Abstract
Pathological angiogenesis associated with wound healing often occurs subsequent to an inflammatory response that includes the secretion of cytokines such as tumor necrosis factor (TNF). Controversy exists on the angiogenic actions of TNF, with it being generally proangiogenic in vivo, but antiangiogenic in vitro. We find that whereas continuous administration of TNF in vitro or in vivo inhibits angiogenic sprouting, a 2- to 3-day pulse stimulates angiogenesis by inducing an endothelial “tip cell” phenotype. TNF induces the known tip cell genes platelet-derived growth factor B (PDGFB) and vascular endothelial cell growth factor receptor-2 (VEGFR2), while at the same time blocking signaling through VEGFR2, thus delaying the VEGF-driven angiogenic response. Notch signaling regulates tip cell function, and we find that TNF also induces the notch ligand jagged-1, through an NFκB-dependent mechanism. Enrichment of jagged-1 in tip cells was confirmed by immunofluorescent staining as well as by laser capture microdissection/quantitative reverse-transcription–polymerase chain reaction (qRT-PCR) of tip cells sprouting in vitro. Thus, in angiogenesis, the temporal expression of TNF is critical: it delays angiogenesis initially by blocking signaling through VEGFR2, but in addition by inducing a tip cell phenotype through an NFκB-dependent pathway, it concomitantly primes endothelial cells (ECs) for sprouting once the initial inflammatory wave has passed.
Introduction
Neovascularization, or the formation of new blood vessels, is a critical component of many physiologic as well as pathologic conditions, including development, reproduction, wound healing, diabetic retinopathy, and tumor growth. During wound healing, new vessel growth by angiogenesis is a necessary early step in rebuilding tissue, however the coordination of angiogenesis with the resolution of the acute inflammatory stage is not well understood. The earliest events after tissue damage include the generation of a fibrin clot and the bursting of platelets to release numerous growth factors. Fibrin provides a provisional matrix that promotes the accumulation of blood-derived monocytes that then differentiate into tissue macrophages. Activated macrophages synthesize several cytokines, including tumor necrosis factor (TNF), which activate local endothelial cells (ECs) and promote leukocyte recruitment. After 3 to 4 days, when the initial infection has been cleared, there is a switch toward tissue repair and concomitant with this is the acceleration of angiogenesis.1,2
TNF is a major inflammatory mediator that induces multiple changes in EC gene expression including induction of adhesion molecules, integrins, and matrix metalloproteinases (MMPs). Its effects on angiogenesis have been the subject of some controversy. For example, TNF blocks EC proliferation and migration in vitro3-5 and has been reported to down-regulate activity6 and expression7,8 of vascular endothelial cell growth factor receptor-2 (VEGFR2). On the other hand, TNF has also been shown to up-regulate VEGFR2 expression9 and promote EC migration.10 In vivo the situation is no clearer: TNF promotes angiogenesis in the cornea,3,11 whereas loss of TNFR1 (p55 receptor) leads to enhanced angiogenesis in both retina12 and wounded skin.13 Further studies with TNF receptor–deficient mice have demonstrated enhanced hind limb angiogenesis after temporary ischemia in TNFR1-knockout (KO) mice, but reduced angiogenesis in TNFR2-KO mice.14
Angiogenesis is a multistep process15-19 involving EC degradation of the adjacent basement membrane, migration (sprouting), proliferation, alignment, tube formation, branching, anastomosis, synthesis of new basement membrane, recruitment of parenchymal cells, and a return to quiescence.15,16,19,20 It is possible that TNF may differentially affect each of these steps. Associated with many of these stages are ECs of distinct phenotype, for example, tip cells, which are highly migratory and lead the extending sprout through the extracellular matrix (ECM), and trunk cells, which form the vessel lumen and recruit support cells. While much is known about growth factors that drive angiogenesis, far less is known about the mechanisms underlying these phenotypic differences. Recently, we and others have shown that notch1 is required in cells autonomously for proper vessel formation21,22 and that notch-delta–like 4 (dll4) signaling blocks branching, and helps define the nonproliferative tip cell phenotype.22-27 In addition, mice lacking notch1,28,29 or the ligands jagged-130 or dll4,31,32 die in utero of vascular defects.
Here we have tested the hypothesis that it is the duration of TNF signaling that critically determines whether an antiangiogenic or proangiogenic response is elicited and find that a 2- to 3-day pulse of TNF primes ECs for rapid sprouting once the TNF is removed. Furthermore, we show that in vitro TNF induces a tip cell phenotype, marked by expression of a distinct set of genes, including the notch ligand jagged-1, and show that this involves the NFκB pathway.
Methods
Cell culture and transfections
Human umbilical vein endothelial cells (HUVECs) were isolated by standard protocols as discarded tissue under University of California (UC) Irvine Institutional Research Board approval, and all experiments were performed with HUVECs at P2 to P4. No identifiers of any kind were collected. ECs were transfected using Lipofectamine 2000. Efficiencies were consistently higher than 50% (and often higher than 70%) as measured by fluorescence-activated cell sorting (FACS) analysis of GFP expression. The constitutively active inhibitor of κB kinase (CA-IKKβ) and the dominant-negative form (DN-IKKβ) were kind gifts of Dr Craig Walsh (UC Irvine).
Fibrin gel angiogenesis assay
Cultures were established and maintained as described previously.33,34 A detailed protocol is available on request. Fibroblasts were a kind gift of Dr Cory Hogaboam (University of Michigan). TNF (R&D Systems, Minneapolis, MN) was added as indicated.
Microvessels were stained in situ after removal of fibroblasts. Cultures were fixed for 10 minutes in 10% formalin and permeabilized with 0.5% Triton X-100 for 5 minutes. Nonspecific binding was blocked using a solution of 5% BSA in PBS for 2 hours. For nuclear staining, DAPI (Sigma-Aldrich, St Louis, MO) was used at a concentration of 1 μg/mL. Antibody to jagged-1 (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:100 dilution. Labeling was detected using an Alexa Fluor 488–conjugated antimouse (1:1000 dilution; Molecular Probes/Invitrogen, Carlsbad, CA). Isotype-specific nonbinding antibody was used as a control. Images were captured on an Olympus IX70 inverted phase contrast/fluorescence microscope (Melville, NY) and an Optronics digital camera (Goleta, CA). High-resolution images were magnified in Adobe Photoshop (Adobe Systems, San Jose, CA) and contrast was adjusted. No other alterations were made. In some cases, confocal fluorescence images were captured on a Carl Zeiss MicroImaging LSM 510 Meta microscopic system (Heidelberg, Germany).
Quantification of vessels in vitro
High-resolution digital images of beads were captured on an IX70 Olympus microscope with a 4×/0.13 NA objective. The increased depth of field attained when capturing images at low magnification results in all of the sprouts being in focus. Images were then magnified in Adobe Photoshop and analyzed in National Institutes of Health (NIH) ImageJ (Bethesda, MD). The number of sprouts per bead was counted, and a minimum of 20 beads were counted for each experimental group. Only sprouts with a minimum length of one bead diameter were included.
Matrigel plug assay
Six-week-old Balb/c mice were injected subcutaneously in the back with 500 μL Matrigel (BD Biosciences, San Jose, CA) supplemented with 10 ng/mL human TNF-α or PBS. Mice then received daily injections of TNF-α or PBS, directly into the gel as indicated. TNF-α (10 ng/mL) or PBS was injected in a total volume of 100 μL. Mice were killed at the indicated times and Matrigel plugs were removed for analysis. All experiments were performed under protocols approved by the UC Irvine Institutional Animal Care and Use Committee. Cryostat sections were stained with rat anti–mouse CD31-biotin–labeled primary antibody (BD Biosciences) followed by streptavidin-Texas Red secondary antibody (Vector Laboratories, Burlingame, CA) and DAPI.
Preparation of peripheral blood mononuclear cell supernatants
Peripheral blood mononuclear cells (PBMCs) were isolated from human blood according to protocols approved by the UC Irvine Institutional Review Board for Human Subjects. Informed consent was obtained in accordance with the Declaration of Helsinki. Cells were resuspended in EGM-2 and 107 cells/well were plated in BD Falcon 6-well plates (BD Biosciences) precoated with or without 1 μg/mL α-CD3ϵ MAb (R&D Systems) and in the presence or absence of 0.5 μg/mL α-CD28 MAb (BD Biosciences). After 48 hours, cell-free supernatants were collected and used as described.
Laser capture microdissection
Bead-containing gels of approximately 500 μL volume were lightly trypsinized to remove fibroblasts (> 99% efficient), fixed in 70% ethanol, and then sequentially dehydrated. Once in 100% ethanol, gels were transferred to 2.0 μm PEN-Membrane slides (Leica, Deerfield, IL) and dried down. The dried gels were approximately 50 to 150 μm thick. Tip cells and trunk cells were identified by location and excised using a Leica LS-LMD system. Microdissected cells were collected and dissolved in TRIZOL. Samples were frozen at − 80°C until roughly 500 cells were collected, and then pooled for cDNA synthesis. Prior to RNA purification, 1 μg tRNA was added to each sample. RNA was isolated and purified according to the manufacturer's directions and standard cDNA synthesis methods were used.
Analysis of gene expression by RT-PCR and qRT-PCR
ECs were cultured in 6-well plates. Where indicated, the following inhibitors were used: p38 inhibitor (SB 203580; Calbiochem, San Diego, CA), used at 0.5 μM, which is 10-fold higher than its IC50; and NFκB inhibitor QNZ (6-amino-4-(4-phenoxyphenylethylamino) quinazoline; Calbiochem), which was used at 100 nM, which is also 10-fold higher than its IC50. Reverse-transcription–polymerase chain reaction (RT-PCR) and quantitative RT-PCR (qRT-PCR) experiments were according to protocols described previously.35 Primers and annealing conditions are available on request. Blocking antibodies to TNF-R1 and TNF-R2 were from R&D Systems and were used at 5× their median neutralization dose (ND50).
Statistical analysis
The differences between experimental groups of equal variance were analyzed using Student t test with P values less than .05 being considered significant. All experiments were performed at least 3 times (except where indicated) with similar results.
Results
A pulse of TNF primes ECs for sprouting
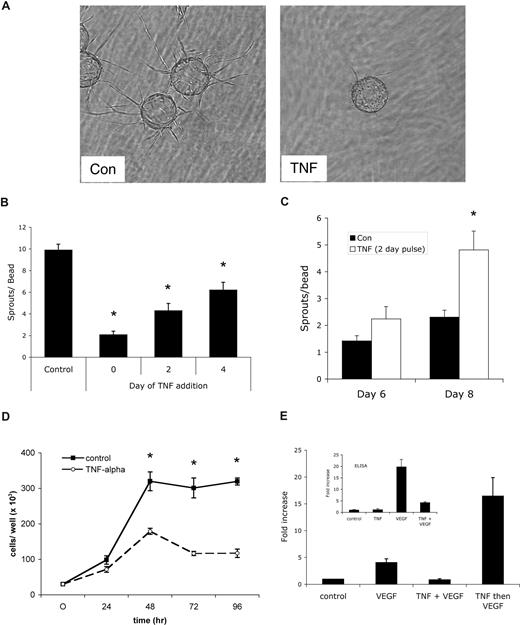
To test the effect of persistent versus pulsed TNF on sprouting angiogenesis, we turned first to an in vitro assay we have developed that uses EC-coated beads embedded in fibrin gels. In this assay, capillary-like sprouts appear by days 1 to 2, lumen formation begins around days 4 to 5, and over the course of 10 to 20 days extensive networks of anastomosed, lumenized capillaries are formed.33,34 Addition of TNF (10 ng/mL) to the fibrin gel assay dramatically blocked sprouting (Figure 1A,B), consistent with reports on the effects of TNF in other in vitro angiogenesis assays.3-5 Delayed addition of TNF resulted in a quantitative reduction in the number of sprouts generated (Figure 1B). Interestingly, however, when cultures were treated with a pulse of TNF for 2 days, followed by fresh medium, and the number of sprouts was counted 4 to 6 days later, there were more than twice as many sprouts by day 8 in TNF-pretreated than in control cultures never receiving TNF. Thus, removal of TNF after 2 days led to robust sprouting that was more vigorous than in cultures that had never seen TNF (Figure 1C). A pulse of TNF between days 2 and 4 also induced sprouting, however, a later pulse had no effect, likely because most of the sprouts had already been primed and initiated (data not shown). We also noted that later addition of TNF, which slows sprouting, did not regress already formed vessels (data not shown). It has previously been reported that TNF down-regulates VEGFR2 activation,6 and thereby blocks EC proliferation. To confirm the antiproliferative effect of TNF on ECs, cells were cultured for 96 hours with or without TNF, and cells were harvested for counting every 24 hours. As shown in Figure 1D, TNF reduced EC proliferation by more than 60%.
A pulse of TNF promotes angiogenesis in vitro. (A) EC-coated beads in fibrin gels were treated with TNF (10 ng/mL) or PBS control and photographed after 4 days on an Olympus IX70 inverted phase-contrast microscope using a 4×/0.13 NA objective and an Optronics digital camera. High-resolution images were magnified in Photoshop. Control cultures showed robust sprouting, whereas TNF almost completely blocked sprouting. One of multiple similar experiments. (B) Cultures were established as in panel A and TNF was added at day 0, 2, or 4. The number of sprouts was counted at day 6. Shown are means and SD; *P < .005 relative to control, by Student t test. One of 2 similar experiments. (C) Cultures were established as above and treated for 2 days with or without TNF (10 ng/mL). TNF-containing medium was then removed and fresh medium lacking TNF was added every 2 days. Sprouts were counted at the indicated times. Shown are means and SD; *P < .005 relative to control, by Student t test. One of 3 similar experiments. (D) ECs (4 × 104) were plated in 24-well plates in the presence or absence of TNF (10 ng/mL) and counted at the indicated times. *P < .005 relative to control, by Student t test. One of 2 similar experiments. (E) TNF blocks VEGF signaling acutely. ECs were pretreated with TNF (10 ng/mL) for 2 days, rested for 1 day, and then given VEGF (10 ng/mL) for 4 hours (TNF then VEGF), or were given TNF for 3 days and then TNF + VEGF for the last 4 hours (TNF + VEGF), or were given VEGF alone for 4 hours (VEGF) or left untreated (control). RNA was harvested for analysis of α2-macroglobulin expression by qRT-PCR. Supernatants were also collected from an independent experiment for analysis of α2-macroglobulin protein level by enzyme-linked immunosorbent assay (ELISA) (inset).
A pulse of TNF promotes angiogenesis in vitro. (A) EC-coated beads in fibrin gels were treated with TNF (10 ng/mL) or PBS control and photographed after 4 days on an Olympus IX70 inverted phase-contrast microscope using a 4×/0.13 NA objective and an Optronics digital camera. High-resolution images were magnified in Photoshop. Control cultures showed robust sprouting, whereas TNF almost completely blocked sprouting. One of multiple similar experiments. (B) Cultures were established as in panel A and TNF was added at day 0, 2, or 4. The number of sprouts was counted at day 6. Shown are means and SD; *P < .005 relative to control, by Student t test. One of 2 similar experiments. (C) Cultures were established as above and treated for 2 days with or without TNF (10 ng/mL). TNF-containing medium was then removed and fresh medium lacking TNF was added every 2 days. Sprouts were counted at the indicated times. Shown are means and SD; *P < .005 relative to control, by Student t test. One of 3 similar experiments. (D) ECs (4 × 104) were plated in 24-well plates in the presence or absence of TNF (10 ng/mL) and counted at the indicated times. *P < .005 relative to control, by Student t test. One of 2 similar experiments. (E) TNF blocks VEGF signaling acutely. ECs were pretreated with TNF (10 ng/mL) for 2 days, rested for 1 day, and then given VEGF (10 ng/mL) for 4 hours (TNF then VEGF), or were given TNF for 3 days and then TNF + VEGF for the last 4 hours (TNF + VEGF), or were given VEGF alone for 4 hours (VEGF) or left untreated (control). RNA was harvested for analysis of α2-macroglobulin expression by qRT-PCR. Supernatants were also collected from an independent experiment for analysis of α2-macroglobulin protein level by enzyme-linked immunosorbent assay (ELISA) (inset).
To confirm previous reports that TNF directly blocks VEGFR2 signaling, we analyzed VEGF induction of α2-macroglobulin in the presence or absence of TNF. Previous microarray data had shown that α2-macroglobulin is a direct target of VEGF signaling in ECs, showing strong up-regulation at 4 hours (M.T.H. and C.C.W.H., unpublished observations, May 2004). We therefore assessed α2-macroglobulin expression by ECs either pulsed with TNF and then given VEGF or stimulated with VEGF in the presence of TNF. As predicted, the presence of TNF completely blocked the ability of VEGF to induce α2-macroglobulin expression, both at the mRNA and protein level (Figure 1E). Interestingly, however, a pulse of TNF, followed by VEGF stimulation in the absence of TNF, augmented α2-macroglobulin mRNA by 4-fold compared with VEGF stimulation of non–TNF-pulsed cells (Figure 1E). This is entirely consistent with the effect of a TNF pulse on sprouting and correlates with TNF induction of VEGFR2 expression (see “TNF induces an endothelial tip cell phenotype”). Thus, TNF acts downstream of VEGF to block expression of a VEGF target gene.
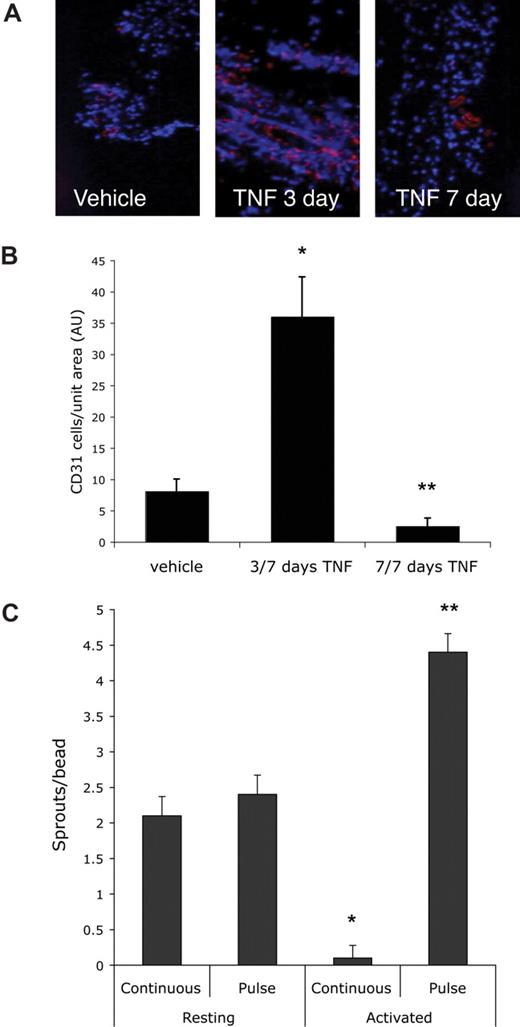
To test whether a defined pulse of TNF had similar effects in vivo, we used the Matrigel plug assay. Mice received a subcutaneous injection of Matrigel (500 μL) and one day later were split randomly into 3 groups of 5 mice each. One group received daily injections of PBS directly into the gel. The second group received daily injections of 10 ng/mL TNF, while the third group received TNF for the first 3 days followed by 4 days of PBS. All mice were killed after 7 days and skin/gel was harvested for immunohistochemical analysis using a CD31 antibody to detect blood vessels (Figure 2A). The continuous presence of TNF suppressed angiogenesis by 70%, similar to what was seen in vitro. In sharp contrast, in mice that received TNF for 3 days and PBS for the next 4, angiogenesis was enhanced 4.7-fold compared with control, and almost 14-fold compared with continuous TNF (Figure 2B). Thus the in vivo findings confirm our in vitro data that whereas continuous TNF is antiangiogenic, a pulse of TNF primes ECs and is proangiogenic.
A pulse of TNF promotes angiogenesis in vivo. Mice (15) were injected intradermally with Matrigel (500 μL) and then split randomly into 3 groups. One group received daily injections into the gel of PBS for 7 days, one group received TNF (1 ng) for 3 days, followed by PBS for 4 days, and the third received daily injections of TNF (1 ng) for 7 days. Skin containing the gel was then harvested and examined by immunohistochemistry for the presence of blood vessels (CD31: pink) and nuclei (DAPI: blue). Images were captured on an Olympus IX70 inverted phase contrast/fluorescence microscope using a 40×/0.60 NA fluorescence objective and an Optronics digital camera. High-resolution images were magnified in Photoshop. (A) Representative sections from each of the groups. (B) The number of ECs present was determined by counting pink (CD31+) cells and this was normalized to area of tissue as determined by DAPI staining. Shown are means and SD of CD31+ cells per unit area. *P < .005 relative to vehicle control; **P < .05 relative to vehicle control, by Student t test. (C) EC-coated beads were established in fibrin gels and incubated with conditioned medium (CM) from either resting or activated PBMCs (CD3 + CD28 for 48 hours). Cultures either received a pulse of CM (2 days of “activated” CM followed by 4 days of “resting” CM) or continuous CM (resting or activated). All cultures had medium changed every 2 days. At 8 days sprouts were counted. Means and SD are shown. *P < .001 for resting compared with activated/continuous; **P < .001 for resting compared with activated/pulse, by Student t test. One of 3 similar experiments.
A pulse of TNF promotes angiogenesis in vivo. Mice (15) were injected intradermally with Matrigel (500 μL) and then split randomly into 3 groups. One group received daily injections into the gel of PBS for 7 days, one group received TNF (1 ng) for 3 days, followed by PBS for 4 days, and the third received daily injections of TNF (1 ng) for 7 days. Skin containing the gel was then harvested and examined by immunohistochemistry for the presence of blood vessels (CD31: pink) and nuclei (DAPI: blue). Images were captured on an Olympus IX70 inverted phase contrast/fluorescence microscope using a 40×/0.60 NA fluorescence objective and an Optronics digital camera. High-resolution images were magnified in Photoshop. (A) Representative sections from each of the groups. (B) The number of ECs present was determined by counting pink (CD31+) cells and this was normalized to area of tissue as determined by DAPI staining. Shown are means and SD of CD31+ cells per unit area. *P < .005 relative to vehicle control; **P < .05 relative to vehicle control, by Student t test. (C) EC-coated beads were established in fibrin gels and incubated with conditioned medium (CM) from either resting or activated PBMCs (CD3 + CD28 for 48 hours). Cultures either received a pulse of CM (2 days of “activated” CM followed by 4 days of “resting” CM) or continuous CM (resting or activated). All cultures had medium changed every 2 days. At 8 days sprouts were counted. Means and SD are shown. *P < .001 for resting compared with activated/continuous; **P < .001 for resting compared with activated/pulse, by Student t test. One of 3 similar experiments.
Inflammatory responses generate a myriad of cytokines, and some of these may have proangiogenic or antiangiogenic effects. Thus, to mimic the complexity of an inflammatory milieu in vitro, we generated supernatants from activated peripheral blood mononuclear cells (PBMCs) and tested these for their ability to prime EC sprouting. Fresh supernatant from activated PBMCs was added every 2 days (10% vol/vol) to provide a continuous stimulus. To provide a pulse stimulus, medium from activated PBMCs was replaced on day 2 with medium from nonstimulated PBMCs and on alternate days thereafter. Supernatant from resting PBMCs, either used as a pulse or as a continuous stimulus, had no effect on sprouting (Figure 2C). In contrast, the continuous presence of supernatant from activated PBMCs suppressed sprouting by more than 95%. Consistent with our previous data, a pulse of the same supernatant increased sprouting by 2-fold compared with supernatant from resting cells and more than 50-fold compared with the continuous stimulus.
TNF induces an endothelial tip cell phenotype
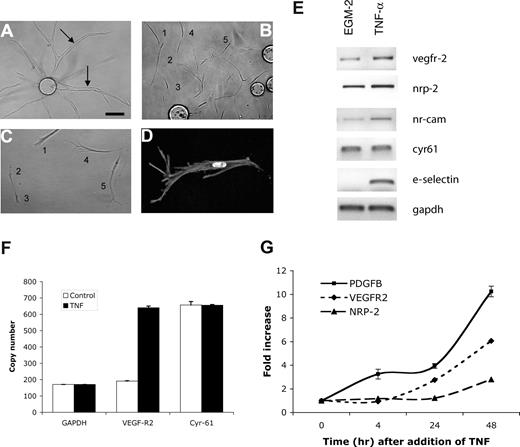
As angiogenic sprouts are led by a tip cell,22,36,37 we hypothesized that TNF treatment might induce tip cells, thereby promoting sprouting. To test this, we examined cultures after TNF treatment and removal. Control cultures showed long sprouts with clearly apparent lumens (Figure 3A arrows). In contrast, whereas a short treatment with TNF leads to enhanced sprouting, we found that a more prolonged treatment followed by removal resulted in the majority of ECs migrating away from the beads with a distinct tip cell phenotype (Figure 3B-D). These cells are clearly polarized in the direction of migration, lack lumens, and have long filopodia that in time-lapse movies are seen to probe the gel ahead of the migrating cell (R.C.A.S., M.T.H., and C.C.W.H., unpublished observations, February 2003). While cells appear to make occasional contacts, these are likely transitory, and no alignment or lumen formation, such as that seen in Figure 3A, is apparent. It appears, therefore, that TNF does indeed induce a tip cell phenotype in ECs, leading to enhanced sprouting once the cytokine is removed.
TNF induces an EC tip cell phenotype. EC-coated beads were established in fibrin gels and treated with (A) PBS or (B-D) TNF (10 ng/mL) for 6 days. Cells were then cultured for a further 4 days in the absence of TNF. (A) Sprouting and lumen formation in control-treated cultures. Lumens are indicated by arrows. (B) Individual migrating tip cells induced by TNF, showing no lumen formation. Some of the tip cells have been numbered to highlight that they are not part of an organized sprout but are migrating with only minimal, and likely transitory, contacts. (C) Higher-power view of 5 tip cells showing polarity and numerous filopodia, but no lumens. (D) High-power view of tip cell stained with phalloidin to show actin fibers, and DAPI to highlight nucleus. Images in panels A-D were captured on an Olympus IX70 inverted phase-contrast/fluorescence microscope using a 10×/0.30 or a 40×/0.60 fluorescence objective and an Optronics digital camera. High-resolution images were magnified in Photoshop. (E) ECs were cultured in the presence or absence of TNF (10 ng/mL) for 2 days and then harvested for analysis of gene expression by RT-PCR. One of 2 similar experiments. (F) Cells were cultured as for panel E, and gene expression was analyzed by qRT-PCR. Copy number was normalized to GAPDH. Means and SD for triplicate samples are shown. One of 3 similar experiments. (G) ECs were cultured in the presence of TNF (10 ng/mL) for the indicated times and then harvested for analysis of gene expression by qRT-PCR. Shown are means and SD for triplicate samples. One of 3 similar experiments.
TNF induces an EC tip cell phenotype. EC-coated beads were established in fibrin gels and treated with (A) PBS or (B-D) TNF (10 ng/mL) for 6 days. Cells were then cultured for a further 4 days in the absence of TNF. (A) Sprouting and lumen formation in control-treated cultures. Lumens are indicated by arrows. (B) Individual migrating tip cells induced by TNF, showing no lumen formation. Some of the tip cells have been numbered to highlight that they are not part of an organized sprout but are migrating with only minimal, and likely transitory, contacts. (C) Higher-power view of 5 tip cells showing polarity and numerous filopodia, but no lumens. (D) High-power view of tip cell stained with phalloidin to show actin fibers, and DAPI to highlight nucleus. Images in panels A-D were captured on an Olympus IX70 inverted phase-contrast/fluorescence microscope using a 10×/0.30 or a 40×/0.60 fluorescence objective and an Optronics digital camera. High-resolution images were magnified in Photoshop. (E) ECs were cultured in the presence or absence of TNF (10 ng/mL) for 2 days and then harvested for analysis of gene expression by RT-PCR. One of 2 similar experiments. (F) Cells were cultured as for panel E, and gene expression was analyzed by qRT-PCR. Copy number was normalized to GAPDH. Means and SD for triplicate samples are shown. One of 3 similar experiments. (G) ECs were cultured in the presence of TNF (10 ng/mL) for the indicated times and then harvested for analysis of gene expression by qRT-PCR. Shown are means and SD for triplicate samples. One of 3 similar experiments.
Tip cells have been reported to show enriched expression of a number of genes. VEGFR2 and PDGF-B are enriched in tip cells in retina,36 and the neuropilin receptors are required for guidance of these cells.38 To determine whether VEGFR2 and neuropilin-2, and the putative tip cell gene NrCAM,39 are induced in ECs by TNF treatment, we harvested RNA for RT-PCR analysis. All 3 genes showed robust induction, whereas the extracellular matrix protein cyr61, used as a control, was not responsive (Figure 3E). The vascular adhesion molecule E-selectin served as a positive control and was strongly induced by TNF. We repeated the analysis of VEGFR2 expression using qRT-PCR and confirmed a 3- to 4-fold increase in response to TNF. Again, cyr61 was not responsive (Figure 3F). The induction of VEGFR2 and nrp2 was relatively slow, with significant induction occurring only after 24 hours (Figure 3G). PDGF-B was also induced by TNF in ECs, with enhanced expression evident at 4 hours and rising to a 10-fold induction by 48 hours (Figure 3G). Thus, TNF induces both a tip cell phenotype and a subset of genes known to be associated with tip cells in vivo.
TNF up-regulates jagged-1 and down-regulates notch4 and notch target genes
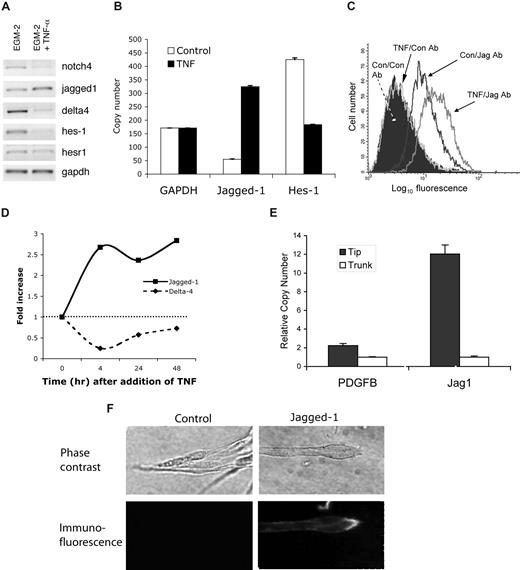
We previously reported a critical role for notch signaling in the maintenance of tip and trunk cell phenotype,22 and others have shown an enrichment of notch ligands at the leading edge of developing retinal vasculature.23 We therefore tested the hypothesis that TNF regulation of vessel sprouting and tip cell phenotype may include the modulation of notch pathway expression and activity. ECs were treated with TNF for 18 hours and then tested for gene expression by RT-PCR. Interestingly, expression of the notch ligand jagged-1 was increased, whereas expression of notch4, a largely vascular-specific notch, was strongly decreased (Figure 4A). This is consistent with findings in other systems where the up-regulation of a notch ligand results in the down-regulation of notch in that cell.40 Interestingly, the expression of a second notch ligand, dll4, was strongly reduced by TNF. Both HES1 and HESR1 are downstream targets of notch signaling, HESR1 being particularly important in the cardiovascular system.41-43 Consistent with a down-regulation of notch4 and an increase in jagged-1 expression, both notch target genes showed decreased expression in response to TNF (Figure 4A). We confirmed a 6-fold up-regulation of jagged-1 mRNA and a greater than 2-fold down-regulation of HES1 by qRT-PCR (Figure 4B). FACS analysis revealed low to absent expression of jagged-1 protein on the surface of resting cultured ECs; however, expression was strongly induced by TNF (Figure 4C). Induction of jagged-1 mRNA by TNF was rapid with maximal levels of message reached by 4 hours (Figure 4D). Expression of dll4 showed a similarly rapid decline. Blocking antibodies to TNF-R1 and TNF-R2 were used to determine which receptor is more important in mediating TNF induction of jagged-1. We found a 7.8-fold increase in jagged-1 mRNA with TNF treatment, and this was blocked by almost 40% by pretreatment with the anti–TNF-RI antibody (data not shown). Pretreatment with the TNF-R2 antibody had no effect on induction of jagged-1, consistent with TNF-R1 being the major mediator of TNF-NFκB signaling. Pretreatment with a combination of both antibodies gave similar blocking to TNF-R1 alone (46%).
TNF induces jagged-1 expression. (A) ECs were cultured in the presence or absence of TNF (10 ng/mL) for 2 days and then harvested for analysis of gene expression by RT-PCR. One of 3 similar experiments. (B) Cells were cultured as for panel A, and gene expression was analyzed by qRT-PCR. Copy number was normalized to GAPDH. Means and SD for triplicate samples are shown. One of 2 similar experiments. (C) ECs were cultured in the presence or absence of TNF (10 ng/mL) for 4 hours and then harvested for analysis of jagged-1 expression by FACS. An isotype-matched, nonbinding antibody was used as a control. (D) ECs were cultured in the presence of TNF (10 ng/mL) for the indicated times and then harvested for analysis of gene expression by qRT-PCR. Data were normalized to GAPDH. One of 3 similar experiments. (E) ECs were allowed to sprout into fibrin gels for 6 days at which time the gels were prepared for laser capture microdissection (LCM). Five hundred tip cells, and considerably more trunk cells, were captured and RNA was prepared for qRT-PCR. Expression of PDGFB and jagged-1 in tip cells versus trunk cells was determined by qRT-PCR. Data were normalized to GAPDH. Means and SD shown are for triplicate samples. For both genes, P < .01, tip versus trunk, by Student t test. (F) Sprouts in fibrin gels were stained in situ for jagged-1 expression. Phase-contrast images of the tips of sprouts are shown in the top panels. The corresponding immunofluorescent images are shown in the bottom panels. Control Ab staining on the left; jagged-1 staining on the right. Confocal fluorescence images were captured on a Carl Zeiss MicroImaging LSM 510 Meta microscopic system (10×/0.45 and 40×/1.20 Apochromat objectives).
TNF induces jagged-1 expression. (A) ECs were cultured in the presence or absence of TNF (10 ng/mL) for 2 days and then harvested for analysis of gene expression by RT-PCR. One of 3 similar experiments. (B) Cells were cultured as for panel A, and gene expression was analyzed by qRT-PCR. Copy number was normalized to GAPDH. Means and SD for triplicate samples are shown. One of 2 similar experiments. (C) ECs were cultured in the presence or absence of TNF (10 ng/mL) for 4 hours and then harvested for analysis of jagged-1 expression by FACS. An isotype-matched, nonbinding antibody was used as a control. (D) ECs were cultured in the presence of TNF (10 ng/mL) for the indicated times and then harvested for analysis of gene expression by qRT-PCR. Data were normalized to GAPDH. One of 3 similar experiments. (E) ECs were allowed to sprout into fibrin gels for 6 days at which time the gels were prepared for laser capture microdissection (LCM). Five hundred tip cells, and considerably more trunk cells, were captured and RNA was prepared for qRT-PCR. Expression of PDGFB and jagged-1 in tip cells versus trunk cells was determined by qRT-PCR. Data were normalized to GAPDH. Means and SD shown are for triplicate samples. For both genes, P < .01, tip versus trunk, by Student t test. (F) Sprouts in fibrin gels were stained in situ for jagged-1 expression. Phase-contrast images of the tips of sprouts are shown in the top panels. The corresponding immunofluorescent images are shown in the bottom panels. Control Ab staining on the left; jagged-1 staining on the right. Confocal fluorescence images were captured on a Carl Zeiss MicroImaging LSM 510 Meta microscopic system (10×/0.45 and 40×/1.20 Apochromat objectives).
Jagged-1 is enriched in endothelial tip cells
To examine spatial expression of jagged-1 in developing capillary-like sprouts in vitro, we turned to laser capture microdissection. Tip and trunk cells were captured and approximately 500 tip cells and considerably more trunk cells were individually pooled for qRT-PCR analysis and comparison with GAPDH (Figure 4E). Previous in situ hybridization studies have indicated that PDGFB is tip-cell specific,36 and we confirmed a 2- to 3-fold enhancement in tip cells in our assay. Jagged-1 was even more highly enriched in tip cells relative to trunk, showing a 10- to 15-fold enhancement (Figure 4E).
To confirm tip cell expression of jagged-1, we immunostained cultures of sprouting vessels. Jagged-1 was expressed at a low level along the trunk of the vessel but was greatly enriched in the tip cells (Figure 4F). We conclude from this series of experiments that in fibrin gels jagged-1 is a tip cell–enriched gene and that it is induced by TNF concomitant with ECs taking on a tip cell phenotype in a proinflammatory setting. We also stained sections from the vascular invasion assay described in Figure 2A, where Matrigel is injected subcutaneously; however, although jagged-1 staining was readily detectable, we could not unequivocally identify tip cells as it was not possible to distinguish between true tip cells and obliquely cut vessels. Finally, we also examined jagged-1 expression on tumor vessels as angiogenesis in this setting has some parallels to angiogenesis in wounds, and tumors have been described as wounds that do not heal. Again, although it was not possible to identify tip cells, we did find much higher jagged-1 expression on vessels in ovarian tumors than in normal ovary (data not shown).
TNF induces jagged-1 through an NFκB-dependent mechanism
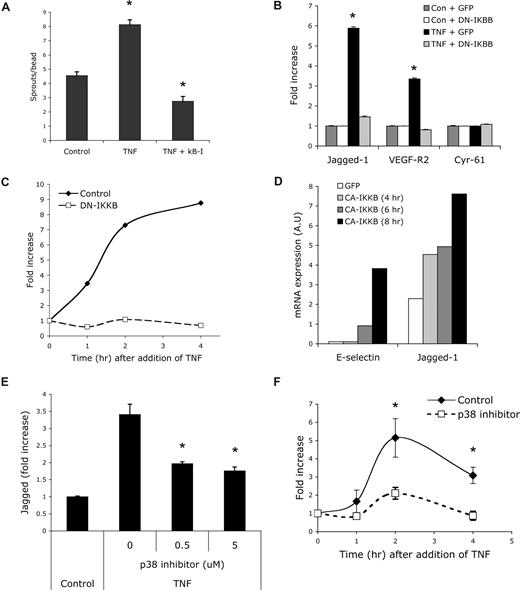
To explore further the induction of a tip cell phenotype by TNF, we took jagged-1 as a model gene and investigated the mechanism of its induction in ECs. First, we used a combination of chemical inhibitors and dominant-negative (DN) and constitutively active (CA) proteins. NFκB has been well characterized as a downstream target of TNF signaling,44 and an important role for NFκB has been identified in angiogenesis.45 In the fibrin gel assay, we found that the augmentation of sprouting by TNF was completely blocked by addition of an NFκB inhibitor (Figure 5A), indicating an important role for this pathway in early growth of new vessels. NFκB is maintained in the cytoplasm in an inactive state by IκB; phosphorylation of IκB by IKK then triggers IκB degradation and consequent release of NFκB. To examine the role of IKK in TNF induction of jagged-1, we transfected cells with a DN-IKKβ, treated them with TNF for 12 hours, and then assessed target gene expression by qRT-PCR. Induction of both jagged-1 and VEGFR2 by TNF was completely inhibited by the DN-IKKβ (Figure 5B), and this inhibition was maintained from the initial induction of jagged-1 mRNA to at least the time of peak expression at 4 hours (Figure 5C). Again, cyr-61 expression was unaffected by TNF or DN-IKKβ (Figure 5B). Thus, IKK activity is necessary for TNF induction of the tip cell genes VEGFR2 and jagged-1.
TNF induction of jagged-1 expression depends on NFκB activity. (A) EC-coated beads were established in fibrin gels and treated with TNF (10 ng/mL) or TNF + NFκB inhibitor (100 nM). Sprouts were counted and data are presented as means plus SD; *P < .005 relative to control, by Student t test. One of 3 similar experiments. (B) ECs were transfected with GFP- or DN-IKKβ expression plasmids and cultured with or without TNF (10 ng/mL). RNA was harvested at 12 hours and gene expression examined by qRT-PCR. Means and SD are shown. *P < .001 relative to control, by Student t test. One of 2 similar experiments. (C) ECs were transfected with GFP- or DN-IKKβ expression plasmids and cultured with TNF (10 ng/mL) for the indicated times. RNA was harvested and gene expression examined by qRT-PCR. One of 2 similar experiments. (D) ECs were transfected with GFP- or CA-IKKβ expression plasmids and cultured for the indicated times. RNA was harvested and gene expression examined by qRT-PCR. One of 2 similar experiments. (E) ECs were cultured in the presence or absence of the p38 inhibitor SB203580 and/or TNF (10 ng/mL) for 18 hours and jagged-1 expression was assayed by qRT-PCR. Means and SD are shown. *P < .01 by Student t test. One of 3 similar experiments. (F) ECs were cultured in the presence or absence of the p38 inhibitor SB203580 and jagged-1 expression was assayed by qRT-PCR at various times after the addition of TNF (10 ng/mL). Means and SD are shown. *P < .02 by Student t test. One of 3 similar experiments.
TNF induction of jagged-1 expression depends on NFκB activity. (A) EC-coated beads were established in fibrin gels and treated with TNF (10 ng/mL) or TNF + NFκB inhibitor (100 nM). Sprouts were counted and data are presented as means plus SD; *P < .005 relative to control, by Student t test. One of 3 similar experiments. (B) ECs were transfected with GFP- or DN-IKKβ expression plasmids and cultured with or without TNF (10 ng/mL). RNA was harvested at 12 hours and gene expression examined by qRT-PCR. Means and SD are shown. *P < .001 relative to control, by Student t test. One of 2 similar experiments. (C) ECs were transfected with GFP- or DN-IKKβ expression plasmids and cultured with TNF (10 ng/mL) for the indicated times. RNA was harvested and gene expression examined by qRT-PCR. One of 2 similar experiments. (D) ECs were transfected with GFP- or CA-IKKβ expression plasmids and cultured for the indicated times. RNA was harvested and gene expression examined by qRT-PCR. One of 2 similar experiments. (E) ECs were cultured in the presence or absence of the p38 inhibitor SB203580 and/or TNF (10 ng/mL) for 18 hours and jagged-1 expression was assayed by qRT-PCR. Means and SD are shown. *P < .01 by Student t test. One of 3 similar experiments. (F) ECs were cultured in the presence or absence of the p38 inhibitor SB203580 and jagged-1 expression was assayed by qRT-PCR at various times after the addition of TNF (10 ng/mL). Means and SD are shown. *P < .02 by Student t test. One of 3 similar experiments.
Next we asked whether IKK activity was sufficient for induction of jagged-1. ECs were transfected with CA-IKKβ, and mRNA was harvested at various times for analysis by qRT-PCR. Expression of CA-IKKβ alone induced jagged-1 by 2-fold at 4 hours and by almost 4-fold at 8 hours (Figure 5D). As a positive control, we examined induction of the known TNF target E-selectin, and this was also induced (Figure 5D). Thus, activation of the NFκB pathway by CA-IKKβ is sufficient for induction of jagged-1 expression in ECs.
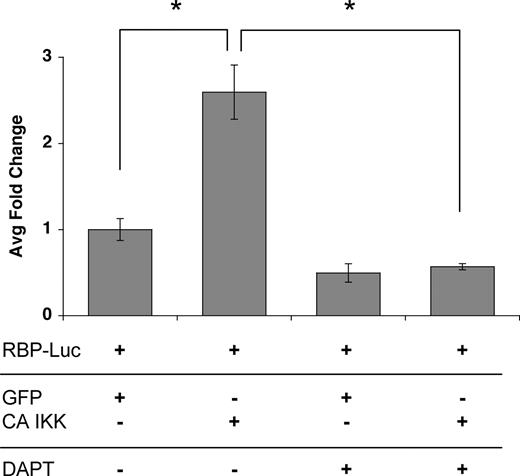
To determine whether the induced jagged-1 was functional we transfected ECs with CA-IKKβ (to induce jagged-1) and then mixed these with cells transfected with a notch reporter consisting of multimerized RBP sites driving luciferase. As anticipated, the IKKβ/jagged-expressing cells induced a significant increase in luciferase activity, and this was completely blocked by a gamma-secretase inhibitor, which blocks notch processing downstream of ligand binding (Figure 6). It has been reported that p38 is activated downstream of TNF and is necessary for activation of a subset of target genes.44,46,47 To test whether p38 might play a necessary role downstream of TNF (a weaker, more physiologic signal than overexpressed CA-IKKβ), we cultured ECs in the presence or absence of the p38 inhibitor SB203580 and examined jagged-1 mRNA expression. SB203580 showed dose-responsive inhibition of jagged-1 expression in response to TNF (Figure 5E), and the inhibitor was effective at all time points examined (Figure 5F). Thus, our data suggest a potential role for p38 in the physiologic induction of jagged-1 in ECs in response to TNF signaling.
Activation of the NFκB pathway induces a notch signal in neighboring cells. ECs were transfected with an expression plasmid for CA-IKKβ, and induction of jagged was confirmed by FACS (data not shown). Cells were then cocultured with ECs transfected with the notch reporter plasmid RBP-Luc, in the presence or absence of the notch signaling inhibitor DAPT (gamma-secretase inhibitor). Cells were harvested at 18 hours for assay of luciferase activity. Mean and SD is shown. *P < .01 by Student t test. One of 2 similar experiments.
Activation of the NFκB pathway induces a notch signal in neighboring cells. ECs were transfected with an expression plasmid for CA-IKKβ, and induction of jagged was confirmed by FACS (data not shown). Cells were then cocultured with ECs transfected with the notch reporter plasmid RBP-Luc, in the presence or absence of the notch signaling inhibitor DAPT (gamma-secretase inhibitor). Cells were harvested at 18 hours for assay of luciferase activity. Mean and SD is shown. *P < .01 by Student t test. One of 2 similar experiments.
Finally, to confirm a role for the TNF-IKK-NFκB pathway in inducing sprouting, we bypassed the membrane proximal effects of TNF on SHP-1 activity48 and transfected ECs with CA-IKKβ and placed these cells into a fibrin gel assay. As predicted, constitutive activation of NFκB induced a 2-fold increase in sprout number by day 4 (data not shown). Thus, TNF downstream of its membrane proximal effects, exerts a proangiogenic action through IKKβ/NFκB.
Discussion
A possible role for TNF in coordinating inflammation and angiogenesis
The role of TNF in angiogenesis has been highly controversial with numerous studies showing that it is either proangiogenic or antiangiogenic, with the preponderance of published studies suggesting that TNF blocks EC activation in vitro, but enhances angiogenesis in vivo.3-7,11,48 We hypothesized that some of these discrepancies might be explained by variations in the local persistence of cytokine. Thus, in vivo, cytokines are rapidly cleared from tissues through diffusion and bulk flow of interstitial fluids, and their concentration falls rapidly once synthesis is stopped. In contrast, addition of TNF to cells in culture leads to persistent high levels. This hypothesis was borne out as we found that continuous TNF stimulation in vitro blocked EC proliferation in monolayer cultures and formation of capillary-like sprouts in a 3-dimensional angiogenesis assay, whereas a 2-day pulse significantly enhanced sprouting. Similarly, we found that continuous TNF administration in vivo blocked angiogenesis, whereas a 2- to 3-day pulse significantly enhanced vascularization. This finding is in agreement with a previous study in mice showing a 5-fold enhancement of VEGF-induced vascularization in animals that were prepulsed with TNF for 3 days before injection of VEGF.9
Interestingly, Luo et al have found a different role for each of the TNF receptors in angiogenesis.14 In a model of temporary hind limb ischemia-induced angiogenesis, they reported enhanced vascularity in TNFR1-KO mice, but reduced angiogenesis in TNFR2-KO mice. How the balance between these opposite effects is regulated in wild-type mice expressing both receptors has not been determined. Conversely, Kociok et al reported that in TNFR p55−/− (TNFR1-KO) mice physiologic angiogenesis is normal, whereas pathologic angiogenesis is defective.49 Our data suggest that TNF is inducing a tip cell phenotype through TNFR1, as we saw significant blocking of jagged-1 induction with an antibody to TNFR1 but no reduction when we blocked TNFR2. This is consistent with TNFR1 being the major receptor for mediating NFκB signals.
NFκB represents a major downstream target of TNF signaling, however a role for p38 has also been reported. For example, induction of ephrin A1 expression on ECs by TNF requires p38 but not NFκB.50 Here we show that in response to a physiologic TNF signal both p38 and NFκB signaling are required for induction of jagged-1 in ECs, although a strong NFκB signal, provided by CA-IKKβ, will also drive expression. A role for NFκB in jagged-1 induction has previously been reported in HeLa cells.51 We have previously reported on a role for p38 in sprouting downstream of VEGF.33,34
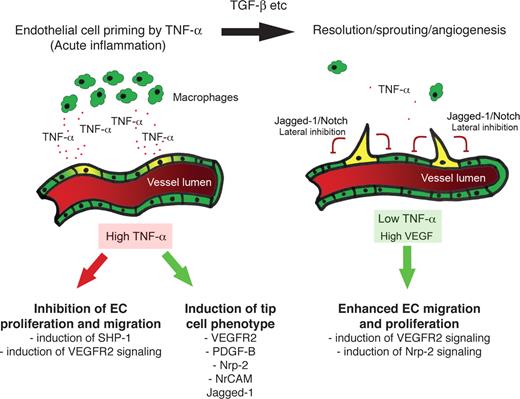
Macrophages are a major source of TNF in inflammatory tissues and have been implicated in angiogenesis during inflammation, wound repair, growth of adipose tissue, and growth of tumors.52-55 In both the mouse cornea and the chick CAM, TNF was shown to be essential for macrophage-induced angiogenesis.11 Furthermore, in CSF-1–null mice, which have severely reduced numbers of macrophages, there was a significant delay in the onset of the angiogenic switch compared with wild-type controls.52 When combined with these data, therefore, our studies suggest that TNF mediates cross-talk between macrophages and ECs at sites of inflammation, which results in an enhanced and temporally regulated burst of angiogenesis once the acute phase of the response begins to wane. Initially, TNF induces expression of proangiogenic genes, such as VEGFR2, PDGFB, and jagged-1, while blocking signaling through VEGFR2 by rapid induction of SHP-1 phosphatase activity.48,56 As the inflammatory response resolves and macrophage numbers fall, the TNF-mediated block of VEGFR2 signaling is relieved and angiogenic sprouting begins. It is also during this phase that macrophages and other cells begin to produce TGF-β, which is also proangiogenic. This hypothesis is summarized in Figure 7. In physiologic, noninflammatory angiogenesis, this additional layer of regulation is not required.
Summary. In the early stages of inflammation, the lesion is macrophage-rich and high levels of TNF are expressed. TNF induces proangiogenic, tip cell genes, including VEGFR2, PDGFB, and jagged-1. TNF also blocks VEGFR2 signaling, likely through induction of SHP-1. The ECs are thus primed for sprouting. Once the inflammatory response subsides, inhibition of VEGFR2 signaling is relieved, and VEGF-driven sprouting angiogenesis begins.
Summary. In the early stages of inflammation, the lesion is macrophage-rich and high levels of TNF are expressed. TNF induces proangiogenic, tip cell genes, including VEGFR2, PDGFB, and jagged-1. TNF also blocks VEGFR2 signaling, likely through induction of SHP-1. The ECs are thus primed for sprouting. Once the inflammatory response subsides, inhibition of VEGFR2 signaling is relieved, and VEGF-driven sprouting angiogenesis begins.
Gene expression in tip cells
In a developing vessel tip, cells have a very different phenotype to trunk cells.22,33,36 They are highly motile, extend numerous lamellipodia and filopodia, generally do not proliferate, and do not develop lumens. Trunk cells by comparison undergo division, at least until they become invested with pericytes, and form lumens. These phenotypic differences are reflected by differences in gene expression: for example, tip cells are enriched for VEGFR2, neuropilin-1 and -2, PDGFB, the netrin receptor Unc5B, and the integrins α6 and β4.36,38,57,58
We and others have shown that notch signaling plays a critical role in the determination of tip cells, and loss of signaling, due to chemical inhibition or loss of dll4 expression, leads to EC hyperproliferation, excessive vascular branching, and an overabundance of tip cells.22-27 Dll4 expression appears, therefore, to mark vessels undergoing active growth in the retina and in zebrafish. Our data show an up-regulation of jagged-1, but not dll4, in TNF-treated ECs undergoing angiogenic sprouting in vitro, and an increase of jagged-1 on ECs during pathologic angiogenesis in vivo. It is possible that during inflammatory angiogenesis jagged-1 plays a role similar to that of dll4 during development. Tumor angiogenesis may represent a mix of physiologic and inflammatory angiogenesis as both dll459,60 and jagged-1 (data not shown) are up-regulated on tumor vasculature. Our data showing jagged-1 expression in angiogenic ECs, and especially in tip cells, is also consistent with the finding of jagged-1 expression at the leading edge of repairing vascular wounds.61 These data suggest a possible paradox—a notch ligand, jagged-1, is up-regulated by a proangiogenic factor, TNF, and yet notch activation limits sprouting. The resolution lies in the timing. TNF acts early to prime angiogenesis by inducing a tip cell phenotype, including the expression of jagged-1. Once TNF levels fall and sprouting begins, notch signaling acts to maintain a single tip cell and limit unnecessary branching.
In vitro versus in vivo angiogenesis
The in vitro angiogenesis assay that we have used here has been optimized for use with HUVECs, which do not differ significantly from adipose-derived microvascular ECs in their ability to sprout or form lumens (unpublished observations). We believe that our results are directly relevant to in vivo angiogenesis, which involves capillary sprouting largely from postcapillary venules, for the following reasons: (1) the effect of short-term TNF treatment is the same in vivo and in vitro, namely enhanced sprouting; (2) the effect of blocking notch signaling (dll4-notch in retina and jagged-1-notch here) is the same in the fibrin gels as in vivo—an increase in tip cells, enhanced branching, and hyperproliferation of tip cells; (3) the major events of angiogenesis, namely sprouting, tube formation, branching, and anastomosis, are all recapitulated by HUVECs in fibrin gels33 ; (4) known tip cell–specific genes such as VEGFR2, PDGFB, and the neuropilins are also tip-cell specific in these cultures; and finally, (5) the gene expression profile of the HUVECs changes dramatically once they begin to grow in the fibrin gels where they turn on microvascular-specific genes62 and turn off large vessel genes (M.T.H. and C.C.W.H., manuscript in preparation).
In conclusion, we have shown that TNF primes ECs for angiogenic sprouting by inducing a tip cell phenotype, while at the same time blocking VEGF-induced proliferation. Our data offer a potential mechanism for coordinating a macrophage-rich inflammatory response with the onset of angiogenesis and tissue repair.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Cory Hogaboam for the gift of fibroblasts. We thank Craig Walsh for the gift of reagents and advice on TNF signaling. We also thank members of the Hughes laboratory for helpful discussions.
This work was supported by NIH grants RO1 HL60067 and HL086959.
National Institutes of Health
Authorship
Contribution: R.C.A.S., D.A.J., M.T.H., M.N.N., and S.P.C. designed, executed, and analyzed experiments; H.C.C., J.D., and E.C. performed experiments under direction; C.C.W.H. was responsible for conception, design, and analysis of the studies and wrote the paper; R.C.A.S., D.A.J., M.T.H., and M.N.N. provided editorial feedback.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. C. W. Hughes, Dept Mol Biol & Biochem, UC Irvine, Irvine, CA 92697; e-mail: cchughes@uci.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal