Abstract
FLT3 internal tandem duplication (FLT3/ITD) is a common somatic mutation in acute myeloid leukemia (AML) with significant variation in the position, length, and number of duplications of the FLT3 gene. We evaluated these physical characteristics in FLT3/ITD-positive patients who were treated on CCG-2941/2961 and correlated them with clinical outcome. Fiftynine of 77 FLT3/ITD-positive patients (77%) had a single ITD, 16 (21%) had 2 ITDs, and 2 (3%) had 3 ITDs. The length of the duplicated region varied from 6 to 51 amino acids, and in all cases amino acid residues Y591–Y597 were duplicated. Structural analysis demonstrated that Y591–Y597 encodes the switch and zipper regions of the juxtamembrane domain of FLT3. In addition, 24 of 77 patients (31%) had duplication of the critical STAT5 docking sites Y589/591. Patients with longer ITDs had a worse relapse-free survival (19% vs 51%, P = .035), while the presence of more than 1 ITD was not clinically significant. Physical characteristics including the length of FLT3/ITD may influence FLT3 activation state by altering its structure and may impact response to therapy.
Introduction
FLT3 internal tandem duplication (FLT3/ITDs) is present in approximately 15% of pediatric and 25% of adult acute myeloid leukemia (AML) patients1-5 and has been associated with inferior outcome.1-3,6,7 However, nearly one-fourth of FLT3/ITD-positive patients survive, suggesting biological and clinical variation among FLT3/ITD-positive patients. FLT3/ITD-positive patients may be risk stratified using mutant/wild-type allelic ratios (ITD-AR).3,8 Other physical characteristics of FLT3/ITD, including length and position of the tandem duplication within the juxtamembrane domain, have been linked to clinical and biologic variation, where a duplication of a single codon may alter biologic activity of the FLT3 gene.9-11 We previously reported that in adults with AML, patients with ITDs of more than 40 nucleotides had a lower complete remission (CR; 35% vs 67%) and worse survival (13% vs 26%) compared with those with shorter ITDs.9 In this report we examine the variability in ITD length, number of ITDs, and the position of the duplication in FLT3 coding sequence and correlate these parameters with clinical outcome in pediatric AML.
Methods
Patients and mutation analysis
The study population has been detailed elsewhere.12 In brief, available samples from 647 patients age 0 to 21 years, enrolled on CCG 2941 and CCG-2961, were tested for FLT3/ITD. FLT3/ITD screening, allelic ratio (ITD-AR) determination, and sequencing was performed as previously described.6,8,13 Informed consents for biology studies were obtained in accordance with the Declaration of Helsinki at study enrollment. This study was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Crystal structure analysis
Statistical methods
Data were analyzed from CCG-2941 and CCG-2961 through April 2005 and June 2005, respectively. Statistical analysis of outcome differences and end points analyzed has been detailed previously.8
Results and discussion
Characteristics of the region of duplication in FLT3/ITD
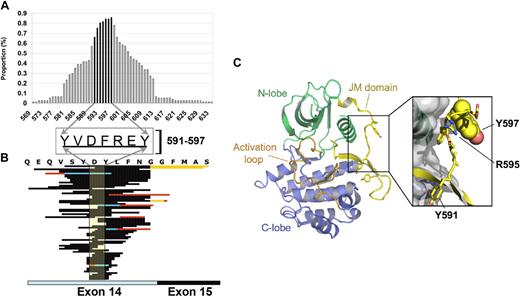
Recent work has highlighted several functional moieties within exon 14 of the FLT3 gene where specific amino acid (aa) residues are involved in receptor activation. As these functional motifs of FLT3 are variably involved in FLT3/ITD, we evaluated the variation of FLT3/ITD in a cohort of pediatric AML patients. FLT3/ITD was identified in 77 of 630 patients tested (12%) and their ITD allelic ratio (ITD-AR, ITD to wild-type ratio) determined as previously described.8 Fifty-nine patients (77%) had a single duplication. The remaining 18 patients (23%) had either 2 ITDs (N = 16) or 3 ITDs (N = 2). All duplications involved exon 14 of the FLT3 gene, and in 5 patients the duplication extended through intron 14 into exon 15. Forty of the 77 patients had a tandem duplication that was identical to native FLT3 coding sequencing, whereas the remaining 37 patients had an insertion of 3 to 21 base pairs (median, 9 base pairs) preceding the duplication. The length of the ITDs ranged from 15 to 174 base pair (bp) with a median of 52 bp. The genomic localization of the duplication varied from patient to patient and overall spanned the entire length of exon 14 (Figure 1). Duplication of at least 1 of 7 aa residues from codon 591 to 597 was observed in all patients, and codon 597 was duplicated in 86% of the patients. In 5 cases, the duplication spanned intron 14 and involved exon 15 and codons 613 to 631. Vempati et al have reported similar results in a cohort of adult patients and have demonstrated that R595 is critical in ITD function.11
Region of duplication in FLT3/ITD. (A) Proportion of patients with involvement of specific aa residues in the duplicated region. Codons 591 to 597 are shown in darker bars, and specific aa residues in this region are shown in the center inset. (B) The region of duplication in all 77 patients are sorted by ITD length (longest to shortest) from top to bottom. The area of duplication of the major ITD peak is shown in dark highlighted area. In patients with additional ITDs, the duplicated region is shown in red with the area of overlap in blue. Codons 591 to 597 are shown in shaded box. (C) FLT3 structure showing the location of the JM domain and Y597. Ribbon representation of the crystal structure of FLT3 previously solved by Griffith et al.14 The kinase domain N-lobe is colored green and the C-lobe, blue. The activation loop is colored orange and the JM domain, yellow. Tyrosines 597, 591, and 589 are shown in stick format. The exploded view shows the molecular surface of the kinase domain and JM domain side-chain residues Y591 to Y597. Tyrosine 597 is depicted with space-filling spheres. This figure was made using the program Pymol (www.pymol.org).
Region of duplication in FLT3/ITD. (A) Proportion of patients with involvement of specific aa residues in the duplicated region. Codons 591 to 597 are shown in darker bars, and specific aa residues in this region are shown in the center inset. (B) The region of duplication in all 77 patients are sorted by ITD length (longest to shortest) from top to bottom. The area of duplication of the major ITD peak is shown in dark highlighted area. In patients with additional ITDs, the duplicated region is shown in red with the area of overlap in blue. Codons 591 to 597 are shown in shaded box. (C) FLT3 structure showing the location of the JM domain and Y597. Ribbon representation of the crystal structure of FLT3 previously solved by Griffith et al.14 The kinase domain N-lobe is colored green and the C-lobe, blue. The activation loop is colored orange and the JM domain, yellow. Tyrosines 597, 591, and 589 are shown in stick format. The exploded view shows the molecular surface of the kinase domain and JM domain side-chain residues Y591 to Y597. Tyrosine 597 is depicted with space-filling spheres. This figure was made using the program Pymol (www.pymol.org).
Structural location of Y597
The crystal structure of autoinhibited FLT3 has revealed distinct regions within the JM domain, the JM binding motif (JM-B, Y572–M578), the JM switch motif (JM-S, V579–V592), and the JM zipper (JM-Z, D593–W60314 ; PDB code 1RJB). The amino acid residues that were observed in ITDs in all patients in this study (Y591–Y597) fall within the JM-S and JM-Z regions (Figure 1C). Tyrosine-597 is exposed on the surface of the protein and does not seem to be critical for maintenance of the autoinhibited conformation of FLT3 seen in the crystal structure. In this study, the residue most frequently observed within an ITD is Y597, however, other residues of the JM domain zipper region (JM-Z) are also frequently duplicated. These duplications are strongly transforming and result in constitutive activation of FLT3. The structural mechanisms of FLT3 activation by these duplications has not yet been elucidated, however, the crystal structure of autoinhibited FLT3 does shed some light on potential activating mechanisms. In this crystal structure the JM-Z region seems to play an important role in directing an optimal orientation of the autophosphorylation “switch” residues Y589 and Y591 and maintaining the autoinhibited conformation. JM-Z ITD insertions are expected to disrupt the autoinhibited conformation of the JM switch (JM-S) region, so preventing proper kinase inhibition by the JM binding (JM-B) region.11,14,18 Interruption of the optimal orientation of Y589 and Y591 is also expected to allow their constitutive phosphorylation. The reason for the prevalence of ITD mutations in the JM-Z region of the JM domain, compared with the JM-S and JM-B regions, has, however, not yet been elucidated.
Prognostic implication of FLT3/ITD length
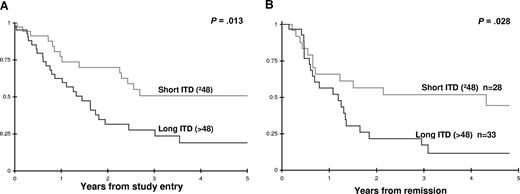
We inquired whether ITD length may impact clinical outcome. We evaluated all ITD-length thresholds between the 10th and 90th quantiles for the ability to differentiate clinical outcome between those with shorter or longer than the particular ITD length. Cox regression analysis was used to compare the survival from diagnosis and relapse from CR for patients with higher or lower than a specific ITD-length threshold. ITD-length threshold of 48 bp had a hazard ratio (HR) of 2.3 for worse overall survival (OS) from study entry (P = .013) with no difference in CR rate (82% vs 81%, P = .927). Actuarial OS at 4 years from study entry for those with shorter ITD (≤ 48 bp) was 51% (± 19%) compared with 17% (± 15%) for those with ITD-length more than 48 bp (P = .011, Figure 2A). Actuarial OS at 4 years from CR for those with a shorter ITD was 67% (± 21%) versus 21% (± 19%) for those with longer ITDs (P = .006). Relapse-free survival at 4 years from CR for those with shorter and longer ITDs was 51% (± 23%) and 19% (± 16%), respectively (P = .035, Figure 2B). Corresponding OS and relapse-free survival (RFS) for patients without FLT3/ITD was 54% and 57%, respectively, similar to those with shorter ITDs. Thus, in FLT3/ITD-positive patients, those with shorter ITDs have a lower relapse rate and more favorable outcome than those with longer ITDs. Although we have demonstrated the significance for ITD length in adult9 and pediatric AML, other studies have not demonstrated such outcome differences based on ITD length,19,20 suggesting that additional factors (eg, nature of duplicated moieties) may modify the significance of ITD length.
Clinical outcome for FLT3/ITD-positive patients with high versus low ITD length based on ITD length threshold of 48 bp. (A) Overall survival from study entry. (B) Relapse-free survival from complete remission.
Clinical outcome for FLT3/ITD-positive patients with high versus low ITD length based on ITD length threshold of 48 bp. (A) Overall survival from study entry. (B) Relapse-free survival from complete remission.
Clinical implications of duplication of Y589–591
Recent studies have identified 2 candidate STAT5 docking sites within the FLT3 juxtamembrane domain coding region that can be disrupted by ITD. Tyrosine to phenylalanine substitution of residues 589 and 591 in the context of the FLT3-ITD maintains tyrosine kinase activity but abrogates STAT5 activation and fails to induce a myeloproliferative process in mice.10 We tested whether involvement of 589 to 591 codons in ITDs correlated with clinical outcome.
In 53 of 77 patients (69%) the ITDs involved the codons 589 to 591. Response to therapy was evaluated in those with and without 589 to 591duplication. The remission induction rate for those with or without Y589/591 duplication was similar (88% vs 93%, P = .6). Relative risk of induction failure or post-remission relapse was 80% for those with Y589 to 591 duplication compared with 54% for those without (P = .7) The corresponding RFS at 5 years from CR was 26% and 46% for those with or without Y589/591 involvement, respectively (P = .42). There was no statistically significant difference in outcome for those with or without Y589/591 duplication, and any perceived difference needs to be evaluated in a larger study population.
Prognostic significance of presence of multiple ITDs
There have been suggestions that patients with multiple ITDs have a worse outcome than those with a single ITD.2 We evaluated whether those with multiple ITDs have a different outcome compared with those with a single ITD. Fifty-nine of the 77 patients (77%) had a single duplication, and the remaining 18 patients (23%) had either 2 ITDs (N = 16) or 3 ITDs (N = 2). Overall survival at 5 years from study entry for those with 1 ITD versus more than 1 ITD was 30% and 31% (P = .52), with a corresponding relapse rate at 5 years from remission of 70% and 69% (P = .35), demonstrating that the presence of multiple ITDs does not carry prognostic significance in this clinical context.
This study suggests that the length of the ITD may be important in disease prognosis. Whether it is the length of the ITD or the involvement of specific residues need yet to be clarified. It is possible that longer ITDs may involve multiple functional domains and more effectively disrupt the autoinhibitory regulation of the kinase activity. It is interesting to note that the lengths of the 3 JM regions, JM-B, JM-S, and JM-Z, are 7, 14, and 11 amino acids in length; a JM domain ITD length greater than 14 amino acids will necessarily duplicate residues from more than one of these regions, disrupting more than one of the structural requirements for autoinhibition. Thus, it is feasible that simultaneous disruption of more than one of the JM domain regions could underlie the prognostic significance of ITD length. Further studies are needed to elucidate the functional dependencies of the JM domain regions on one another. We also show that STAT5 binding sites are duplicated in one-third of the cases and may impact outcome, although the latter does not meet statistical significance. Collectively, these findings indicated that among the physical parameters that delineate between various FLT3-ITDs, increased length of the ITD is a poor prognostic indicator and may warrant alternative approaches to therapy in this cohort of pediatric patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institute of Health grants nos. K23 CA92405, CA18029, CA32102, CA114563, R01 CA114563, and R21 CA102624. T.J.B. is the recipient of an American Society of Hematology Junior Faculty Scholar Award.
National Institutes of Health
Authorship
Contribution: S.M. designed the study, contributed to analysis, and wrote manuscript. D.L.S. assisted in data analysis and assisted in writing the manuscript. T.A.A., senior statistician, assisted in statistical analysis and interpretation of data. T.J.B. provided structural analysis and wrote the paper. R.B.G., statistician, performed statistical analysis. J.L.R. assisted in design of the study. B.J.L., clinical study PI, assisted in data analysis. D.G.G. assisted in design of study. J.P.R. provided valuable resources, assisted in data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Soheil Meshinchi, Fred Hutchinson Cancer Research Center, Clinical Research Division, D5-380, 1100 Fairview Ave N, Seattle, WA 98103; e-mail: smeshinc@fhcrc.org.