Abstract
Evidence from population genetics, gene expression microarrays, and assays of ex vivo T-cell function indicates that the cytotoxic T lymphocyte (CTL) response to human T-lymphotropic virus type 1 (HTLV-1) controls the level of HTLV-1 expression and the proviral load. The rate at which CTLs kill autologous HTLV-1–infected lymphocytes differs significantly among infected people, but the reasons for such variation are unknown. Here, we demonstrate a strong negative cor-relation between the frequency of CD4+FoxP3+ Tax− regulatory T cells (Tregs) in the circulation and the rate of CTL-mediated lysis of autologous HTLV-1–infected cells ex vivo. We propose that the frequency of CD4+FoxP3+ Tax− Tregs is one of the chief determinants of the efficiency of T cell–mediated immune control of HTLV-1.
Introduction
Human T-lymphotropic virus type 1 (HTLV-1) is a persistent retrovirus that infects 10 to 20 million people worldwide. The majority of infected individuals remain lifelong asymptomatic carriers (ACs) of the virus. However, 2% to 3% of infected individuals develop a progressive inflammation of the central nervous system called HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP1 ). HTLV-1 causes leukemia or lymphoma in approximately 4% of seropositive individuals.2
HTLV-1 encodes a number of regulatory proteins,3,4 the most studied of which is Tax. Tax is a pleiotropic transcriptional transactivator and is central to the HTLV-1 life cycle. Tax increases the expression of many cellular genes, including several genes involved in T-cell activation and proliferation.5 Tax expression is thus promitotic and drives CD4+ T-cell proliferation to increase HTLV-1 proviral load.6-8 Tax is also the immunodominant HTLV-1 antigen recognized by the strong CD8+ cytotoxic T-cell (CTL) response.9,10 The host cellular immune response counteracts this Tax-mediated proliferation and increase in proviral load. The level of spontaneous HTLV-1 gene expression in naturally infected peripheral blood mononuclear cells (PBMCs) during short-term culture can be increased if CD8+ cells are removed.11 In this study, we used Tax protein as a marker of HTLV-1 expression in naturally infected PBMCs.
The steady-state HTLV-1 proviral load in vivo is therefore likely to be determined by proliferation of infected cells balanced by the clearance of productively infected cells by CD8+ cells.12-14
Because the immune response plays a major role in the control of HTLV-1 infection, it is important to study the possible role of immune regulation in the control of HTLV-1 infection. One of the major components of immune regulation is the regulatory T cell (Treg).15,16 Tregs are specialized subsets of CD4+ T cells that suppress effector T-cell responses in chronic disease, including retroviral infection.17,18 Tregs can suppress the function of antigen-presenting cells (APCs) and CD4+ and CD8+ effector T cells16 by cell-cell contact or by the secretion of cytokines such as IL-10 and TGF-β1.
Identification of Tregs by flow cytometry remains ambiguous, because more than one subpopulation of T cell is capable of suppressive function,19 and because most markers of Tregs proposed until now are also expressed by activated CD4+ T lymphocytes: CD25, CTLA-4, GITR, and CD103.20,21 The significance of CD25hi expression, which has been widely used as part of a phenotypic definition of Tregs (CD4+ CD25hi FoxP3+), is especially uncertain in HTLV-1 infection because CD25 expression is strongly induced by the HTLV-1 Tax protein.22,23 Recently it was reported that the absence of expression of CD127 on CD4+ cells can be used as a marker of Tregs.24 It appears that the best current single marker of Tregs in CD4+ cells in the human, as in the mouse, is the forkhead transcription factor FoxP3, and the phenotype CD4+FoxP3+ is now increasingly used to identify a major population of Tregs.15
In this study, we tested the hypothesis that the efficiency of the CTL response to HTLV-1–infected cells25 correlates with the frequency of CD4+FoxP3+ cells. We conclude that the CD4+FoxP3+ Tax− cell population is a major determinant of the efficiency of immune control of HTLV-1 infection.
Methods
Cells and cultures
PBMCs were isolated by density centrifugation on Histopaque (Sigma, Poole, United Kingdom) from EDTA-anticoagulated blood samples taken from HTLV-1–infected individuals. All individuals attended the HTLV-1 clinic at St Mary's Hospital, London, and informed consent was obtained in accordance with the Declaration of Helsinki. This study was approved by St Mary's National Health Service Trust Local Research Ethics committee. PBMCs were cryopreserved and thawed when required. Cells were cultured in complete medium (RPMI-1640, 10% FCS, pen/strep l-glutamine at 37°C, 5% CO2 for 18 hours. If CD8+ cell–depleted PBMCs were required, CD8+ cells were removed by positive selection using magnetic microbeads following the manufacturer's instructions (Miltenyi Biotec, Surrey, United Kingdom). The median CD8+ cell depletion achieved was 93% (range: 84%-98%).
Flow cytometry
To detect Tax expression and FoxP3 in HTLV-1–infected cells, whole PBMCs or CD8+ cell–depleted PBMCs were incubated for 18 hours. The cells were then surface stained with monoclonal antibodies to CD4 and CD8 (each at 15 μg/mL; Beckman Coulter, Marseille, France). Cells were then fixed and permeabilized with a commercial kit (Insight Biotechnology, Wembley, United Kingdom), following the manufacturer's protocol. Finally, cells were stained intracellularly with the FITC-conjugated antibody anti-Tax protein Lt-426 diluted 1/100 and antihuman FoxP3-PE antibody (clone 236A/E7; Insight Biotechnology) in permeabilization buffer (Insight Biotechnology) following the manufacturer's protocol. Following staining, cells were analyzed on a Coulter Epics XL flow cytometer. Thirty thousand events were routinely collected. Viable lymphocytes were gated for closer analysis using Expo32 analysis software (Beckman Coulter).
Proviral load measurement
DNA was extracted from PBMCs following the manufacturer's protocol (DNeasy Tissue Kit; Qiagen, Crawley, United Kingdom) and eluted in polymerase chain reaction (PCR) grade H2O. Three dilutions of eluted DNA (1:4, 1:8, 1:16) were amplified for HTLV-1 DNA (Tax-specific primers as in Tosswill et al27 ) and β-actin by real-time quantitative PCR (Q-PCR) in a Roche light cycler using SYBR Green 1 dye incorporation (Roche Diagnostics, Burgess Hill, United Kingdom). Incorporation was detected at 85°C at the end of each of 45 amplification cycles. Standard curves were generated using DNA from the TARL-2 cell line, which carries a single HTLV-1 provirus copy per cell.28 The sample copy number was estimated by interpolation from the standard curve, calculated as an average of the 3 dilutions and expressed as the proportion of PBMCs infected.
FoxP3 mRNA measurement
CD4+ cells were positively selected from PBMCs on a magnetic-activated cell sorting (MACS) column (Miltenyi Biotec) using magnetic microbeads according to the manufacturer's instructions. Purity was confirmed by flow cytometry to be more than 90% in most cases. Total RNA was extracted from freshly isolated cells using the RNeasy Method (Qiagen). RNA was reverse transcribed using an high-performance liquid chromatography (HPLC)–purified oligo(dT) primer with a T7 RNA pol site attached to the 5′ end (Transgenomic Bioconsumables, Glasgow, United Kingdom), with the SuperScript Double-Stranded cDNA Synthesis kit (Invitrogen Life Technologies, Paisley, United Kingdom). BIRC2 was chosen as a housekeeping gene because it exhibited the most uniform expression across all patient samples in the microarray data. HTLV-1 tax mRNA expression was assayed using the same method, except that cDNA from the Tax+ cell line MT2 was used to generate the standard curve.29
Gene-specific primers for the constitutively expressed (“housekeeping”) gene BIRC2 were designed using sequences deposited in GenBank and obtained from Invitrogen Life Technologies. The following primer set was used: FoxP3-for, 5′-TCC CAG AGT TCC TCC ACA AC-3′ and FoxP3-rev, 5′-ATT GAG TGG TGT CCG CTG CTT CT-3′. Real-time kinetic PCR was performed on a LightCycler (Roche Diagnostics) using the LightCycler FastStart DNA Master SYBR Green I kit as recommended by the manufacturer.
CD8+ cell lytic efficiency assay
The rate (or “efficiency”) of CD8+ cell–mediated lysis of HTLV-1–infected cells was estimated as recently described.25 PBMCs were thawed and washed, and then CD8+ cells were positively selected with anti-CD8-coupled magnetic microbeads and titrated back into the CD8-depleted fraction at CD8+/CD8− ratios above, below, and including the physiological ratio for that individual. Cells were then cocultured at 37°C for 18 hours, harvested, and stained for Tax, FoxP3, CD4, and CD8 as described above. The proportion of Tax+CD4+ cells surviving coculture was plotted against the proportion of CD8+ cells present, and a mathematic model25 was then fitted to the data. CD8+ cell lytic efficiency (expressed as the proportion of Tax-expressing CD4+ cells killed per CD8+ cell per day) was calculated for each HTLV-1–infected individual tested. All assays were done in duplicate and the results are presented as the mean CD8+ cell lytic efficiency (percentage CD4+ Tax+ cells killed/CD8+ cell/day). The results of a typical assay are shown in Figure S1A (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Statistical analysis
Nonparametric statistical tests were used as appropriate taking the null hypothesis and the sample size into account. The Spearman rank-order correlation coefficient was calculated when the significance of observed changes in 2 parameters across all HTLV-1–infected individuals was tested. The measure of rate of lysis was calculate with the software SPSS 12-0 for Windows (Chicago, IL).
Quantification of cytokine concentrations
Using Luminex multibead antibody kit technology (Millipore, Southampton, United Kingdom), we quantified the concentration of cytokines in supernatant of PBMCs after 18 hours of incubation at the physiological ratio of CD4+/CD8+ cells point (with no depletion of CD8). The supernatant was centrifuged at 10 000 rpm (3000g) for 3 minutes to remove all debris and cells, and frozen at −80°C. For detection of TGF-β1 the supernatant was first acidified, according to the manufacturer's indications.
For each patient, we also quantified the concentration of the same cytokines in plasma, taken at the time of purification of PBMCs. The detection limits of these cytokines were 12 to 14 pg/mL.
Results
FoxP3 expression in HTLV-1 patients
Flow cytometric analysis of samples from 8 patients with HAM/TSP confirmed that CD4+ Tax+ cells were also CD25+ (Figure S1C). This result has been previously described,30 and suggests that it is not appropriate to use high CD25 expression as a marker of Tregs in HTLV-1 infection. For this reason, we chose to characterize Tregs by the expression of FoxP3 in CD4+ T cells.
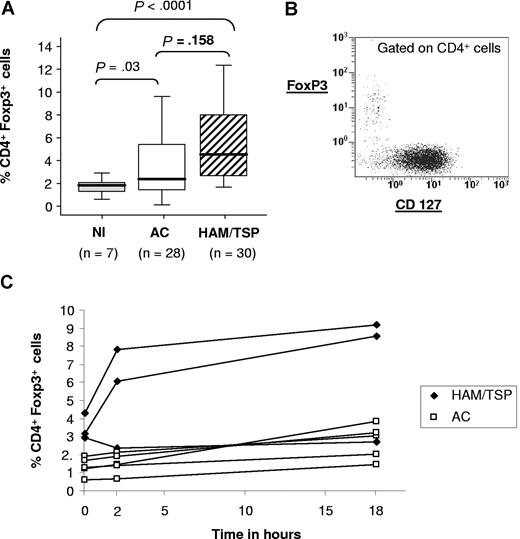
In each HTLV-1–seropositive patient, we measured the frequency of FoxP3 expression in CD4+ cells after 18 hours of incubation in vitro at the physiological ratio of CD4+/CD8+ cells. In Figure 1A, we show the expression of FoxP3 in total CD4+ T cells from 7 uninfected patients and 58 HTLV-1–seropositive patients (28 ACs and 30 patients with HAM/TSP). The results show a significant difference in the frequency of expression of FoxP3 between HTLV-1–infected individuals (ACs and HAM/TSP patients combined) and uninfected patients. But there was no significant difference in the frequency of FoxP3 expression between ACs and HAM/TSP patients (P = .158).
FoxP3 expression in HTLV-1–seropositive subjects. (A) FoxP3 expression in CD4+ cells in 7 uninfected subjects, 28 ACs, and 30 HAM/TSP patients gated on the CD4+. The P value was calculated by an unpaired t test (2-tailed). (B) Dot plot showing FoxP3 and CD127 expression in PBMCs, gated on the CD4+ population, from a representative HAM/TSP patient. (C) Time course of FoxP3 expression in CD4+ cells, for 4 different ACs and 4 HAM/TSP patients.
FoxP3 expression in HTLV-1–seropositive subjects. (A) FoxP3 expression in CD4+ cells in 7 uninfected subjects, 28 ACs, and 30 HAM/TSP patients gated on the CD4+. The P value was calculated by an unpaired t test (2-tailed). (B) Dot plot showing FoxP3 and CD127 expression in PBMCs, gated on the CD4+ population, from a representative HAM/TSP patient. (C) Time course of FoxP3 expression in CD4+ cells, for 4 different ACs and 4 HAM/TSP patients.
Expression of the IL-7 receptor (CD127) was recently reported to be down-regulated in CD25hi FoxP3+ regulatory T cells.24 Flow cytometric analysis confirmed that all CD4+FoxP3+ were also CD127low (Figure 1B).
In PBMCs from 8 HTLV-1–infected individuals (4 ACs and 4 HAM/TSP patients) we measured the expression of FoxP3 in CD4+ T cells during in vitro incubation for 18 hours (Figure 1C). The results showed no significant variation in mean FoxP3 expression between 0 and 18 hours, although FoxP3 rose between 0 and 2 hours in 2 patients with HAM/TSP.
HTLV-1 infection in FoxP3+ T cells
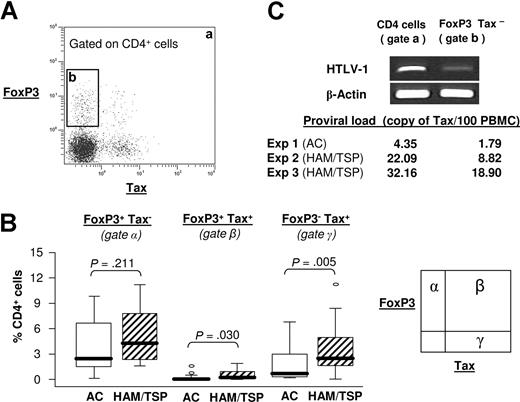
A typical expression of FoxP3 and Tax in CD4+ T cells from an HTLV-1–infected patient is shown in Figure 2A.
FoxP3 expression and Tax expression. (A) Density plot showing Tax and FoxP3 expression in CD4+ cells from a representative AC patient after 18-hour incubation. The dot plot was divided in 2 gates: a indicates total CD4+ cells; b, FoxP3+Tax−CD4+ cells. (B) Percentage of CD4+ cells that express FoxP3 alone (gate α), FoxP3 and Tax (gate β), and Tax alone (gate γ). The P value was calculated by an unpaired t test (2-tailed). This percentage was measured on 28 ACs and 30 HAM/TSP patients. (C) Classic PCR for one AC and quantification of proviral load in 3 independent HTLV-1–seropositive patients. FoxP3+Tax− cells were isolated by cell sorting of the CD4+ population (gate b) and compared with the total CD4+ cell population (gate a). The data were normalized to the level of beta-actin cDNA, and expressed as Tax copy number per 100 PBMCs.
FoxP3 expression and Tax expression. (A) Density plot showing Tax and FoxP3 expression in CD4+ cells from a representative AC patient after 18-hour incubation. The dot plot was divided in 2 gates: a indicates total CD4+ cells; b, FoxP3+Tax−CD4+ cells. (B) Percentage of CD4+ cells that express FoxP3 alone (gate α), FoxP3 and Tax (gate β), and Tax alone (gate γ). The P value was calculated by an unpaired t test (2-tailed). This percentage was measured on 28 ACs and 30 HAM/TSP patients. (C) Classic PCR for one AC and quantification of proviral load in 3 independent HTLV-1–seropositive patients. FoxP3+Tax− cells were isolated by cell sorting of the CD4+ population (gate b) and compared with the total CD4+ cell population (gate a). The data were normalized to the level of beta-actin cDNA, and expressed as Tax copy number per 100 PBMCs.
FoxP3 was expressed in both Tax+ and Tax− populations. The frequency of FoxP3 expression was higher in Tax+ CD4+ T cells than in Tax− CD4+ T cells (Figure S2), but the population of FoxP3+ Tax+ cells was small (< 2% of CD4+ T cells) compared with the size of FoxP3+ Tax− population (≤ 14% of CD4+ T cells) (Figure 2B).
Finally, we also separated the different populations expressing FoxP3 by flow sorting and isolated the Tax− FoxP3+ population (Figure 2C gate b). By Q-PCR we then measured the proviral load in 3 independent experiments in the Tax− FoxP3+ population and in the total CD4+ T-cell population (Figure 2C gate a). The results (Figure 2C) show that the proviral load was lower in the population of CD4+FoxP3+ Tax− cells than in the total CD4+ population. This result suggests that HTLV-1 does not preferentially infect FoxP3+ T cells, and that the majority of FoxP3+ Tax− CD4+ T cells do not carry the provirus of HTLV-1.
Correlation between the rate of lysis and percentage of CD4+FoxP3+ cells
We previously described a method25 to quantify the capacity of CD8+ cells to kill autologous CD4+ Tax+ cells in vitro. Figure S1A represents the results of typical experiments showing Tax and FoxP3 expression in samples from 2 HAM/TSP patients (codes TAT and TBS). Each quadrant shows the percentage of Tax+ and FoxP3+ cells in the CD4+ population, for an increasing frequency of CD8+ cells. The point marked “normal” corresponds to the physiological frequency of CD8+ cells for that patient. The regression curve is calculated using a mathematic model,25 and this permits the determination of the per-cell rate or “efficiency” of lysis of CD4+ Tax+ cells by CD8+ cells, denoted epsilon (ϵ). In this example the patient with HAM/TSP, coded TAT, had a lower rate of lysis (ϵ = 0.035) than the patient TBS (ϵ = 0.184). Note that the patient with a low rate of lysis had a high percentage of CD4+ FoxP3+ cells, and conversely the patient with a high rate of lysis had a low percentage of CD4+ FoxP3+ cells.
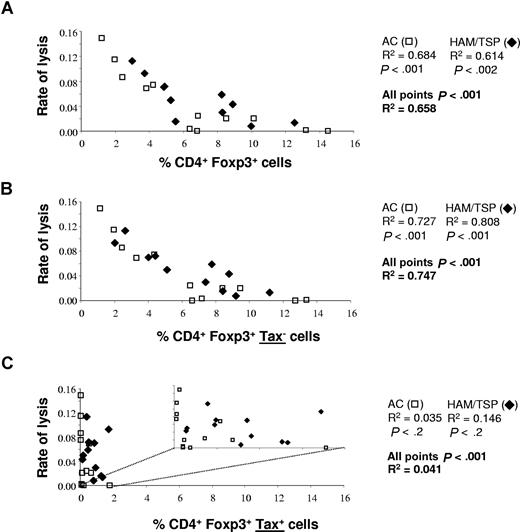
We then wished to test the hypothesis that the percentage of CD4+ FoxP3+ cells correlates with the rate of lysis of CD4+ Tax+ cells in vitro. The results (Figure 3A) revealed a significant negative correlation between the rate of lysis and the percentage of CD4+ FoxP3+ cells in patients infected with HTLV-1 (ACs alone: P < .001; HAM/TSP patients alone: P < .002; ACs and HAM/TSP patients combined: P < .001). As previously observed,25 the range of variation of lysis rate was similar in ACs and HAM/TSP patients.
Correlation between FoxP3 expression and the rate of CD8+ cell–mediated lysis. The percentage of FoxP3+ cells in all CD4+ cells (A), the percentage of CD4+FoxP3+ Tax− cells (B), and the percentage of CD4+FoxP3+ Tax+ cells were plotted against the efficiency of lysis. The data represent the result obtained with samples from 15 ACs and 19 HAM/TSP patients. The P values were determined by a 2-tailed Spearman test. For the percentage of CD4+FoxP3+ Tax+ cells, we have also represented the correlation with the efficiency of lysis on a smaller scale, to clarify the lack of correlation here (C).
Correlation between FoxP3 expression and the rate of CD8+ cell–mediated lysis. The percentage of FoxP3+ cells in all CD4+ cells (A), the percentage of CD4+FoxP3+ Tax− cells (B), and the percentage of CD4+FoxP3+ Tax+ cells were plotted against the efficiency of lysis. The data represent the result obtained with samples from 15 ACs and 19 HAM/TSP patients. The P values were determined by a 2-tailed Spearman test. For the percentage of CD4+FoxP3+ Tax+ cells, we have also represented the correlation with the efficiency of lysis on a smaller scale, to clarify the lack of correlation here (C).
To evaluate the influence of FoxP3 expression in Tax+ cells and in Tax− cells, respectively, in determining the efficiency of lysis, we compared the correlation between the efficiency of lysis and the percentage of CD4+FoxP3+ Tax− cells (Figure 3B) and CD4+FoxP3+ Tax+ cells (Figure 3C). As described above, the percentage of CD4+FoxP3+ Tax+ cells was consistently lower than the percentage of CD4+FoxP3+ Tax− cells.
The results showed that the percentage of CD4+FoxP3+ Tax+ population did not correlate with the rate of lysis (ACs alone: P < .2; HAM/TSP patients alone: P < .2; ACs and HAM/TSP patients combined: P < .5; Figure 3C). However, there was a strong negative correlation between the rate of lysis and the percentage of CD4+FoxP3+ in the Tax− population. Furthermore, the rate of lysis in HAM/TSP patients correlated more strongly with the frequency of FoxP3 expression in Tax− cells (R2 = 0.747; P < .001) than with the frequency of FoxP3 expression in all CD4+ cells (Tax+ and Tax− combined) (R2 = 0.658; P < .002) (Figure 3A,C). There was an even stronger correlation between the rate of lysis and log10 (% CD4+FoxP3+ Tax−) (R2 = 0.911).
CD4+FoxP3+ cell frequency correlated with Tax expression and proviral load
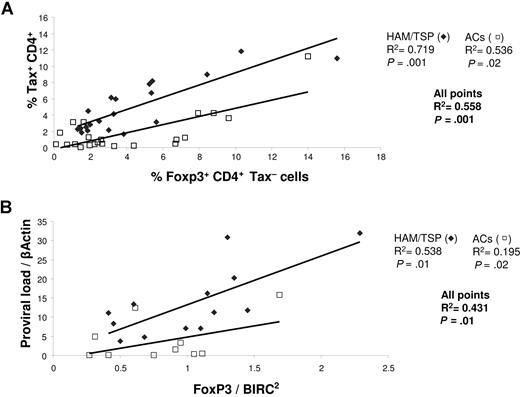
In previous work, we concluded that the rate of CD8+ cell–mediated lysis controls the rate of Tax expression and thus also the proviral load of HTLV-1.8,25 Because the expression of FoxP3 correlates with the rate of lysis, we wished to determine the impact of FoxP3 expression on both Tax expression and the proviral load. We observed (Figure 4A) a correlation between the percentage of CD4+FoxP3+ Tax− cells and the percentage of CD4+ cells that expressed Tax. This correlation was statistically significant in both ACs and HAM/TSP patients. However, the 2 respective correlations were distinct (Figure 4A). That is, at a given frequency of FoxP3 expression, the level of Tax expression was systematically higher in HAM/TSP patients than in ACs (Figure S1B). This is similar to our previous observation that Tax expression at a given proviral load was systematically greater in HAM/TSP patients than in ACs.8 These results confirm the importance of CD4+FoxP3+ cells in the control of Tax expression and in particular the importance of CD4+FoxP3+ Tax− cells.
Correlation between FoxP3 expression, Tax expression, and proviral load. (A) The percentage of FoxP3 expression in CD4+Tax− cells was correlated with the percentage of CD4+Tax+ cells both in ACs (N = 23) and in HAM/TSP patients (N = 22). The P values were calculated by a 2-tailed Spearman test. (B) The proviral load was correlated with the level of FoxP3 mRNA expression in CD4+ cells (measured by Q-PCR) both in ACs (N = 11) and in patients with HAM/TSP (N = 13).
Correlation between FoxP3 expression, Tax expression, and proviral load. (A) The percentage of FoxP3 expression in CD4+Tax− cells was correlated with the percentage of CD4+Tax+ cells both in ACs (N = 23) and in HAM/TSP patients (N = 22). The P values were calculated by a 2-tailed Spearman test. (B) The proviral load was correlated with the level of FoxP3 mRNA expression in CD4+ cells (measured by Q-PCR) both in ACs (N = 11) and in patients with HAM/TSP (N = 13).
By quantitative PCR, we measured the level of expression of FoxP3 in CD4+ cells and correlated it with the proviral load (Figure 4B). The results show a significant positive correlation between FoxP3 expression and the proviral load and, as observed with the level of Tax expression (Figure 4A), 2 distinct correlations were seen (one for ACs and one for HAM/TSP patients).
Quantification of cytokines in lysis assay supernatant
Using fluorescent microbead technology, we measured the concentration of the cytokines TNF-α, IL-2, IL-6, IL-10, IL-12(p70), IL-17, IFN-γ, and TGF-β1 in the supernatant of PBMCs after in vitro incubation for 18 hours (Figure S3). Neither IL-12(p70) nor IL-17 was detected in any sample.
The PBMCs from some HAM/TSP patients had a high IL-10 secretion, but the median level of IL-10 expression in the 15 HAM/TSP patients was not significantly different from the median level in samples from uninfected control subjects. In contrast, there was a significantly lower rate of spontaneous secretion of TGF-β1 in both ACs and HAM/TSP patients than in samples from uninfected controls (Figure S3A). For both IL-10 and TGF-β1, we did not observe a correlation between the level of cytokine expression and either the frequency of FoxP3 expression in CD4+ cells or the rate of lysis (results not shown). As a further test of the possible effects of IL-10, in 2 separate experiments we added a high concentration of IL-10 during the lysis assay. The results showed that the addition of the IL-10 did not alter the rate of CD8+ cell–mediated lysis (Figure S3B).
Discussion
We report here 2 principal findings. First, HTLV-1 infection was associated with an abnormally high frequency of expression of FoxP3 in circulating CD4+ cells. Second, the frequency of CD4+FoxP3+ cells in the circulation showed a strong negative correlation with the rate of CD8+ cell–mediated lysis of autologous HTLV-1–infected cells.
The high frequency of FoxP3 expression in HTLV-1–infected subjects suggests that HTLV-1 directly or indirectly induces FoxP3 expression in the CD4+ T-cell population. In a recent study of Tregs in HTLV-1 infection,31 it was reported that the fraction of cells that expressed FoxP3 in the CD4+ CD25+ population was lower in HTLV-1–infected subjects, in apparent conflict with the results reported here. The authors concluded that the frequency of Tregs was abnormally low in HTLV-1 infection. However, HTLV-1 Tax protein is known to induce strong expression of CD2523,30,32 (Figure S1C). It is therefore likely that the frequency of FoxP3+ cells in the CD4+ CD25+ population is reduced, in HTLV-1 infection, by Tax-induced expression of CD25. This produces an apparent reduction in the frequency of Tregs (ie, when they are defined as CD4+CD25+FoxP3+) in HTLV-1 infection. This reduction is especially marked in HAM/TSP patients because Tax expression is typically greater in HAM/TSP patients than in ACs at a given proviral load8,33 (Figure S1B). We confirmed this hypothesis by flow cytometric analysis of PBMCs from 4 patients with HAM/TSP, 2 ACs, and 2 uninfected controls. The results (Table 1) showed that in patients with HAM/TSP there was indeed an increase in the percentage of FoxP3+ cells in the total CD4+ population compared with that in uninfected individuals, but at the same time the percentage of FoxP3+ cells in the CD4+CD25+ population was lower than in uninfected individuals. We conclude that it is inappropriate to use CD25 as a marker of Tregs in HTLV-1 infection. In the present study, we avoided the use of CD25 in the working phenotypic definition of Tregs and instead quantified the frequency of CD4+FoxP3+ cells.
The effect of HTLV-1 infection on the frequency of Tregs depends on the phenotypic definition of Tregs
| Patients . | % FoxP3+ in CD4+ cells . | % FoxP3+ in the CD4+CD25+ fraction . |
|---|---|---|
| Uninfected 1 | 1.1 | 33.1 |
| Uninfected 2 | 0.7 | 33.8 |
| AC 1 | 1.2 | 22.6 |
| AC 2 | 2.2 | 35.6 |
| HAM/TSP 1 | 4.6 | 13.4 |
| HAM/TSP 2 | 6.1 | 11 |
| HAM/TSP 3 | 5.6 | 5 |
| HAM/TSP 4 | 3.4 | 15.3 |
| Patients . | % FoxP3+ in CD4+ cells . | % FoxP3+ in the CD4+CD25+ fraction . |
|---|---|---|
| Uninfected 1 | 1.1 | 33.1 |
| Uninfected 2 | 0.7 | 33.8 |
| AC 1 | 1.2 | 22.6 |
| AC 2 | 2.2 | 35.6 |
| HAM/TSP 1 | 4.6 | 13.4 |
| HAM/TSP 2 | 6.1 | 11 |
| HAM/TSP 3 | 5.6 | 5 |
| HAM/TSP 4 | 3.4 | 15.3 |
HTLV-1 infection was associated with an increase in the proportion of CD4+ T cells that express FoxP3 (column 1). This increase was especially marked in patients with HAM/TSP, who have a high proviral load of HTLV-1 and a high rate of proviral expression. However, the proportion of CD4+CD25+ cells that express FoxP3 was lower in patients with HAM/TSP than in uninfected individuals. The explanation for this is that HTLV-1 infection of CD4+ T cells strongly induces CD25 expression, thereby increasing the denominator of the fraction FoxP3+/CD4+CD25+. Because of the confounding effect of HTLV-1–induced expression of CD25, in the present study we avoided the use of CD25 in the working definition of the phenotype of Tregs, and used instead the simpler definition CD4+FoxP3+.
The percentage of CD4+ FoxP3+ cells in the circulation was proportional to the level of HTLV-1 expression (Figure 4A). However, there was significant variation between individuals in the percentage of Tax-expressing cells at a given percentage of CD4+FoxP3+ cells. Further, the finding that the percentage of Tax-expressing cells was systematically greater in patients with HAM/TSP than in ACs at a given percentage of CD4+ FoxP3+ cells (Figure 4A) implies that additional factors act in patients with HAM/TSP to increase the Tax expression at a given percentage of FoxP3+ cells. This conclusion is consistent with previous studies8,33 that showed that the level of Tax expression was greater in patients with HAM/TSP than in asymptomatic carriers at a given proviral load. The factors that determine the rate of Tax expression at a given proviral load remain unknown; possible factors include epigenetic modifications and the genomic integration site of HTLV-1 (Kiran N. Meekings, G.P.T., C.R.M.B., material submitted).
A small proportion of CD4+ FoxP3+ cells was also found to express HTLV-1 Tax protein (Figure 2A). The fraction of FoxP3+ cells that expressed Tax typically exceeded the fraction of FoxP3− cells that expressed Tax (Figure S2A). There are 2 possible explanations for this observation, which are not mutually exclusive. First, HTLV-1 might preferentially (although not exclusively) infect FoxP3+ cells. This possibility cannot be excluded on the basis of the present data. However, the majority of FoxP3+ cells were Tax− (Figure 2B); and cell sorting and quantitative PCR demonstrated that the majority of these FoxP3+Tax− cells did not carry the HTLV-1 provirus (Figure 2C). Second, HTLV-1 expression might induce FoxP3 expression in the infected cell. Human T cells transiently express FoxP3 upon activation34,35 ; therefore, FoxP3 expression in these cells might result from Tax-induced T-cell activation. However, it was recently reported that Tax transfection of CD4+CD25+ cells purified from uninfected PBMCs reduced FoxP3 expression at the mRNA level.36 Finally Tao et al37 have suggested that histone deacetylases (HDACs) may control FoxP3 expression, and we have previously demonstrated that HDACs also play a role in HTLV-1 infection.38 But whatever the interaction between FoxP3 and Tax in the infected cell, the present data show that, in contrast with the CD4+ FoxP3+ Tax− population, the size of the CD4+FoxP3+Tax+ population was small (< 0.2% vs ≤ 4% of circulating CD4+FoxP3+ T cells, respectively; Figures 2B and 3), and was unrelated to the CD8+ cell–mediated lysis rate (compare Figure 3B,C).
The data reported here revealed a strong negative correlation between the percentage of CD4+FoxP3+ Tax− cells and the rate of lysis of naturally infected CD4+ T cells by autologous CD8+ cells isolated from the fresh blood of HTLV-1–infected individuals. This correlation might result either from suppression of CTL activity by FoxP3+ cells or conversely from suppression of FoxP3+ cell proliferation by CTLs, presumably by CD8+ T cell–mediated lysis of HTLV-1–infected FoxP3+ cells. We propose that the first possibility is true, for 3 reasons. First, the present data show a strong (negative) correlation between the CD8+ T cell–mediated lysis rate (epsilon) and the frequency of FoxP3+ cells that are Tax− (ie, that do not express HTLV-1 antigens and therefore cannot serve as targets for HTLV-1–specific CTLs). Second, there was no significant difference between HAM/TSP patients and ACs in the mean (or median) frequency of FoxP3+ cells in the CD4+ population (Figure 1A), whereas the cells from HAM/TSP patients consistently expressed greater levels of HTLV-1 antigens than those from ACs at the same proviral load.8,33 Third, evidence from in vitro experiments demonstrates that FoxP3+ cells can suppress both the replication and the activity of CTLs.16,39 Finally, recent reports from other laboratories have demonstrated suppression of CTLs by FoxP3+ T cells.40,41
Quantification of cytokines in the PBMC culture supernatant confirmed a high rate of secretion of IFN-γ, TNF-α, and IL-2 in cells from HTLV-1–seropositive patients (Figure S3A) consistent with T-cell activation and with previous results.42 The measurement of concentration of IL-10 and TGF-β1 in the supernatant of the lysis assays in this study did not suggest a role of these cytokines in the control of the rate of CD8+ cell–mediated lysis (Figure S3B). IL-10 is well known to be associated with the control of the immune response.16,43 In 2 experiments, addition of recombinant IL-10 to the culture medium had no effect on the efficiency of lysis. However, we cannot exclude such a role in vivo, where CTL activity might be influenced by the relative concentrations of cytokines in the local milieu. It is possible that CD4+FoxP3+ cells exert their regulatory effect on HTLV-1–specific CD8+ T cells by a mechanism that depends on cell contact, with or without the involvement of cytokines.19 But at present no means exist of testing this possibility in our model, because there is no known appropriate surface marker of the CD4+FoxP3+ cells that might allow their selection and separation. As noted above, CD25 is not a good marker, because its expression is strongly induced by HTLV-1 Tax protein.
We previously reported a significant negative correlation between the rate of CD8+ cell–mediated lysis of HTLV-1–infected cells in vitro and the proviral load in vivo.25 We hypothesized that CTL surveillance limits HTLV-1 replication in vivo. This implies that Tax+ T cells turn over abnormally rapidly in vivo as a result of CTL-mediated lysis. Recently, measurement of the turnover rate of CD4+ T-cell populations in vivo, by labeling proliferating cells with deuterated glucose, demonstrated that the lifespan of a Tax+ CD4+ T cell in vivo was indeed reduced to approximately 1 day from the normal approximately 30 days.14 The conclusion that CTLs limit proviral load in vivo by killing HTLV-1–expressing cells is consistent with data from host population immunogenetics,44-46 gene expression microarrays,29 evidence of positive selection,47,48 and CTL escape mutations49 in the tax gene, which encodes the immunodominant antigen recognized by HTLV-1–specific CTLs.9,10
We conclude that HTLV-1 infection is associated with abnormal expression of FoxP3 in circulating CD4+ cells and that the frequency of CD4+FoxP3+ Tax− T cells is an important determinant of the rate of CD8+ cell–mediated immune surveillance of HTLV-1–infected cells in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Wellcome Trust (United Kingdom) and by funding under the Sixth Research Framework Program of the European Union, Project INCA (LSHC-CT-2005-018704).
Wellcome Trust
Authorship
Contribution: F.T. designed and carried out experiments, analyzed data, and wrote the paper; A.H. carried out assays of HTLV-1 proviral load; Y.T. supplied anti-Tax antibody (Lt-4) and provided technical advice; G.P.T. carried out clinical work and supplied blood samples; and C.R.M.B. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frederic Toulza, Department of Immunology, Imperial College, Norfolk Place, London W2 1PG, United Kingdom; e-mail: f.toulza@imperial.ac.uk; or Charles Bangham, Department of Immunology, Imperial College, Norfolk Place, London W2 1PG, United Kingdom; e-mail: c.bangham@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal