We previously reported that bortezomib inactivates hypoxia-inducible factor-1α (HIF-1α) C-terminal transactivation domain (CAD) via factor-inhibiting HIF-1 (FIH).1 Dr Kaluz and colleagues raise several issues regarding concentrations of bortezomib and the mechanism underlying FIH stimulation. We thank them for their critical comments on our paper.
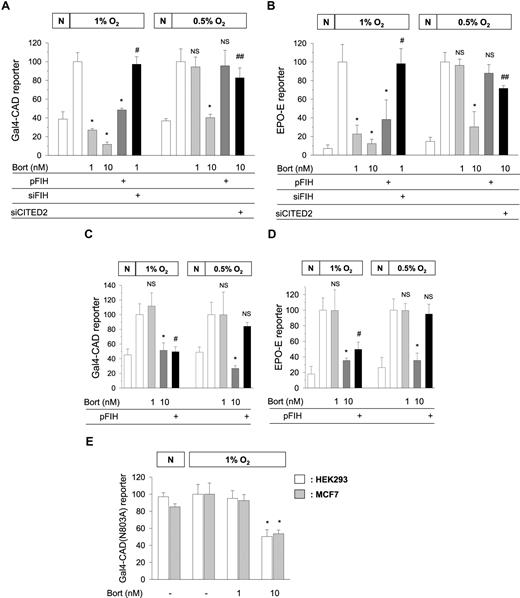
Kaluz et al demonstrated that Gal4−CAD was inactivated by bortezomib only above 10 nM. However, it should be recalled that FIH requires oxygen to hydroxylate CAD. For this reason, we used 1% oxygen to achieve CAD activation, whereas they used 0.5% oxygen. As previously reported, 1 nM bortezomib noticeably inhibited CAD in 1% oxygen, but not in 0.5% oxygen. However, 10 nM bortezomib inhibited CAD in both oxygen tensions (Figure 1A). FIH overexpression inhibited CAD and FIH knock-down recovered CAD repressed by 1 nM bortezomib in 1% oxygen. However, FIH overexpression failed to inhibit CAD in 0.5% oxygen. In EPO-enhancer reporter, we obtained the same results as above (Figure 1B). It appears that FIH requires at least 1% oxygen to inhibit CAD, and thus, FIH-mediated CAD inhibition by bortezomib cannot occur in more severe hypoxia. Then, how does 10 nM bortezomib inhibit CAD in 0.5% oxygen? Figure 1A and B show that CITED2 (cAMP-responsive element–binding protein [CBP]/p300-interacting transactivators with glutamic acid [E] and aspartic acid [D]–rich tail 2) contributed to CAD inhibition by high concentrations of bortezomib. Indeed, we previously reported that CITED2 interfered with CAD/p300 binding and was also up-regulated by bortezomib above 10 nM.1,2
Oxygen-dependent effects of bortezomib and FIH on the transcriptional activity of HIF-1. Gal4-CAD/Gal4-luciferase reporter plasmids (A,C) or EPO enhancer-luciferase reporter plasmid (B,D) were cotransfected with cytomegalovirus-β-gal plasmid into HEK293 (A,B) or MCF7 (C,D) cells. Gal4-CAD(N803A)/Gal4-luciferase reporter plasmids were cotransfected with the β-gal plasmid into HEK293 and MCF7 cells (E). HA-tagged FIH (pFIH) plasmid, FIH siRNA (siFIH), or CITED2 siRNA (siCITED2) was also cotransfected into these cells. After being stabilized for 48 hours, transfected cells were incubated under normoxic (N) or hypoxic (1% or 0.5% O2) conditions for 16 hours with 1 or 10 nM bortezomib (4 hours prior to hypoxia), and then luciferase activities were measured. The results shown are presented as relative values versus the hypoxic control, and are plotted as means (± SD) of 8 or more experiments. *P < .01 versus the hypoxic control; #P < .01 versus the 1 nM bortezomib group; ##P < .01 versus the 10 nM bortezomib group; NS, not significant versus the hypoxic control.
Oxygen-dependent effects of bortezomib and FIH on the transcriptional activity of HIF-1. Gal4-CAD/Gal4-luciferase reporter plasmids (A,C) or EPO enhancer-luciferase reporter plasmid (B,D) were cotransfected with cytomegalovirus-β-gal plasmid into HEK293 (A,B) or MCF7 (C,D) cells. Gal4-CAD(N803A)/Gal4-luciferase reporter plasmids were cotransfected with the β-gal plasmid into HEK293 and MCF7 cells (E). HA-tagged FIH (pFIH) plasmid, FIH siRNA (siFIH), or CITED2 siRNA (siCITED2) was also cotransfected into these cells. After being stabilized for 48 hours, transfected cells were incubated under normoxic (N) or hypoxic (1% or 0.5% O2) conditions for 16 hours with 1 or 10 nM bortezomib (4 hours prior to hypoxia), and then luciferase activities were measured. The results shown are presented as relative values versus the hypoxic control, and are plotted as means (± SD) of 8 or more experiments. *P < .01 versus the hypoxic control; #P < .01 versus the 1 nM bortezomib group; ##P < .01 versus the 10 nM bortezomib group; NS, not significant versus the hypoxic control.
Compared with HEK293, MCF7 was less sensitive to bortezomib because 1 nM bortezomib failed to inhibit either Gal4−CAD or EPO-enhancer activity (Figure 1C,D). These results support the cell type-specific sensitivity to bortezomib even in terms of FIH activation. However, FIH showed the oxygen-dependent action in MCF7, as was found in HEK293. In addition, Kaluz et al suggested that the CAD inhibition by bortezomib is independent of FIH because bortezomib inhibited CAD(N803A) mutant. However, this was also the result obtained in 0.5% oxygen and at concentrations higher than 10 nM. In 1% oxygen, CAD(N803A) was inactivated by 10 nM bortezomib, but not by 1 nM bortezomib (Figure 1E).
FIH has a much higher affinity for oxygen than HIF-1α proline hydroxylases (PHDs), and thus retains its activity even in 1% oxygen,3 which was also found during our study.1 Therefore, CAD hydroxylation could be reinforced by bortezomib in 1% oxygen, and this may occur in the cytoplasm before nuclear translocation of HIF-1α.4
Finally, Kaluz et al mentioned that our speculation concerning CAD inactivation by subnanomolar bortezomib may not be attributable to proteasome inhibition. Despite its substantial inhibition of proteasome, 10 μM MG132 failed to stimulate FIH binding to HIF-1α. As they suggested, we also found that 1 nM bortezomib inhibited proteasome over 50%.1 Nevertheless, it is possible that the remaining proteasome activity is sufficient to degrade proteins, which is in-line with higher concentrations of bortezomib used by Dr Kaluz. Yet we did not clarify the proteasome-independent mechanism of bortezomib, and thus, we just speculated on this possibility in the Discussion. In terms of targets of bortezomib, the possibility of new targets cannot be excluded, although no targets other than proteasome have been identified to date.
In conclusion, both FIH and CITED2 mediate CAD inhibition, but their contributions to the action of bortezomib may be variable and dependent on oxygen level and bortezomib concentration. Furthermore, chemosensitivity to bortezomib also varies among cell lines. The mechanism underlying CAD inhibition by bortezomib is complicated.
Authorship
This work was supported by a grant from The National Research and Development Program for Cancer Control, Korean Ministry of Health & Welfare Research Fund (#0520260-2).
Contribution: D.H.S. performed research, Y.S.C. analyzed data, and J.W.P. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jong-Wan Park, MD, PhD, Department of Pharmacology, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul 110-799, Korea; e-mail: parkjw@snu.ac.kr.
References
Wellcome Trust

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal