Abstract
Natural killer (NK) cells have been originally defined by their “naturally occurring” effector function. However, only a fraction of human NK cells is reactive toward a panel of prototypical tumor cell targets in vitro, both for the production of interferon-γ (IFN-γ) and for their cytotoxic response. In patients with IL12RB1 mutations that lead to a complete IL-12Rβ1 deficiency, the size of this naturally reactive NK cell subset is diminished, in particular for the IFN-γ production. Similar data were obtained from a patient with a complete deficit in IL-12p40. In addition, the size of the subset of effector memory T cells expressing CD56 was severely decreased in IL-12Rβ1– and IL-12p40–deficient patients. Human NK cells thus require in vivo priming with IL-12/23 to acquire their full spectrum of functional reactivity, while T cells are dependent upon IL-12/23 signals for the differentiation and/or the maintenance of CD56+ effector memory T cells. The susceptibility of IL-12/23 axis–deficient patients to Mycobacterium and Salmonella infections in combination with the absence of mycobacteriosis or salmonellosis in the rare cases of human NK cell deficiencies point to a role for CD56+ T cells in the control of these infections in humans.
Introduction
Natural killer (NK) cells have been initially described as non-T, non-B lymphocytes that are “naturally” elicited to mediate their effector functions (ie, cytotoxicity and cytokine production) without prior sensitization.1 Both arms of NK cell effector functions participate in the direct innate defense and in the shaping of the adaptive immune response.2 In several mouse models, NK cells limit the development of tumors and microbial infections.3-5 In particular, NK cells control the early steps of mouse cytomegalovirus (MCMV) infection, both by directly killing virus-infected cells and by producing IFN-γ.6
The natural acquisition of NK cell effector function has recently been challenged through the demonstration that only a minor fraction of circulating human NK cells or splenic mouse NK cells is reactive toward prototypical NK cell targets in single-cell assays.7-13 It is thus becoming increasingly clear that NK cells are following various steps of maturation, culminating into the final effector stage.10-15 In mice, the production of interleukin (IL)–15 by dendritic cells is one of the factors that primes naive NK cells into effectors.9,13
These results suggest that the fraction of NK cells that qualifies as effectors in vitro corresponds to the NK cells that had been exposed to in vivo priming prior to the in vitro assays. This hypothesis prompted us to determine the host genetic factors that contribute to NK cell reactivity in humans. We focused our interest on the IL-12 family of cytokines, as IL-12 had been initially identified on the basis of its ability to enhance NK cell cytotoxicity and interferon-γ (IFN-γ) production.16-19 A number of studies have indeed demonstrated that IL-12 affects NK cell effector function,20-23 especially with respect to NK cell activation by dendritic cells. IL-12 (IL-12p40:IL-12p35) and IL-23 (IL-12p40:IL-23p19) are structurally related heterodimeric cytokines that regulate cell-mediated immune responses and Th1-type inflammatory reactions.24 The IL-12 receptor is composed of 2 chains, IL-12Rβ1 and IL-12Rβ2, the former being also part of the IL-23R.24 In mice, numerous studies have shown a critical role for IL-12 in protective immunity to various pathogens.25 In contrast, the description of human patients with inherited IL-12 or IL-12R deficiencies has revealed that IL-12 is redundant for human defense against most microorganisms.26-30 Noticeable exceptions include Mycobacterium, such as environmental Mycobacterium, BCG vaccines, and M tuberculosis, as well as Salmonella infections, which critically depend on IL-12/23.26,27 Overall, patients with mutations in molecules involved in the IFN-γ/IL-12/23–dependent pathway are affected by the syndrome of Mendelian susceptibility to mycobacterial disease (MSMD).26,27,30,31 This syndrome is biologically characterized by deeply impaired or absent IFN-γ production or function, and is clinically defined by the susceptibility to mycobacteriosis and salmonellosis. Here, we analyzed the phenotypic and functional features of circulating NK and NK-like CD56+ T cells in a group of 9 patients who present a complete IL-12Rβ1 or IL-12p40 deficiency.
Methods
Patients and controls
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation (GE Healthcare, Little Chalfont, United Kingdom) from whole blood samples obtained from healthy volunteer donors, and IL-12Rβ1– and IL-12p40–deficient patients described in Table 1. These human studies were performed and informed consent from all participating subjects was obtained in accordance with the Declaration of Helsinki.
Patient characteristics
| Patient . | Age, y . | Sex . | Onset . | Mutations . | Historical clinic status . | Experimental time clinic status . |
|---|---|---|---|---|---|---|
| 1* | 25 | F | Morocco | IL12RB1 K305X | BCGite + Salmonella | Salmonella suspicion |
| 2*† | 34 | F | France | [IL12RB1 1745]−1746insCA+1483+182-1619-1073del | BCGite + Salmonella | Asymptomatic |
| 3* | 4 | F | France | IL12RB1 Q32X | BCGite | Asymptomatic |
| 4* | 16 | F | Belgium | IL12RB1 Q32X | Asymptomatic | Asymptomatic |
| 5 | 11 | M | Turkey | IL12RB1 R173P | Salmonella | Asymptomatic |
| 6* | 6 | M | Israel | IL12RB1 700+362-1619-944del | Salmonella | Asymptomatic |
| 7 | 9 | M | Saudi Arabia | IL12RB1 1190-1G>A | BCGite + Salmonella | Salmonella |
| 8 | 13 | M | Saudi Arabia | IL12RB1 1190-1G>A | Salmonella | Salmonella |
| 9 | 5 | M | Tunisia | IL12 297del8 | Salmonella | Salmonella + asymptomatic |
| Patient . | Age, y . | Sex . | Onset . | Mutations . | Historical clinic status . | Experimental time clinic status . |
|---|---|---|---|---|---|---|
| 1* | 25 | F | Morocco | IL12RB1 K305X | BCGite + Salmonella | Salmonella suspicion |
| 2*† | 34 | F | France | [IL12RB1 1745]−1746insCA+1483+182-1619-1073del | BCGite + Salmonella | Asymptomatic |
| 3* | 4 | F | France | IL12RB1 Q32X | BCGite | Asymptomatic |
| 4* | 16 | F | Belgium | IL12RB1 Q32X | Asymptomatic | Asymptomatic |
| 5 | 11 | M | Turkey | IL12RB1 R173P | Salmonella | Asymptomatic |
| 6* | 6 | M | Israel | IL12RB1 700+362-1619-944del | Salmonella | Asymptomatic |
| 7 | 9 | M | Saudi Arabia | IL12RB1 1190-1G>A | BCGite + Salmonella | Salmonella |
| 8 | 13 | M | Saudi Arabia | IL12RB1 1190-1G>A | Salmonella | Salmonella |
| 9 | 5 | M | Tunisia | IL12 297del8 | Salmonella | Salmonella + asymptomatic |
Indicated IL-12Rβ1– or IL-12p40–deficient patients (n = 9, 13.7 ± 10 years old, M/F ratio: 5:4) were analyzed in comparison with healthy control individuals (n = 16, 26.1 ± 12.0 years old, M/F ratio: 4:12 for the phenotypic analysis; n = 13, 29.5 ± 8.4 years old, M/F ratio: 3:10 for the functional analysis).
The patients P1, P2, P3, P4, and P6 were previously described in Fieschi et al27 as 1.II.2, 19.II.1, 20.II.1, 21.II., and 10.II.1, respectively.
The patient contracted hepatitis C virus (HCV) after a blood transfusion.
Reagents
The following monoclonal antibodies (mAbs) were used: PE-conjugated anti-CD16 (mouse IgG1, 3G8), anti-CD25 (IgG2a, B1.49.9), anti-CD62L (IgG1, Dreg 56), anti-CD94 (IgG2a, HP-3B1), anti-CD158a,h (IgG1, EB6), anti-CD158b1/b2/j (IgG1, GL183), anti-CD158e1 (IgG1, Z27), anti-CD158i (IgG2a, FESTR172), anti-CD161 (IgG2a, 191B8), anti-NKp30 (IgG1, Z25), anti-NKp44 (IgG1, Z231), anti-NKp46 (IgG1, Bab281), anti-NKG2A (IgG2b, Z199); FITC-conjugated anti-CD3 (IgG1, UCHT1); PECy5-conjugated anti-CD56 (IgG1, NKH-1); APC-conjugated anti-CD56 (NKH-1; Beckman Coulter Immunotech, Marseille, France); PE-conjugated anti-CD69 (IgG1, FN50), antiperforin (IgG2b, 27–35), anti–IFN-γ (IgG1, 4S-B3); FITC-conjugated anti-CD107a (IgG1, H4A5), anti-CD107b (IgG1, H4B4); PerCP-Cy5.5–conjugated anti-CD3 (IgG1, SK7; Becton Dickinson, Lincoln Park, NJ); purified anti–IL-12 (IgG1, 24910; R&D Systems, Minneapolis, MN), biotin-conjugated anti-CD162R (IgM, 5H10; Innate Pharma, Marseille, France); and PE-labeled streptavidin (SouthernBiotechnology Associated, Birmingham, AL). Human recombinant IL-12 (219-IL) and IL-23 (1290-IL) were purchased from R&D Systems; human IL-2 (Proleukin), from Chiron (Emeryville, CA); human IL-15(200–15), from Peprotech (Rocky Hill, NJ); and human IL-18 (B003–5), from MBL (Watertown, MA).
NK cell analysis
PBMCs were analyzed by 3-color flow cytometry using a FACSCalibur cytometer (Becton Dickinson). NK cells were defined as CD3−CD56+ cells within the lymphocyte gate. Natural cytotoxicity was assessed using the MHC class I− human erythroleukemic K562 target cells, as well as fibroblastic hamster CHO and human HeLa target cells. Antibody-dependent cell cytotoxicity (ADCC) was assessed using the P815 mouse mastocytoma cells coated with rabbit antimouse lymphocyte antibodies (Accurate Biochemicals, Westbury, NY). NK cell effector functions were tested in a single-cell assay using CD107 mobilization and IFN-γ production, as previously described.7 In these assays, PBMCs were incubated for 4 hours at 37°C in the presence of GolgiStop (1/1500; Becton Dickinson), anti-CD107 mAb, and various stimuli. The effector-target ratio was 2.5:1. Cells were then washed in PBS supplemented with 2% FCS, 1 mM EDTA and stained for 30 minutes at 4°C with PerCP-Cy5.5–conjugated anti-CD3, APC-conjugated anti-CD56, and normal mouse serum 2%. After fixation in paraformaldehyde 2% and permeabilization (PermWash; Becton Dickinson), the expression of IFN-γ was detected by incubation with PE-conjugated anti–IFN-γ for 30 minutes at 4°C. As a negative control, species- and isotype-matched control mAbs were used for all stainings.
Generation of IL-2–activated NK cells
NK cell–enriched PBMCs were obtained using the RosetteSep Human NK Cell kit (StemCell Technologies, Vancouver, BC). Then, NK cells were resuspended in RPMI 10% FCS containing human IL-2 at 100 U/mL and PHA (Invitrogen, Frederick, MD) at 10 μg/mL in 96-well U-bottom plate. For expansion, NK cells needed previously irradiated (50 gray) allogeneic PBMCs at the concentration 2 × 106 cells/mL. Every 2 days, the medium was replaced by RPMI 10% FCS supplemented with IL-2 100 U/mL.
Whole-blood activation by live BCG
Venous blood samples of healthy donors were collected into heparinized tubes. Blood (500 μL) was dispensed into wells of a 6-well plate for a final volume of 1 mL/well (dilution with RPMI 1640 supplemented with 100 U/mL penicillin and 100 μ/mL streptomycin). The diluted blood sample then incubated in a 2-stage procedure during 24 and 48 hours at 37°C in an atmosphere containing 5% CO2 and under 3 conditions of activation: with medium alone, with live bacillus Calmette-Guerin (M bovis BCG, Pasteur substrain) at an MOI of 20 BCG/leukocytes,32 and with BCG plus IL12 (20 ng/mL; R&D Systems). Six hours before the end of activation, GolgiStop (1/1500; Becton Dickinson) was added in each well. The production of IFN-γ was detected by intracellular staining as described in “NK cell analysis” and analyzed by flow cytometry.
Statistical analysis
Graphic representation and statistical analysis of NK cell distribution were performed using GraphPad Prism software (GraphPad Software, San Diego, CA). Comparison of distributions was performed using Mann Whitney test. *P was less than .05; **P was less than .01; ns indicates not significant. The statistical analysis never included the IL-12p40−/− patient together with the IL-12Rβ1−/− patients. Age-matched statistical analysis was performed as described in Table 1 (L.A.).
Results
NK cell phenotype in IL-12Rβ1–deficient patients
The role of IL-12 and IL-23 on human NK cells in vivo was first tested by analyzing circulating NK cell counts in a cohort of IL-12Rβ1–deficient patients presenting a complete IL-12Rβ1 deficiency (Table 1). Normal PBMC counts have been previously reported in a large cohort of IL-12Rβ1–deficient patients.27 No alteration in the percentage CD3−CD56+ NK cells within PBMCs was detected here in our cohort of 8 IL-12Rβ1–deficient patients (Figure 1A). Human NK cells can be divided in 2 reciprocal subsets, based on the cell surface expression of CD56. CD56bright NK cells represent a minority of blood NK cells, but are prominent in secondary lymphoid organs.33 CD56bright NK cells readily produce IFN-γ in response to proinflammatory cytokines such as IL-12, IL-18, and IL-15.7,34 In contrast, most circulating NK cells have a CD56dim phenotype; they initiate their cytolytic and cytokine production programs upon interaction with tumor cell targets.7 No difference between the size of the CD56bright and CD56dim NK cell subsets was detected when control and IL-12Rβ1–deficient patients were compared (data not shown). The NK cell surface phenotype of IL-12Rβ1–deficient patients was also indistinguishable from that of control individuals, for the expression of MHC class I–specific receptors (killer cell Ig-like receptors: CD158/KIR, CD94, CD159a/NKG2A), of a panel of activating and cell adhesion receptors (CD16, CD161/NKR-P1, CD162R/PEN5, CD62L/L-selectin) as well as of NK cell activation markers (CD25 and CD69). Importantly, the intracytoplasmic NK cell content in perforin was comparable between control and IL-12Rβ1–deficient individuals (Figure 1B). In control individuals, CD56bright NK cells expressed slightly lower cell surface levels of NKp30 and higher levels of NKp46 than CD56dim NK cells (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). In IL-12Rβ1–deficient patients, a slight decrease in NKp30 cell surface density was observed mainly on CD56dim NK cells (Figure S1A,B). A minor down-regulation of NKp46 expression was also observed (Figure S1B), but this trend did not reach statistical significance. Thus, circulating NK cells did not present gross abnormalities in counts or in their phenotype, including the repertoire of MHC class I receptors, showing that IL-12 and IL-23 are dispensable for the phenotypic development of human NK cells in vivo.
Normal NK cellularity and phenotype in IL-12/23 axis–deficient patients. (A) The percentages of NK cells present in peripheral blood of indicated individuals were computed from the percentages of CD3−CD56+ cells within the lymphocyte. Each dot indicates the value obtained from one individual. (B) Circulating NK cells from indicated individuals were explored for their cell surface phenotype (except for perforin, where an intracytoplasmic staining was performed). Each dot indicates the value obtained from one individual.
Normal NK cellularity and phenotype in IL-12/23 axis–deficient patients. (A) The percentages of NK cells present in peripheral blood of indicated individuals were computed from the percentages of CD3−CD56+ cells within the lymphocyte. Each dot indicates the value obtained from one individual. (B) Circulating NK cells from indicated individuals were explored for their cell surface phenotype (except for perforin, where an intracytoplasmic staining was performed). Each dot indicates the value obtained from one individual.
