is the role of this population of neutrophils in tissue injury, repair, and defense in humans? Le Guyader et al have shed a new light on the origin and regulation of developmental vertebrate myelopoiesis.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal