To the editor:
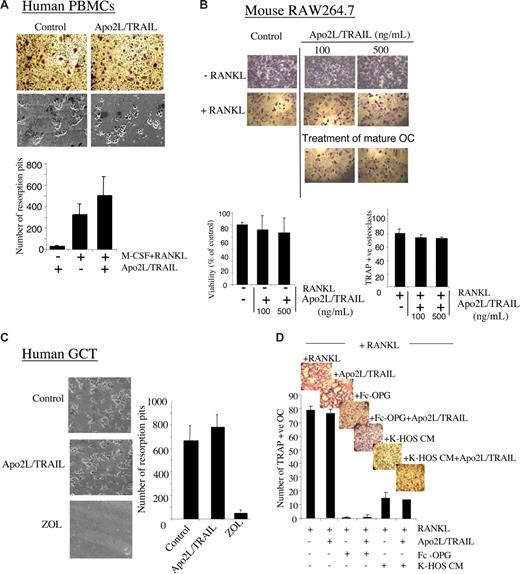
Apo2L/TRAIL is a promising new cancer therapeutic with demonstrated efficacy against a variety of malignancies.1 A number of recent studies have demonstrated the ability of different versions of recombinant Apo2L/TRAIL to inhibit both human and mouse osteoclastogenesis, and influence the survival of mature osteoclasts.2-4 Recent work by Zauli and colleagues also demonstrated that injection of mice with recombinant TRAIL induced a significant increase in tibial trabecular thickness and total bone mass, suggesting that Apo2L/TRAIL may regulate normal bone metabolism via inhibition of host osteoclast differentiation and bone resorption.4 In contrast, other studies have suggested that different versions of recombinant Apo2/TRAIL might promote osteoclastogenesis5 and different molecular mechanisms to explain these observations have been proposed, with the interplay between TRAIL and the RANKL/RANK/OPG system being central to the cause.5 We have shown that the version of Apo2L/TRAIL that is currently being used in phase 1b clinical trials, inhibited breast cancer growth within bone and protected against cancer-induced osteolysis in a murine model.6 These results could be explained by the potential of Apo2L/TRAIL to either (1) directly induce apoptosis of breast cancer cells within the bone microenvironment, or, based on the above evidence, (2) block osteoclastogenesis or osteoclast activity via direct inhibitory effects. We have examined in detail the effect of the clinical grade recombinant Apo2L/TRAIL on bone histology in mice, and also its effect on osteoclast differentiation and bone resorption in 3 independent in vitro models of osteoclastogenesis. Firstly, we found that Apo2L/TRAIL did not block RANKL+ macrophage–colony stimulating factor (M-CSF)–mediated osteoclast differentiation or bone resorption from human peripheral blood mononuclear cells (PBMC; Figure 1A) or from the murine monocytic cell line, RAW264.7 (Figure 1B). Furthermore, Apo2L/TRAIL had no effect on bone resorption by mature osteoclasts isolated from human giant cell tumors of bone (GCT; Figure 1C). In addition, Apo2L/TRAIL could not reverse the antiosteoclastogenic effect of recombinant Fc-OPG or native OPG, a finding that has now been substantiated by Zauli and colleagues7 (Figure 1D). Secondly, extensive histomorphometric analysis of bones from Apo2L/TRAIL–treated animals showed no changes in any bone histomorphometric parameter, compared with the untreated animals (data not shown). It is now well accepted that various preparations of recombinant Apo2L/TRAIL may have different biologic activities, perhaps explaining the findings in osteoclasts,2-4 in addition to the toxicity reported previously in human hepatocytes.8 The specificity and purity of the soluble Apo2L/TRAIL protein used in our study has been verified both in vitro,1 in animal models in vivo,9 and in human clinical trials.10 In conclusion, our results argue against a direct effect of Apo2L/TRAIL on osteoclast differentiation and activity and suggest that the protective effects of this agent on cancer-induced osteolysis are due to direct actions on cancer cells themselves within the bone microenvironment, resulting in the abrogation of the “vicious cycle” of cancer-induced bone destruction.
Effect of Apo2L/TRAIL on osteoclast differentiation and bone resorption. (A) Human peripheral blood mononuclear cells (PBMC) from healthy donors were isolated from buffy coats acquired from the Australian Red Cross Blood Service. The cells were diluted in Hanks balanced salt solution (HBSS) and separated by gradient centrifugation with Lymphoprep (Nycomed Pharma, Oslo, Norway). Isolated cells (2.5 × 105 cells/well) were then plated in minimal essential medium (MEM), supplemented with 10% fetal calf serum, l-glutamine (2 mM), Hepes (20 mM), recombinant human M-CSF (25 ng/mL; Genetics Institute, Cambridge, MA), 1α,25(OH)2vitamin D3 (10 nM; Wako Pure Chemicals, Osaka, Japan) and dexamethasone (10 nM; Fauldings, Adelaide, Australia) into 96-well plates containing slices of sperm whale dentine, for the bone resorption assay, or directly into wells for tartrate resistant acid phosphatase (TRAP) staining. The following day, media were removed and replaced with media as above, additionally supplemented with recombinant human RANKL (50 ng/mL; Roche, Indiannapolis, IN), in the presence or absence of 100 ng/mL of Apo2L/TRAIL (Genentech, South San Francisco, CA). Media and treatments were replaced every 3 days. Cells were fixed on day 9 and stained histochemically for TRAP (Sigma-Aldrich, St Louis, MO), and TRAP-positive cells were visualized by light microscopy. To assess bone resorption, dentine slices were washed in 0.1% Extran detergent, rinsed with distilled water, washed in 70% ethanol, and air-dried overnight. The dentine slices were then mounted on stubs, carbon-gold coated, and examined on a Philips XL-20 scanning electron microscope. Images were then analyzed and resorption pits counted using ImageQuant software (GE Healthcare, Little Chalfont, United Kingdom; quadruplicate dentine slices for each treatment). Results shown are average numbers of pits (± SEM) and the significant differences between treatments determined using Student t tests (2-tailed, unpaired). (B) RAW264.7 cells were left untreated or cultured in the presence of increasing concentrations of Apo2L/TRAIL with or without RANKL for 5 days. Shown are representative fields of the cell cultures treated as indicated after TRAP staining. The number of TRAP-positive multinucleated cells (containing 3 or more nuclei) was scored. The viability of identically-treated cells was assessed by crystal violet staining. Data represent the means (± SD) of 3 independent experiments. Osteoclasts generated after 5 days of RANKL-stimulation were treated with Apo2L/TRAIL for a further 24 hours as indicated to assess the effect of Apo2L/TRAIL on the survival of the mature osteoclasts. Apo2L/TRAIL had no effect on the survival of mature osteoclasts. (C) To determine the effect of Apo2L/TRAIL on bone resorption by mature osteoclasts, we used cells isolated from human primary giant cell tumors of bone (GCT) specimens known to contain abundant numbers of osteoclast-like cells, as we have described previously. GCT cells were plated onto dentine slices in 96-well plates at a density of 105/well and treated for 5 days with 100 ng/mL of Apo2L/TRAIL or with 25 μM of the osteoclast-ablating bisphosphonate zoledronic acid (ZOL). Medium and treatments were replaced on day 3 and pit formation was determined, as described above, after 5 days. Whereas treatment with ZOL completely inhibited bone resorption, Apo2L/TRAIL treatment was without effect. (D) RAW264.7 cells were cultured in the presence of RANKL (100 ng/mL) with or without recombinant Fc-OPG (100 ng/mL) or conditioned media from cultured K-HOS human osteosarcoma cells in the presence or absence of 100 ng/mL of Apo2L/TRAIL. The number of TRAP+ osteoclasts were assessed as described in panel A. The data represent the means (± SD) of 3 different experiments.
Effect of Apo2L/TRAIL on osteoclast differentiation and bone resorption. (A) Human peripheral blood mononuclear cells (PBMC) from healthy donors were isolated from buffy coats acquired from the Australian Red Cross Blood Service. The cells were diluted in Hanks balanced salt solution (HBSS) and separated by gradient centrifugation with Lymphoprep (Nycomed Pharma, Oslo, Norway). Isolated cells (2.5 × 105 cells/well) were then plated in minimal essential medium (MEM), supplemented with 10% fetal calf serum, l-glutamine (2 mM), Hepes (20 mM), recombinant human M-CSF (25 ng/mL; Genetics Institute, Cambridge, MA), 1α,25(OH)2vitamin D3 (10 nM; Wako Pure Chemicals, Osaka, Japan) and dexamethasone (10 nM; Fauldings, Adelaide, Australia) into 96-well plates containing slices of sperm whale dentine, for the bone resorption assay, or directly into wells for tartrate resistant acid phosphatase (TRAP) staining. The following day, media were removed and replaced with media as above, additionally supplemented with recombinant human RANKL (50 ng/mL; Roche, Indiannapolis, IN), in the presence or absence of 100 ng/mL of Apo2L/TRAIL (Genentech, South San Francisco, CA). Media and treatments were replaced every 3 days. Cells were fixed on day 9 and stained histochemically for TRAP (Sigma-Aldrich, St Louis, MO), and TRAP-positive cells were visualized by light microscopy. To assess bone resorption, dentine slices were washed in 0.1% Extran detergent, rinsed with distilled water, washed in 70% ethanol, and air-dried overnight. The dentine slices were then mounted on stubs, carbon-gold coated, and examined on a Philips XL-20 scanning electron microscope. Images were then analyzed and resorption pits counted using ImageQuant software (GE Healthcare, Little Chalfont, United Kingdom; quadruplicate dentine slices for each treatment). Results shown are average numbers of pits (± SEM) and the significant differences between treatments determined using Student t tests (2-tailed, unpaired). (B) RAW264.7 cells were left untreated or cultured in the presence of increasing concentrations of Apo2L/TRAIL with or without RANKL for 5 days. Shown are representative fields of the cell cultures treated as indicated after TRAP staining. The number of TRAP-positive multinucleated cells (containing 3 or more nuclei) was scored. The viability of identically-treated cells was assessed by crystal violet staining. Data represent the means (± SD) of 3 independent experiments. Osteoclasts generated after 5 days of RANKL-stimulation were treated with Apo2L/TRAIL for a further 24 hours as indicated to assess the effect of Apo2L/TRAIL on the survival of the mature osteoclasts. Apo2L/TRAIL had no effect on the survival of mature osteoclasts. (C) To determine the effect of Apo2L/TRAIL on bone resorption by mature osteoclasts, we used cells isolated from human primary giant cell tumors of bone (GCT) specimens known to contain abundant numbers of osteoclast-like cells, as we have described previously. GCT cells were plated onto dentine slices in 96-well plates at a density of 105/well and treated for 5 days with 100 ng/mL of Apo2L/TRAIL or with 25 μM of the osteoclast-ablating bisphosphonate zoledronic acid (ZOL). Medium and treatments were replaced on day 3 and pit formation was determined, as described above, after 5 days. Whereas treatment with ZOL completely inhibited bone resorption, Apo2L/TRAIL treatment was without effect. (D) RAW264.7 cells were cultured in the presence of RANKL (100 ng/mL) with or without recombinant Fc-OPG (100 ng/mL) or conditioned media from cultured K-HOS human osteosarcoma cells in the presence or absence of 100 ng/mL of Apo2L/TRAIL. The number of TRAP+ osteoclasts were assessed as described in panel A. The data represent the means (± SD) of 3 different experiments.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Andreas Evdokiou, Discipline of Orthopaedics and Trauma, Level 4, Bice Building, Royal Adelaide Hospital, North Terrace, Adelaide 5000, South Australia, Australia; e-mail: andreas.evdokiou@adelaide.edu.au.