NK cell effector functions in IL-12Rβ1–deficient patients
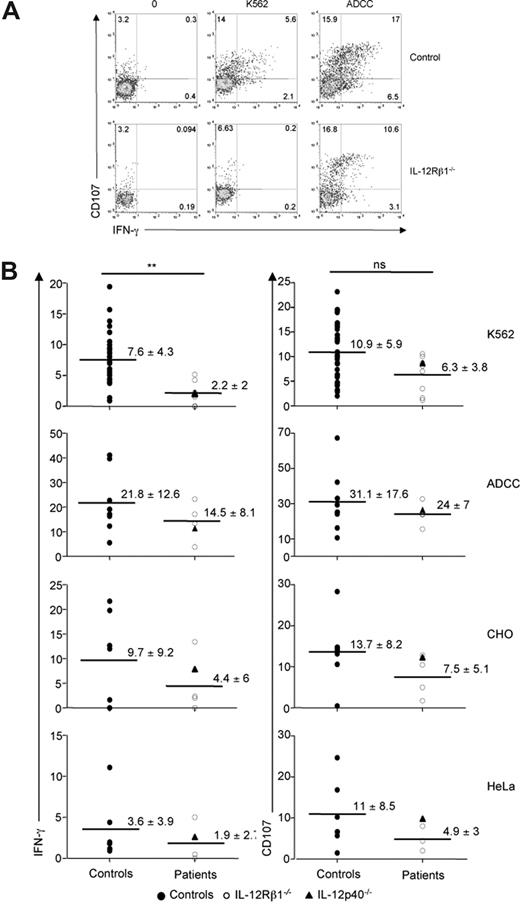
We then analyzed NK cell effector functions using single-cell assays. We quantified the IFN-γ production and the cytotoxicity potential (via the CD107 degranulation assay), using peripheral blood NK cells from patients and control individuals, in response to a panel of tumor cell lines. The response of patients' NK cells to the prototypical MHC class I− tumor cell target K562 was diminished compared with control individuals (Figure 2A). The reduction in NK cell response was more pronounced for IFN-γ production than for the CD107 degranulation assay, as only the former reached statistical significance in these experimental settings (Figure 2B). A trend toward a decrease in NK cell effector function (both IFN-γ production and degranulation) was also observed in response to 2 other tumor cell lines (CHO and HeLa), as well as upon antibody-dependent cell cytotoxicity (ADCC) challenge (Figure 2B). It is likely that the small size of our cohort of IL-12Rβ1–deficient patients was responsible for the fact that the decrease in NK cell reactivity did not reach statistical significance. K562, HeLa, and CHO cells are recognized by a combination of NK cell receptors including NKp30 (data not shown). However, the slight decrease in NKp30 expression observed in patients' NK cells was unlikely to be solely responsible for the decreased NK cell reactivity observed with IL-12Rβ1–deficient cells. Indeed, the ADCC response of IL-12Rβ1–deficient NK cells followed the same trend, but is CD16 dependent and NCR independent. In addition, no correlation could be found between the extent of NKp30 down-regulation and the reduced reactivity observed with NK cells from IL-12Rβ1–deficient patients (data not shown). Therefore our data rather suggest that signaling via IL-12Rβ1 partially controls critical transduction components that are downstream of and common to various NK cell activating pathways. Patients included in this study were symptomatic or asymptomatic (Table 1), and no correlation between the decrease in IFN-γ production upon K562 stimulation and the clinical status could be established (data not shown).
NK cell hyporesponsiveness in IL-12/23 axis–deficient patients. (A) A representative experiment comparing the in vitro reactivity of NK cells from healthy control individuals and IL-12Rβ1–deficient patients is shown. PBMCs were incubated for 4 hours in the presence or absence of K562 cells and assessed for CD107 and IFN-γ expression. (B) PBMCs prepared from a cohort of healthy control individuals, IL-12Rβ1–deficient patients and one IL-12p40–deficient patient were analyzed for their NK reactivity in the presence of indicated tumor cells; ADCC: antibody-coated P815 cells. Values indicate mean plus or minus SD. Each dot represents the data obtained from one individual.
NK cell hyporesponsiveness in IL-12/23 axis–deficient patients. (A) A representative experiment comparing the in vitro reactivity of NK cells from healthy control individuals and IL-12Rβ1–deficient patients is shown. PBMCs were incubated for 4 hours in the presence or absence of K562 cells and assessed for CD107 and IFN-γ expression. (B) PBMCs prepared from a cohort of healthy control individuals, IL-12Rβ1–deficient patients and one IL-12p40–deficient patient were analyzed for their NK reactivity in the presence of indicated tumor cells; ADCC: antibody-coated P815 cells. Values indicate mean plus or minus SD. Each dot represents the data obtained from one individual.
NK cells in an IL-12p40–deficient patient
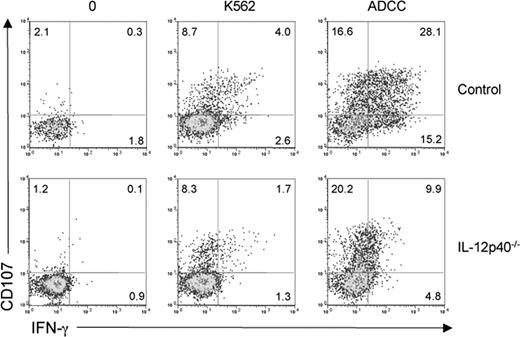
We further tested the role of IL-12Rβ1–dependent signals on NK cells by analyzing the reactivity of circulating NK cells isolated from a patient presenting a genetic deficiency in IL-12p40 (IL12B). NK cells from the IL-12p40–deficient patient were hyporesponsive to K562 and ADCC challenge (Figure 3). The IL-12p40–deficient patient was tested under symptomatic and asymptomatic conditions, and no correlation between the decrease in NK cell reactivity and the clinical status was detected (data not shown). As for IL-12Rβ1–deficient patients, no gross abnormalities in circulating NK cell counts and phenotype were observed in the IL-12p40–deficient patient (Figure 1A,B closed triangles). The lack of other IL-12p40–deficient patients available prevented us from analyzing whether the intensity of the NK cell defect was different in IL-12p40– and IL-12Rβ1–deficient patients. Nevertheless, the NK cell hyporesponsiveness in both the IL-12p40– and the IL-12Rβ1–deficient patients strongly advocates for a role of IL-12/23 in the acquisition NK cell effector function (ie, in NK cell priming in vivo in humans). In contrast to IL-12,25 we could not detect a significant in vitro effect of IL-23 treatment on healthy NK cell IFN-γ production (Figure 4), suggesting that the decrease in NK cell IFN-γ production in IL-12Rβ1–deficient patients was due to IL-12 rather than IL-23.
NK cell hyporesponsiveness in an IL-12p40–deficient patient. A representative experiment comparing the in vitro reactivity of NK cells from one control individual and one IL-12p40–deficient patient is shown. PBMCs were incubated for 4 hours in the presence or absence of K562 cells and assessed for CD107 and IFN-γ expression.
NK cell hyporesponsiveness in an IL-12p40–deficient patient. A representative experiment comparing the in vitro reactivity of NK cells from one control individual and one IL-12p40–deficient patient is shown. PBMCs were incubated for 4 hours in the presence or absence of K562 cells and assessed for CD107 and IFN-γ expression.
Differential role of IL-12 and IL-23 on IFN-γ production by NK cells in vitro. PBMCs prepared from healthy control individuals were cultured for 4 hours in vitro with the indicated concentrations of human recombinant IL-12 or IL-23, and then assayed for IFN-γ production. Results are expressed as mean plus or minus SD of 3 independent experiments.
Differential role of IL-12 and IL-23 on IFN-γ production by NK cells in vitro. PBMCs prepared from healthy control individuals were cultured for 4 hours in vitro with the indicated concentrations of human recombinant IL-12 or IL-23, and then assayed for IFN-γ production. Results are expressed as mean plus or minus SD of 3 independent experiments.
Role of IL-12 in NK cell priming
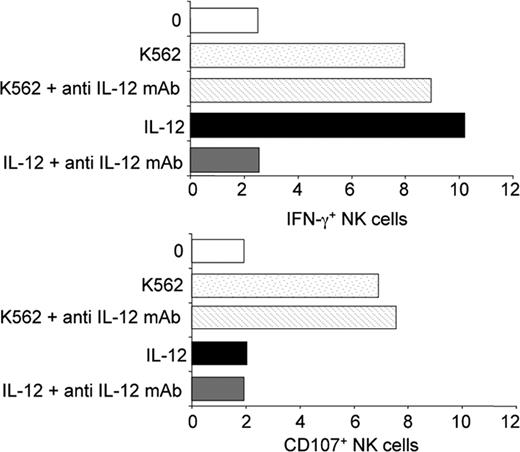
We then tested whether IL-12 was required during the contact between NK cells present in PBMCs and the tumor cell target or whether IL-12 was one of the factors that contributes to human NK cell priming in vivo. As shown in Figure 5, the addition of a blocking anti–IL-12 mAb during the 4-hour incubation between healthy PBMCs and K562 target cells did not influence NK cell response. The NK cell defect observed in IL-12Rβ1–deficient patients was thus most likely not the consequence of a role for IL-12 during the 4-hour in vitro assay, but resulted from a role of IL-12 in vivo prior to the isolation of peripheral blood cells.
No detectable role for endogenous IL-12 during in vitro NK cell stimulation by K562 cells. PBMCs from healthy control individuals were incubated with K562 target cells for 4 hours at 37°C, in the presence or absence of anti–hIL-12 mAb (10 μg/mL). IFN-γ production and CD107 mobilization were assessed in a 4-hour K562 stimulation assay.
No detectable role for endogenous IL-12 during in vitro NK cell stimulation by K562 cells. PBMCs from healthy control individuals were incubated with K562 target cells for 4 hours at 37°C, in the presence or absence of anti–hIL-12 mAb (10 μg/mL). IFN-γ production and CD107 mobilization were assessed in a 4-hour K562 stimulation assay.
Complementation of IL-12–dependent NK cell defects
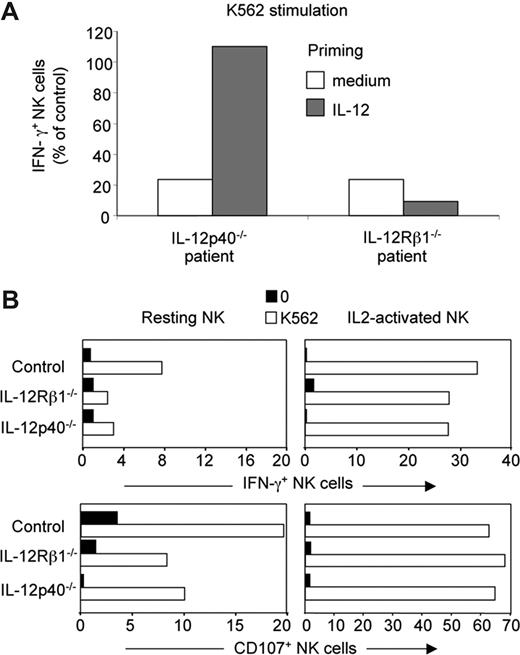
To further address the role of IL-12 in NK cell function, PBMCs prepared from the IL-12p40–deficient patient and IL-12Rβ1–deficient patients were treated in vitro with recombinant human IL-12, and the reactivity of NK cells to K562 was assessed. Exogenous IL-12 complemented the defect in NK IFN-γ production of the IL-12p40–deficient patient, but not of IL-12Rβ1–deficient patients, as expected (Figure 6A). By contrast, no difference in the reactivity to K562 was observed in IL-2–cultured NK cells from control, IL-12p40–deficient, and IL-12Rβ1–deficient patients (Figure 6B), showing that IL-12 played a redundant role in the priming of NK cells, when grown in IL-2.
Complementation of the IL-12–dependent NK cell hyporesponsiveness. (A) PBMCs from one representative control individual, one representative IL-12Rβ1–deficient patient, and one IL-12p40–deficient patient were cultured for 24 hours in vitro with human recombinant IL-12 (1 ng/mL), and then assayed for IFN-γ production in response to 4-hour K562 stimulation. Results are expressed as the percentage of IFN-γ+ NK cells in patients normalized to the percentage of IFN-γ+ NK cells in the control individual (set to 100%). (B) NK cell cultures of indicated origin (healthy controls, IL-12Rβ– and IL-12p40–deficient patients) were generated by incubating NK cell–enriched PBMCs with recombinant human IL-2 (100 U/mL) for 3 weeks. Resting NK cells or IL-2–cultured NK cells of the same individuals were then compared in parallel in a 4-hour K562 stimulation.
Complementation of the IL-12–dependent NK cell hyporesponsiveness. (A) PBMCs from one representative control individual, one representative IL-12Rβ1–deficient patient, and one IL-12p40–deficient patient were cultured for 24 hours in vitro with human recombinant IL-12 (1 ng/mL), and then assayed for IFN-γ production in response to 4-hour K562 stimulation. Results are expressed as the percentage of IFN-γ+ NK cells in patients normalized to the percentage of IFN-γ+ NK cells in the control individual (set to 100%). (B) NK cell cultures of indicated origin (healthy controls, IL-12Rβ– and IL-12p40–deficient patients) were generated by incubating NK cell–enriched PBMCs with recombinant human IL-2 (100 U/mL) for 3 weeks. Resting NK cells or IL-2–cultured NK cells of the same individuals were then compared in parallel in a 4-hour K562 stimulation.
Lack of CD56+ T cells in IL-12/23 axis–deficient patients
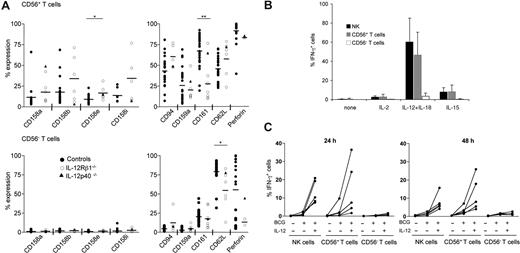
During their maturation, T cells can acquire some NK cell attributes, such as the cell surface expression of NK cell receptors.35 In contrast to the lack of major NK cell phenotypic alteration in IL-12/23 axis–deficient patients, the size of the subset of T cells that expresses CD56 was severely reduced in both IL-12Rβ1– and IL-12p40–deficient patients (Figure 7A,B). The small size of the subset of CD56+ T cells in patients prevented us from precisely analyzing their functional characteristics in great detail. Nevertheless, in control individuals CD56+ T cells were mainly CD8+ T cells, whereas a few consisted of Vα24 invariant NKT cells and γδ T cells (data not shown). The low fraction of invariant Vα24+ T cells in CD56+ T cells (from 1% to 5% of CD56+ T cells) is consistent with previous results,36 and makes it unlikely to be responsible for the drastic reduction in the size of the CD56+ T-cell subset in IL-12/23 axis–deficient patients (from 4.2% ± 2.6% to 1.6% ± 1.5% of total lymphocytes in control individuals vs patients, respectively, Figure 7B). In control individuals, CD56+ T cells also included a substantial fraction of T cells expressing other NK cell phenotypic features such as KIR, CD94/NKG2A, and CD161 (Figure 8A). CD56 surface expression on T cells correlated with high intracytoplasmic perforin content (Figure 8A), consistent with previous results.37 Importantly, CD56+ T cells were not only equipped as cytolytic effectors, but they also shared with NK cells the capacity to produce IFN-γ upon IL-12 + IL-18 treatment,38 and to a lesser extent upon IL-15 stimulation (ie, in absence of TCR engagement; Figure 8B). In addition, a substantial fraction of NK cells and CD56+ T cells, but barely detectable CD56− T cells, produced IFN-γ in vitro in presence of live BCG (Figure 8C) and in response to Salmonella typhimurium–infected macrophages (N. Lapaque and J. Trowsdale, personal communication, December 17, 2007). The IL-12/23 axis deficiency was also associated with a lower expression of CD161 on CD56+ T cells. Since the size of the CD56+ T-cell subset increases with aging and most of the IL-12/23 axis–deficient patients comprised infants and young adults,39 a careful statistical analysis was conducted to find out whether age had a confounding effect on our results. However, the restriction of the cohort of healthy control individuals to age-matched patients still revealed a statistically significant reduction in the size of the CD56+ T-cell subsets in IL-12/23–deficient patients (data not shown). Thus, IL-12/23 was mandatory for the expansion of a subset of T cells, mainly CD8+, that presents features shared by both NK cells and effector memory T cells: cell surface expression of CD56, intracytoplasmic expression of perforin, and IFN-γ production in response to IL-12 + IL-18. IL-12/23 was critical for the final CD8+ T-cell maturation steps and/or for the maintenance of this CD56+ T-cell subset in PBMCs.
Reduced size of the CD56+ T-cell subset in IL-12/23 axis–deficient patients. (A,B) The percentages of CD56+ T cells present in peripheral blood of indicated individuals were computed within the total lymphocyte gate. Each dot represents the value obtained from one individual (B).
Reduced size of the CD56+ T-cell subset in IL-12/23 axis–deficient patients. (A,B) The percentages of CD56+ T cells present in peripheral blood of indicated individuals were computed within the total lymphocyte gate. Each dot represents the value obtained from one individual (B).
Altered T-cell phenotype in IL-12/23 axis–deficient patients. (A) Circulating CD56+ T cells (top panel) and CD56− T cells (bottom panel) from indicated individuals were explored for their cell surface phenotype (except for perforin, where an intracytoplasmic staining was performed). Each dot indicates the value obtained from one individual. (B) Circulating CD56− T cells, CD56+ T cells, and NK cells from 4 representative healthy control individuals were assayed for their IFN-γ production in response to 24-hour treatment in the presence or absence of indicated cytokines: IL-2 (50 U/mL), IL-15 (10 ng/mL), IL-18 (20 ng/mL), IL-12 (5 ng/mL). (C) Circulating CD56− T cells, CD56+ T cells, and NK cells from 5 healthy individuals were assayed for their IFN-γ production in response to live BCG alone or BCG plus IL-12 (20 ng/mL) during 24 and 48 hours. Each line represents the response obtained with one individual.
Altered T-cell phenotype in IL-12/23 axis–deficient patients. (A) Circulating CD56+ T cells (top panel) and CD56− T cells (bottom panel) from indicated individuals were explored for their cell surface phenotype (except for perforin, where an intracytoplasmic staining was performed). Each dot indicates the value obtained from one individual. (B) Circulating CD56− T cells, CD56+ T cells, and NK cells from 4 representative healthy control individuals were assayed for their IFN-γ production in response to 24-hour treatment in the presence or absence of indicated cytokines: IL-2 (50 U/mL), IL-15 (10 ng/mL), IL-18 (20 ng/mL), IL-12 (5 ng/mL). (C) Circulating CD56− T cells, CD56+ T cells, and NK cells from 5 healthy individuals were assayed for their IFN-γ production in response to live BCG alone or BCG plus IL-12 (20 ng/mL) during 24 and 48 hours. Each line represents the response obtained with one individual.
Discussion
IL-12 and IL-23 are cytokines that represent a functional bridge between the early resistance and the subsequent antigen-specific adaptive immunity.24,26,32,40 Here we have shown that IL-12/23 was differentially required by 2 subsets of effector lymphocytes in vivo in humans: NK cells and CD56+ T cells. While acting on NK cells as a priming factor, IL-12/23 was required for the differentiation and/or the maintenance of CD56+ effector memory T cells.
Previous observations had revealed that NK cells were present in normal numbers in IL-12Rβ1–deficient patients.21,41 We confirmed these observations, and extended the phenotypic analysis to a large panel of receptors expressed at the NK cell surface. All described NK cells subsets develop normally in vivo in absence of IL-12 and IL-23 stimulation. In particular, we did not detect alterations in the CD56dim or CD56bright circulating NK cells subsets in IL-12Rβ1–deficient patients, contrasting with a role for IL-12 in the maturation of CD56bright NK cells, suggested earlier by in vitro experiments.42 Furthermore, the repertoire of Ig-like and lectin-like MHC class I receptors did not present any gross abnormalities in IL-12/23 axis–deficient patients. Thus, the variegation at the KIR locus, which is still poorly understood, occurs in an IL-12– and IL-23–independent manner. A defect in NK cell IFN-γ production was also reported in the pioneering description of one IL-12Rβ1–deficient patient.21 The high variability of NK cell reactivity in vitro, combined with the large variations in peripheral NK cell counts, prompted us to complete this first characterization, by increasing the number of patients and the number of tumor cell targets, and by using single NK cell assays. We confirmed in these 4-hour short-term stimulation protocols, the low IFN-γ production by NK cells from IL-12Rβ1–deficient patients in response to the prototypical MHC class I− K562 tumor cells. We also showed a trend toward a broader hyporesponsiveness of NK cells for IFN-γ production and for cytotoxicity to a lesser extent to various human tumors as well as to antibody-coated target cells. This phenotype was recapitulated with NK cells from an IL-12p40–deficient patient and complemented with exogenous IL-12. Consistent with an earlier report,43 we did not detect much impact of IL-23 of NK cell effector function in vitro, suggesting, but not formally proving, that IL-12 and not IL-23 was responsible for the weak reactivity of NK cells from IL-12Rβ1– and IL-12p40–deficient patients. Recent data in humans and mice point to a reappraisal of the “natural” effector function of NK cells. In mice, IL-15 and MHC class I participate in the acquisition of the full spectrum of NK cell reactivity.7,9-13 Thus, NK cells do not distinguish themselves from classical T and B cells by their naturally occurring reactivity with targets, but rather by the presence of a substantial fraction of primed and broadly reactive NK cells in the circulation. Yet, the factors that contribute to NK cell priming in vivo may vary between humans and mice. Indeed, we showed here that IL-12/23 is one of the NK cell priming factors in humans. In contrast, IL-12 was recently shown to be redundant for mouse NK cell priming,9 despite the moderate but detectable defect in NK cell antitumor cytolytic activity detected in Il-12– (data not shown), Il-12rb1–, or Il-12rb2–deficient mice.44-48
The size of the subset of T cells expressing surface CD56 was drastically reduced in IL-12/23 axis–deficient patients. Much confusion exists regarding the characterization and the function of the subsets of T cells that share phenotypic similarities with NK cells.35,49 In particular, CD56+ T cells have been too often referred as to NKT cells. There is, however, a consensus defining NKT cells as a subset of CD4+ or CD4−CD8− T cells that express invariant TCRs, such as CD1d-restricted Vα24 T cells in humans, CD1-restricted Vα14 T cells in mice, or MR1-restricted mucosal associated invariant T (MAIT) in both species.50,51 CD56+ T cells are clearly different from aforementioned invariant NKT cells, as they are mainly CD8+TCRαβ+ cells with a high cytolytic potential in absence of in vitro maturation.37 CD56+TCRαβ+ cells express a diverse TCR repertoire, which tends to oligoclonality, and the size of this subset expands with aging.39 CD56+ T cells thus have attributes of effector memory CD8 T cells, although the precise steps of differentiation of CD56+ T cells from naive CD8 T cells are still unknown. In vitro data have argued for a role for IL-12 in their development and/or expansion,52-55 but one report disputed the in vivo relevance of these findings for the pool of hepatic CD56+ T cells.55 We also previously showed that most CD56+ T cells constitutively express IL-12Rβ1.56 Similarly, IL-12 priming during primary antigenic challenge increased the population of memory CD8+ T cells in mice.57,58 Our data unambiguously show that IL-12/23 is required for the maturation of CD8+ T cells into circulating CD8+CD56+ T cells and/or for the maintenance of the latter in vivo in PBMCs in humans. Although IL-12/23 plays a necessary role in the determination of the size of CD56+ T cells, it is not sufficient. Indeed, addition of IL-12 in vitro did not lead to the induction or expansion of CD56+ T cells (data not shown), consistent with results obtained from the monitoring of IL-12–treated patients.59 Along this line, TCR, IL-2, and/or IL-15 stimulations have been show to be involved in the induction/maintenance of CD56+ T cells.55,60-62 Altogether, the presence of CD56+ T cells correlates with several conditions of chronic inflammation such as celiac disease63 or melanoma.64 In cirrhotic livers, a decreased number of CD56+ T cells may be related to their susceptibility to hepatocellular carcinoma.65
Although we favor the possibility that IL-12/23 acts directly on NK cells and CD56+ T cells, the effect of IL-12/23 deficiency might be indirect (ie, function through a different cell type as opposed to directly these lymphocytes). Irrespective of this possibility, IL-12/23 is involved in the priming of NK cell effector function and in the differentiation and/or the maintenance of CD56+ effector memory T cells. The IL-12/IFN-γ axis is a critical molecular pathway in the susceptibility of mycobacteriosis and salmonellosis. Yet, the precise identification of the cells that produce protective IFN-γ in vivo in response to IL-12 during natural Mycobacterium or Salmonella infection in human is still lacking. In the case of Mycobacterium, the in vitro production of IFN-γ by whole blood cells upon live BCG stimulation is shown to be specific and sensitive to identify disease-causing genes in MSMD patients. Importantly, IFN-γ production by whole blood upon live BCG stimulation was abrogated in patients lacking NK cells or NK and T cells.32 In the same study, the production of IFN-γ by whole blood from IL-12p40– and IL-12Rβ1–deficient patients is abolished or severely reduced, respectively.32 Taken together with the strong genetic epidemiologic data showing that IFN-γ/IL-12/23 axis is critical for the protection against Mycobacterium and Salmonella in vivo in humans,30 these results indicate that NK cells and T cells are the source of IFN-γ and that IL-12p40 and IL-12Rβ1 are required for this production. In the case of Salmonella, NK and CD56+ T cells produce IFN-γ in response to Salmonella typhimurium–infected macrophages in vitro (N. Lapaque and J. Trowsdale, personal communication, December 17, 2007). Although the NK cell hyporesponsiveness observed in IL-12/23 axis–deficient patients is moderate, the biologic consequences of this defect should not be hastily underestimated. A quantitative difference in NK cell reactivity in vitro might be translated in vivo by a delay in the early control of microbial replication and/or in the arming of the immune response (eg, myeloid cell activation as well as T- and B-cell activation by IFN-γ production). In such a situation of competition between the onset of the immune response and the development of an aggression, the consequences of a reduction and/or a postponement of the NK cell response might be more severe that intuitively thought. Moreover, the clinical consequences might be limited to certain disease conditions. For instance, MHC class I deficiency in mice leads to a targeted deficit in the rejection of MHC class I− tumors or hematopoietic grafts, but does not compromise the ability of NK cells to keep in check MCMV infections.66 However, the potential role for mouse NK cells in the control of M tuberculosis in vivo43 is disputed.67 Furthermore, the rare cases of true NK cell–selective deficiencies do not advocate for a role of NK cells in MSMD. No mycobacteriosis nor salmonellosis has been described in these patients, although mouse NK cells have been recently reported to control Salmonella enterica serovar Typhimurium infections.68 The recent description of 4 children with a novel primary NK cell immunodeficiency rather showed that these patients developed Epstein-Barr virus–driven lymphoproliferative disorder or severe respiratory illnesses of probable viral etiology.69 Other clinical reports are also consistent with a role of NK cells in defense against human herpesviral infection.70 By contrast, few studies have analyzed the impact of CD56+ T cells during Mycobacterium or Salmonella infections, but the size of this T-cell subset in PBMCs is increased in both conditions.71,72 In the presence of live BCG and Salmonella typhimurium–infected macrophages in vitro, CD56+ T cells, but not CD56− T cells, appear to produce IFN-γ in absence of TCR stimulation. Thus, consistent with other reports on mouse memory CD8 T-cell subsets, a major functional feature of the subset of CD56+ T cells resides in their “NK-like” effector functions.73 Interestingly, high counts of circulating CD56+ T cells at diagnosis of pulmonary tuberculosis correlated significantly with negative sputum culture after 8 weeks of treatment.74 Taken together with their expansion in a limited set of inflammatory conditions and their high effector potential (both IFN-γ production and cytotoxicity), these data pave the way to dissect whether NK-like CD56+ T cells might be critical players in the protective IL-12/23/IFN-γ–dependent immune response against Mycobacterium and Salmonella in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicolas Lapaque and John Trowsdale (Cambridge) for sharing unpublished results, and Corinne Beziers-Lafosse (CIML) for her help in the illustrations.
This work was supported by Inserm, CNRS, the European Community (“ALLOSTEM,” E.V.), Ligue Nationale contre le Cancer (“Equipe labellisée La Ligue”), the Agence Nationale de la Recherche (“Réseau Innovation Biotechnologies” and “Microbiologie Immunologie–Maladies Emergentes”), Institut National du Cancer, Ministère de l'Enseignement Supérieur et de la Recherche, and Institut Universitaire de France.
Authorship
Contribution: S.G., C.C., J.-L.C., and E.V. designed the experiments and wrote the paper; M.S.T. and L.B. performed experiments in mice (data not shown); L.deB., E.J., C.F., J.F., O.F.-S., Y.C., J.L., J.-L.S., C.B., S.A.J., and S.A.-H. collected patient materials; and L.A. performed statistical analysis.
Conflict-of-interest disclosure: E.V. is a founder and shareholder of Innate-Pharma. All other authors declare no competing financial interests.
Correspondence: Eric Vivier, Centre d'Immunologie de Marseille-Luminy, Case 906, 13288 Marseille cedex 9, France; e-mail: vivier@ciml.univ-mrs.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal